Summary
Sleep spindles, defining oscillations of non-rapid eye movement stage 2 sleep (N2), mediate memory consolidation. Spindle density (spindles/minute) is a stable, heritable feature of the sleep electroencephalogram. In schizophrenia, reduced spindle density correlates with impaired sleep-dependent memory consolidation and is a promising treatment target. Measuring sleep spindles is also important for basic studies of memory. But overnight sleep studies are expensive, time-consuming and require considerable infrastructure. Here we investigated whether afternoon naps can reliably and accurately estimate nocturnal spindle density in health and schizophrenia. Fourteen schizophrenia patients and eight healthy controls had polysomnography during two overnights and three afternoon naps. Although spindle density was lower during naps than nights, the two measures were highly correlated. For both groups, naps and nights provided highly reliable estimates of spindle density. We conclude that naps provide an accurate, reliable and scalable alternative to measuring spindle density overnight.
Keywords: endophenotype, clinical trials, cognition, intervention, thalamocortical circuitry
Introduction
Sleep spindles, defining oscillations of stage 2 non-rapid eye movement sleep (N2), mediate memory consolidation (Fogel & Smith, 2011). Spindle activity is a stable, heritable feature of the electroencephalogram (EEG, Purcell et al., 2017), leading to its description as an electrophysiological fingerprint (De Gennaro, Ferrara, Vecchio, Curcio, & Bertini, 2005). Schizophrenia patients and their first-degree relatives have reduced spindle density (spindles/minute) that correlates with impaired sleep-dependent memory consolidation, positive symptoms, executive dysfunction and lower IQ (Manoach, Pan, Purcell, & Stickgold, 2016). Sleep spindles are the product of thalamocortical circuits and spindle density inversely correlates with thalamocortical connectivity (Baran et al., 2019). In schizophrenia, lower spindle density is associated with abnormally increased connectivity, consistent with other evidence of thalamocortical circuit dysfunction (Manoach & Stickgold, 2019). Given that spindle deficits may reflect the pathophysiology of schizophrenia and contribute to its manifestations, spindles are a promising novel treatment target for clinical trials (Wamsley et al., 2013). Measuring sleep spindles is also important for basic studies of memory. A major impediment to measuring spindles, however, is that overnight sleep studies are expensive, time-consuming and require considerable infrastructure and participant burden. Afternoon naps may provide a more cost-effective, scalable alternative to overnights, if they allow reliable measurement of spindles. Following lunch there is an increase in sleep propensity making napping feasible (Monk, 2005). Naps can enhance memory, sometimes as much as a night (Mednick, Nakayama, & Stickgold, 2003), and post-nap memory improvement correlates with spindle density (Schmidt et al., 2006). In the present study, we investigated whether naps can reliably estimate nocturnal spindle density in health and schizophrenia.
Methods
Participants
Fourteen schizophrenia outpatients and eight healthy controls were included based on having successfully completed both overnight and napping sleep studies. Patient and control groups did not differ in age, mean parental education or estimated premorbid verbal IQ, although the control group had proportionately more females (Table 1). Patients had been maintained on stable doses of antipsychotic and adjunctive medications and one was unmedicated for at least six weeks prior to enrollment in both studies (See Table S1 for individual patient characteristics). Diagnoses were confirmed with Structured Clinical Interviews for DSM-IV. Controls were screened to exclude a history of mental illness or a family history of schizophrenia spectrum disorders or psychosis. All participants were screened to exclude diagnosed sleep disorders, treatment with sleep medications, pregnancy and a history of head injury, neurological disorder or substance abuse or dependence within the past six months. Participants gave written informed consent and were paid for participation. The study was approved by the Partners Human Research Committee.
Table 1.
Participant characteristics and group comparisons of demographic data.
| Schizophrenia Patients (n = 14) | Healthy Controls (n = 8) | |||
|---|---|---|---|---|
| M ± SD | M ± SD | t | p | |
| Age (yrs) | 33 ± 6 | 31 ± 6 | −0.8 | .43 |
| Sexa | 11M/3F | 4M/4F | N/A | .34 |
| Mean Parental Education (yrs) | 14 ± 3 | 14 ± 2 | 0.1 | .92 |
| Premorbid Verbal IQb | 104 ± 10 | 109 ± 8 | 1.3 | .21 |
| PANSS Totalc | 63 ± 22 | N/A | Severity: mild | |
Group difference in sex tested with Fisher’s exact test; M=Males, F=Females.
Estimate based on standard scores on the reading subtest of the Wide Range Achievement Test 3.
PANSS (Positive and Negative Syndrome Scale) symptom severity.
Procedure
Participants completed four nights of polysomnography (PSG) at the Massachusetts General Hospital Clinical Research Center as a part of a double-blind, randomized, placebo-controlled study of eszopiclone. Placebo and eszopiclone visits were separated by one week and took place on two consecutive weeknights, with the first night of each visit serving as a baseline night and the second a memory night. On the memory night participants were trained on the Motor Sequence Task (MST, Wamsley et al., 2012) 2h before bedtime. Bedtime was 10:30pm and participants slept up to 10 hours. The present report includes data from the two placebo nights only.
Then, 7.4±8.7 (M±SD, range: .4–34.4) months later, the same participants completed three afternoon nap visits, separated by at least a week, with simultaneous EEG and magnetoencephalography (MEG; Elekta-Neuromag) recording of sleep. The first (adaptation) visit acclimated the participant to napping in the MEG scanner. Baseline and memory visits followed in a counter-balanced order. During the memory visit, participants trained on the MST and then had a 90-minute nap opportunity starting at 2:00pm.
Polysomnography:
Overnight PSG was acquired at 400Hz using an Aura LTM64 system (Grass Technologies, Astro-Med Inc., RI) and a 58 channel EEG cap (Easycap GmbH, Herrsching, Germany). During naps, PSG was acquired at 600Hz using a 70 channel Easycap. Impedances were kept below 10kOhms at the start of each recording. Submental EMG (electromyography) and EOG (electrooculography) were also acquired.
PSG recordings were divided into 30s epochs and visually scored as WAKE, REM, N1, N2 or N3 according to standard criteria (Iber, Ancoli-Israel, Chesson, & Quan, 2007) by raters blind to diagnosis. Data were re-referenced to a common average and pre-processed using BrainVision Analyzer 2.0 (BrainProducts, Germany) for overnights and MNE (Gramfort et al., 2014) and custom Matlab scripts for naps. Data were filtered at 0.3–35Hz. Electrodes displaying significant artifacts were interpolated with spherical splines. Data were visually inspected and epochs with artifacts were removed. Five PSG records (5% of total) were excluded from further analyses, including three patient naps, one control nap and one control overnight, based on having either <2 mins of total sleep time (TST), no N2 remaining after artifact rejection or artifacts throughout the recording.
Spindle detection:
Spindles were automatically detected in the 12–15Hz band at Cz using an automated wavelet-based algorithm that was validated against hand-counted spindles in healthy and schizophrenia samples from a prior study (Wamsley et al., 2012). These samples were used to set our threshold for spindle detection to 9 times the median signal amplitude of artifact-free epochs based on its maximization of between-class (‘spindle’ vs. ‘non-spindle’) variance. We chose to measure N2 spindle density since it most consistently shows deficits in schizophrenia and correlates with memory (Manoach & Stickgold, 2019).
Control analyses:
To examine whether MST training affected spindle density or sleep architecture, we used mixed-effects linear models for nights and naps separately with Subject as a random effect and Visit (baseline, memory), Group (controls, patients) and their interaction as fixed effects. In this model, we substituted Nap Order (1st, 2nd, 3rd) for Visit to examine its effects. Since there were no significant effects of MST or nap order on either spindle density or any index of sleep architecture (all ps>.07), these factors were omitted from subsequent analyses.
Nap vs. night effects:
We characterized differences in spindle density and sleep architecture between naps and nights using a linear mixed-effects model with Subject as a random effect and Group, Condition (nights, naps) and their interaction as fixed effects. We also correlated average spindle density for naps and nights.
Nap vs. night reliability of spindle density:
To calculate Intraclass Correlation Coefficients, we estimated between- and within-subject variances in spindle density from regression models with Subject as a random effect. To compare the reliability of spindle density between nights and naps we estimated the 95% Confidence Intervals (CIs) of ICCNight, ICCNap and their difference (ICCNight-ICCNap) based on 10,000 bootstrap samples.
Results
Nap vs. Night Sleep Quality and Architecture
Patients had less TST than controls during naps (Table 2; Naps: F(1,19)=7.5, p=.01) but not nights (F(1,20)=.3, p=.58; Group by Condition: F(1,103)=4.3, p=.04). In both groups, percent of wake time after sleep onset (WASO) was greater during naps than nights (Controls: F(1,30)=6.6, p=.02; Patients: F(1,54) = 55.1, p<10−3; Combined: F(1,84)=42.9, p<10−3) and more so in patients (Group by Condition: F(1,84)=7.7, p=.007).
Table 2.
Nap vs. night effects on sleep quality and architecture.
| Night | Nap | Group | Condition | Group × Condition | |||
|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | ||||
| M ± SD | M ± SD | M ± SD | M ± SD | p | p | p | |
| Sleep Quality | |||||||
| 511 ± 47 | 500 ± 34 | 49 ± 17 | 70 ± 18 | .52 | < 10−3* | .04* | |
| 5 ± 4 | 4 ± 5 | 37 ± 38 | 14 ± 13 | .10 | < 10−3 * | .01* | |
| Sleep Architecture (%) | |||||||
| 9 ± 5 | 10 ± 5 | 34 ± 18 | 24 ± 11 | .24 | < 10−3* | .03* | |
| 58 ± 8 | 51 ± 5 | 60 ± 13 | 54 ± 11 | .11 | .47 | .91 | |
| 18 ± 8 | 19 ± 6 | 5 ± 8 | 17 ± 16 | .07 | < 10−3* | .01* | |
| 16 ± 3 | 20 ± 5 | 1 ± 3 | 5 ± 5 | .01* | < 10−3* | .76 | |
| Sleep Architecture (min) | |||||||
| 44 ± 23 | 47 ± 22 | 16 ± 10 | 16 ± 6 | .79 | < 10−3* | .74 | |
| 296 ± 50 | 257 ± 33 | 30 ± 13 | 37 ± 13 | .12 | < 10−3* | .001* | |
| 89 ± 39 | 96 ± 31 | 3 ± 4 | 13 ± 13 | .29 | < 10−3* | .75 | |
| 82 ± 13 | 101 ± 28 | 1 ± 2 | 4 ± 4 | .01* | < 10−3* | .01* | |
Significant effects
WASO (%) was measured as WASO/(TST+WASO).
TST = Total Sleep Time; WASO = Wake time After Sleep Onset.
Both groups had a higher N1% (F(1,84)=77, p<10−3) and minutes (F(1,84)=98, p<10−3) during naps than nights, and the N1% difference was greater in patients (Group-by-Condition: F(1,84)=4.9, p=.03). While N2% did not differ between groups or conditions, a Group-by-Condition interaction (F(1,82)=11.1, p=.001) reflected that patients had more N2 minutes than controls at night (F(1,20)=3.8, p=.07) and fewer during naps (F(1,20)=1.7, p=.20). Patients (F(1,54)=28.1, p<10−3), but not controls (F(1,30)=.2, p=.66), had a lower N3% during naps than nights (Group-by-Condition: F(1,84)=7.2, p=.01). Only 14/40 patient naps (35%) contained N3 compared with 18/23 control naps (78%; χ2=10.9, p<10−3). REM% was lower in naps than nights for both groups (F(1,84)=297.8, p<10−3) and lower for patients than controls (F(1,21)=8.4, p=.01). Only 4/40 patient naps (10%) and 10/23 control naps (44%) contained REM sleep (χ2=9.5, p=.002).
Nap vs. Night Spindle Density
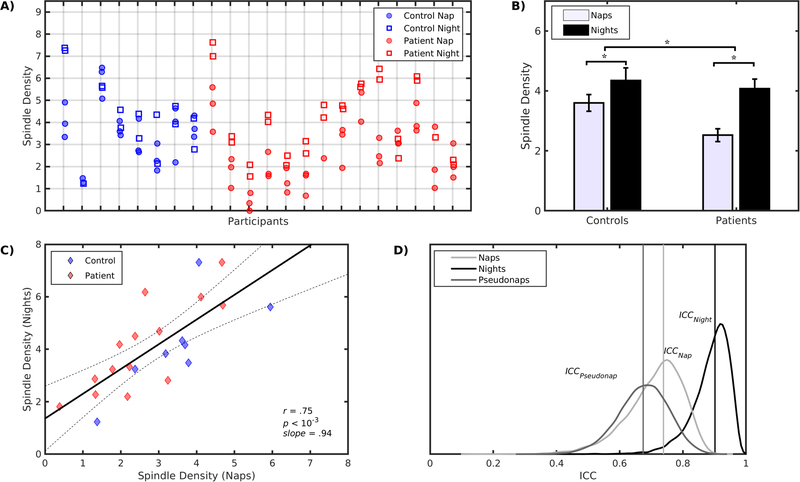
Although spindle density was lower during naps than nights (F(1,81)=40.3, p<10-3) and more so for patients (Group-by-Condition: F(1,81)=5.4, p=.02; Figure 1A,B), average spindle density across naps and nights was highly correlated in the combined group (r=.75, p<10−3; slope=.94; Figure 1C) and for controls (r=.76, p=.03, slope=1.02±.36) and patients (r=.81, p<10−3, slope=1.08±.23) separately. The slopes did not differ between groups (p=.89).
Figure 1.
A) Dot plot of spindle density for each subject during naps (circles) and nights (squares). B) Differences of spindle density between naps and nights for controls and patients. Patients showed a larger nap/night spindle density difference than controls. C) Relation of average spindle density during naps (x-axes) and nights (y-axes). The solid line is the least squares line for the combined groups and 95% confidence intervals are indicated by dotted lines. D) Spindle density reliability across naps (ICCNap), nights (ICCNight) and pseudonaps (ICCPseudonap) are indicated by vertical lines along with their bootstrap distributions.
Nap vs. Night Reliability
The ICCs for naps were the same across groups, but higher for patients for nights (p=.007; Table 3). We combined groups to increase statistical power and found that spindle density reliability was higher for nights (ICCNight=.89, p<10−3; Figure 1D) than naps (ICCNap=.74, p<10−3) and this difference approached significance (ICCNight-ICCNap=.16; p=.09).
Table 3.
Intraclass Correlation Coefficients (ICC) and their 95% Confidence Intervals.
| Night | Nap | Pseudonap | Night - Nap | Nap - Pseudonap |
|---|---|---|---|---|
| .97* [.92, .98] | .72* [.39, .84] | .69* [.46, .86] | .25* [.11, .57] | .03 [−.35, .28] |
| .72* [.07,.91] | .72* [.15, .89] | .66* [.40, .87] | −.006 [−.79,.6] | .06 [−.55, .37] |
| .89* [.74, .97] | .74* [.50, .85] | .67* [.51, .82] | .17 [−.03, .39] | .07 [−.21, .27] |
Significance (p<.05) based on 10,000 bootstrap samples.
We evaluated whether this difference in reliability was simply due to having less N2 during naps. For each participant, we measured spindle density in random samples of N2 from the baseline night that matched the N2 duration of each nap (pseudonap). We repeated this procedure 10,000 times to estimate the mean and 95% CI of the ICCPseudonap and compared its reliability to actual naps. Spindle density reliability across pseudonaps (ICCPseudonap=.67, p<10−3) did not differ from actual naps in the combined group (ICCNap-ICCPseudonap=.07, p=.71; Table 3; Figure 1D).
Discussion
Measures of N2 spindle density during naps are highly reliable in both health and schizophrenia. Moreover, spindle density during naps and nights is strongly correlated, supporting the use of naps as an accurate and reliable alternative to measuring overnight spindle density, with a few caveats.
Spindle density reliability was marginally lower for naps than nights, a difference that was driven by patients. This less reliable estimate may simply reflect less N2 during naps than nights. When nocturnal N2 was sampled in nap long segments (pseudonaps), spindle reliability for nocturnal sleep and naps did not differ.
Although N2% did not differ between nights and naps, naps significantly underestimated nocturnal spindle density. This is consistent with prior work and may reflect that melatonin secretion at night increases spindles (Knoblauch, Martens, Wirz-Justice, Krauchi, & Cajochen, 2003) or that worse sleep quality during naps impeded spindle generation (although WASO% did not correlate with spindle density). Reduced sleep quality during naps than nights in the present study may reflect having to sleep lying supine in the bore of an MEG scanner instead of a bed.
Despite having similar sleep architecture and quality during nights as controls, patients had poorer sleep quality during naps. Nevertheless, the groups did not differ in N2% or in the reliability of spindle density during naps. Although the small sample left us underpowered to examine group differences in spindle density or correlations with memory, studies with larger samples have shown that spindle density during naps is reduced in schizophrenia and correlates with memory improvement in health (Mylonas et al., 2017; Schmidt et al., 2006)
In summary, these findings provide additional evidence that spindle density is a reliable and robust trait of the nocturnal sleep EEG (Purcell et al., 2017) and extend these findings to naps and to schizophrenia. Despite the limitations of the present study, including the long interval between overnight and nap studies and their different settings, naps still provided reliable and accurate estimates of nocturnal sleep spindle density. We conclude that naps provide a feasible, efficient and scalable alternative to measuring spindle density in nocturnal sleep for basic and clinical studies.
Supplementary Material
Acknowledgements:
George and Marie Vergottis Postdoctoral Fellowship (DM); National Institutes of Health R01-MH48832 (DSM, RS); K24-MH099421 (DSM); T32-HL07901 (WGC); K01-MH114012 (BB); National Center for Research Resources UL1TR001102-01 (Harvard Clinical and Translational Science Center), Shared Instrumentation Grants 1S10RR023401, 1S10RR019307, 1S10RR023043.
Footnotes
Disclosure Statement:
Financial Disclosure: none
Non-financial Disclosure: none
References
- Baran B, Karahanoglu FI, Mylonas D, Demanuele C, Vangel M, Stickgold R, … Manoach DS (2019). Increased Thalamocortical Connectivity in Schizophrenia Correlates With Sleep Spindle Deficits: Evidence for a Common Pathophysiology. Biol Psychiatry Cogn Neurosci Neuroimaging. doi: 10.1016/j.bpsc.2019.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Vecchio F, Curcio G, & Bertini M (2005). An electroencephalographic fingerprint of human sleep. Neuroimage, 26(1), 114–122. [DOI] [PubMed] [Google Scholar]
- Fogel SM, & Smith CT (2011). The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev, 35(5), 1154–1165. [DOI] [PubMed] [Google Scholar]
- Gramfort A, Luessi M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, … Hamalainen MS (2014). MNE software for processing MEG and EEG data. Neuroimage, 86, 446–460. doi: 10.1016/j.neuroimage.2013.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, & Quan SF (2007). The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, Ill: American Academy of Sleep Medicine. [Google Scholar]
- Knoblauch V, Martens W, Wirz-Justice A, Krauchi K, & Cajochen C (2003). Regional differences in the circadian modulation of human sleep spindle characteristics. Eur J Neurosci, 18(1), 155–163. doi: 10.1046/j.1460-9568.2003.02729.x [DOI] [PubMed] [Google Scholar]
- Manoach DS, Pan JQ, Purcell SM, & Stickgold R (2016). Reduced Sleep Spindles in Schizophrenia: A Treatable Endophenotype That Links Risk Genes to Impaired Cognition? Biol Psychiatry, 80(8), 599–608. doi: 10.1016/j.biopsych.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, & Stickgold R (2019). Abnormal sleep spindles, memory consolidation and schizophrenia. . Annu Rev Clin Psychol, 15, 451–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S, Nakayama K, & Stickgold R (2003). Sleep-dependent learning: a nap is as good as a night. Nat Neurosci, 6(7), 697–698. [DOI] [PubMed] [Google Scholar]
- Monk TH (2005). The post-lunch dip in performance. Clin Sports Med, 24(2), e15–23, xi-xii. [DOI] [PubMed] [Google Scholar]
- Mylonas D, Demanuele C, Baran B, Kohnke EJ, Tocci C, Stickgold R, … Manoach DS (2017). Spindle activity related to motor procedural learning in patients with schizophrenia. Paper presented at the American Physiological Sleep Society, Boston. [Google Scholar]
- Purcell SM, Manoach DS, Demanuele C, Cade BE, Mariani S, Cox R, … Stickgold R (2017). Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat Commun, 8, 15930. doi: 10.1038/ncomms15930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Munch M, … Cajochen C (2006). Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci, 26(35), 8976–8982. doi: 10.1523/JNEUROSCI.2464-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley E, Tucker MA, Shinn AK, Ono KE, McKinley S, Ely AV, … Manoach DS (2012). Reduced sleep spindles and spindle coherence in schizophrenia: Mechanisms of impaired memory consolidation? Biol Psychiatry, 71(2), 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Shinn AK, Tucker MA, Ono KE, McKinley SK, Ely AV, … Manoach DS (2013). The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep, 36(9), 1369–1376. doi: 10.5665/sleep.2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.