Abstract
Background
Prescription opioids (PO) have been widely used for chronic non-cancer pain, with commensurate concerns for overdose. The long-term effect of these medications on non-overdose mortality in the general population remains poorly understood. This study’s objective was to examine the association of prescription opioid use and mortality in a large cohort, accounting for gender differences and concurrent benzodiazepine use, and using propensity score matching.
Methods
29,025 US community-dwellers were enrolled in the REasons for Geographic and Racial Differences in Stroke cohort between 2003 and 2007, and followed through December 31, 2012. At baseline there were 1,907 participants with PO; 1,864 of them were matched to participants without PO, based on the model-derived propensity to receive opioid prescriptions. Causes of death were expert-adjudicated.
Results
Over median follow-up of 6 years there were 4428 deaths (413 among persons with PO). The risk for all-cause mortality was 12% higher, in absolute terms, for persons with PO compared to those without PO in the overall sample, with gender differences (interaction p=0.0008). The risk of death was increased for women with PO (hazard ratio [HR] 1.21 [95% Confidence Interval (CI) 1.04–1.40]), but not men (HR 0.92 [0.77–1.10]). Women with PO were at higher risk of cardiovascular disease (CVD) death (HR 1.43 [1.12–1.84]), sudden death (HR 2.02 [95%CI 1.29–3.15]) (a subset of CVD death), and accidents (HR 2.18 [1.03–4.60]). These risks were not observed for men with PO.
Conclusion
Over 6 years of follow-up, women but not men who had opioid prescriptions were at higher risk of all-cause mortality, CVD death, sudden death, and accidents. Special caution in prescribing opioids for women may be warranted until these findings are confirmed.
Introduction
Half of older Americans suffer from chronic non-cancer pain that limits their daily activities, and management of chronic pain remains a challenge.1,2,3 Between 1999 and 2011, prescription of opioids for chronic non-cancer pain quadrupled4–6, with the parallel increase in opioid use disorder and overdose related mortality, a phenomenon that is widely described as the opioid epidemic or opioid crisis.7 Although per capita prescriptions have fallen since 2012–2013,8 the volume of opioids prescriptions has not returned to its pre-1999 level.9 Approximately 3–4% of US community-dwelling adults reported the long-term therapeutic use of opioids.10,11,12
While opioid use disorder and overdose-related mortality have been studied,8,13–16 less is known about non-overdose-related all-cause and cause-specific mortality, particularly from major somatic comorbidities such as cardiovascular disease, chronic pulmonary disease, and infection, among persons with opioid prescriptions in the community. 17–19 Some evidence from retrospective studies or prescription records does suggest that long-term use of prescription opioids may be associated with increased non-overdose-related morbidity, hospital utilization20 and mortality, especially for older adults21–26 and women .27 However, data on mortality and causes of death among adults prescribed opioids in prospective general population-based cohorts are quite limited.17,28,29
Therefore, the objective of this study was to examine the association of prescription opioid use (POU) and mortality among older (age 45 and above) adults enrolled in REasons for Geographic And Racial Differences in Stroke (REGARDS), a large prospective national cohort study, controlling for potential confounding by indication with the help of propensity score-matched analyses, and accounting for gender differences. Additionally, we explored association of POU with specific causes of death, grouped by causal category as infectious, chronic pulmonary, cardiovascular, sudden (a subset of cardiovascular), cancer and accident-related deaths.
Methods
This analysis is built upon previous data from the REGARDS study that showed increased incident coronary heart disease (CHD) and cardiovascular death (CVD) among females who had PO receipt history.27
Study Cohort and Procedures
The REGARDS study is a prospective national ongoing cohort of 30,239 community-dwelling adults from all 48 continental US states. It was designed to examine regional and racial influences on stroke mortality. Details are described elsewhere; briefly, English-speaking adults aged 45 years and older residing in the continental US were enrolled between 2003 and 2007, with the help of commercially available lists combining mail and telephone contacts.30 Active treatment for cancer was an exclusion criterion.30 Race and gender were balanced by design, with oversampling from the Southeastern US; the final cohort composition was 58% women and 42% African American. Baseline data collection included computer-assisted telephone interviews on socio-demographics, medical history, and health status. In-home examinations by trained staff followed standardized, quality-controlled protocols to collect fasting blood and urine samples; electrocardiograms; blood pressure (BP), anthropometric measures; and pill bottle review of medications. Blood and urine samples were centrally analyzed at the University of Vermont and electrocardiograms at Wake Forest University. Living participants or their proxies were telephoned every 6 months with retrieval of medical records for reported hospitalizations. The REGARDS study procedures were approved by the Institutional Review Boards at the participating centers and all participants provided written informed consent.30
Mortality
Deaths were recorded through December 31, 2012 by report of next of kin or through online sources (e.g., Social Security Death Index) and the National Death Index. Proxies or next of kin were interviewed about the circumstances surrounding death. Death certificates, medical records, and autopsy reports were also obtained to adjudicate cause of death.31 Data from all sources were reviewed by trained experts to adjudicate the underlying cause of death. Cause-specific deaths were classified as infectious, chronic pulmonary disease, CVD, including sudden deaths and non-sudden CVD deaths, cancer and accidents. CVD death was defined as death from CHD, stroke, heart failure, sudden death, vascular pathology, and other CVD causes. Sudden death was defined as unexpected death with rapid collapse and without extra-cardiac cause. Accidental deaths included injuries, suicide, homicide, and other accidents (including poisoning and overdoses). For all adjudicated endpoints, κ for agreement between independent adjudicators was >0.80.31
Main Exposure: Prescription Opioids
We have searched REGARDS baseline medications database, compiled as a result of the pill bottle review during the in-home study visit at study initiation, for any prescription opioids (PO). The following medications were identified and included in the exposure: diphenoxylate, hydrocodone, hydromorphone, meperidine, morphine sulfate, methadone, oxycodone, pentazocine, tramadol, fentanyl, and codeine. Persons with propoxyphene prescriptions were excluded from the analyses because of known cardiotoxicity and propoxyphene’s withdrawal from the US market in 2010.32 We distinguished short and long-acting opioids (methadone; fentanyl; and extended release preparations of oxycodone, morphine, and hydromorphone). We also defined a group of REGARDS participants who were treated with two or more different PO. Additionally persons with prescriptions for benzodiazepines were identified.
Propensity score calculation and matching
To minimize confounding by indication for PO, we matched persons who were prescribed opioids to those who did not have such prescriptions utilizing a propensity score, constructed from 56 baseline characteristics, presented in the supplement (Figure 1) and considered as potential confounders of any association between POU and mortality.33 The propensity score estimated the probability of receiving PO vs. not, and was calculated in a multivariable logistic regression model estimating odds of receiving PO as a function of these 56 characteristics, which included socio-demographics, baseline medical conditions and symptoms, physiological measures, other medication use, health behaviors, and chronic pain levels in the past 4 weeks. Socio-demographics, medication adherence, and health behaviors were self-reported. Use of other medications was determined via pill bottle review or self-report. Diabetes was defined as use of insulin or oral antiglycemic agents, fasting blood glucose concentration of 126 mg/dL or higher, or non-fasting random plasma glucose concentration of 200 mg/dL or higher. Atrial fibrillation was ascertained from self-report or via baseline electrocardiograms. Left ventricular hypertrophy (LVH) was identified using Sokolow-Lyon criteria on electrocardiogram.34 Baseline CHD was defined as electrocardiogram evidence of myocardial infarction (MI) or self-reported history of coronary artery bypass surgery, percutaneous coronary intervention, or MI.31
Depressive symptoms were assessed using the 4-item version of the Center for Epidemiological Studies Depression (CES-D) dichotomized at a threshold of 4.35 Health status was defined using the Short Form 12 (SF-12) physical component summary (PCS) and mental component summary (MCS) scores.36 Pain was measured by asking: “During the past 4 weeks how much did pain interfere with your normal work, including both work outside and housework?”36 with responses dichotomized as moderate to severe chronic pain (pain interfered with work ‘extremely,’ ‘quite a bit’ or ‘moderately’) versus no or low pain (‘not at all’ or ‘a little bit’). QT intervals were corrected for heart rate using the formula QT + (154*[1 – (60/heart rate)]).37 All other medical conditions and symptoms were self-reported.38,39 Physiological measures were assessed using established assays.30
To maximize the sample retention, missing data in baseline characteristics were replaced using multiple imputation by chained equations40 in 30 datasets in STATA 12.
Matching was performed using the ‘Matching’ package in R, where each PO user was propensity-score matched to non-POU using caliper width of 0.01. Multiple matches were weighted, e.g. if 5 non-users matched to a PO user, they were each weighted by a factor of 0.2,41which was equivalent to a 1:1 match. This method used matching with replacement and retained ties. Other methods may match without replacement, which then renders the matching sensitive to the ordering of the dataset, or they try to find a unique match within the specified caliper by randomly breaking ties or finding the closest match within the caliper. To check the sensitivity of our findings to the method of matching we performed a simulation study of the unadjusted analysis. For 1000 random samples of the dataset we repeated the analysis using matching with and without replacement and with and without ties. Overall results were very similar across all methods.
Overall, 1,864 out of 1,907 POU were matched (97.7%). Observations not included in the matched datasets were dropped from further analyses. We created three matched analytic cohorts: overall, men and women. Each gender-specific cohort was created using a propensity score developed within that gender. All subsequent analyses accounted for weighting, and regression analyses included the matched groupings as a clustering factor to compute appropriate standard errors.
Statistical analysis
Chi-square and Student t-tests were used to compare baseline characteristics of persons who has PO receipt vs those who did not before and after propensity score matching to assess whether sample balance was achieved between two groups for the tested covariates. Kaplan-Meier curves and log rank tests were constructed to examine unadjusted associations of PO use with all-cause mortality. One model examined any PO use and the other model contrasted long-acting, short-acting, and use of two or more opioids vs no use. Participants were censored at time of death, last follow-up date, or end of follow up (December 31, 2012).
Cox proportional hazards regression models estimated hazard ratios (HR) and 95% confidence intervals (CI) for all-cause and cause-specific mortality as a function of PO use and were fitted within matched pairs of persons who had PO and participants who did not have opioid prescriptions. Additional models specifically adjusted for concurrent benzodiazepine use. This modeling exercise was first done overall and then repeated separately for men and women. Gender*POU interaction terms were assessed for significance in the overall models. The proportionality assumption was tested by assessing POU*log of follow-up time interactions and was satisfied for all mortality endpoints.
Analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC), R version 3.01, and STATA version 12 (STATA incorporated, College Station, TX).
Results
After excluding 569 individuals missing follow-up data, 69 participants missing medication data, and 576 propoxyphene users, the analytic study sample included 29,025 participants. Over a median of 6 years of follow-up (interquartile range 4.5–7.3 years) there were 4,428 deaths (15.2% of the cohort). We identified 1,907 (6.6%) persons who had PO receipt (Table 1). Hydrocodone was the most commonly reported opioid (46%), followed by tramadol (20.4%), and oxycodone (14.8%); 9.8% had two or more PO and 8.9% had long-acting PO (Figure 1).
Table 1.
Baseline characteristics of REGARDS participants who had prescription opioids (PO) vs those who did not, overall and by gender (before propensity score matching)
| Overall | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics, n (%) | No PO n=27118 | Persons with PO n=1907 | P-value | No PO n=14,668 | Persons with PO n=1227 | P-value | No PO n=12,450 | Persons with PO n=680 | P-value |
| Socio-demographics | |||||||||
| Age, mean years, (SD) | 64.9 ± 9.4 | 63.9 ± 9.3 | <.001 | 65.7 +− 9.3 | 63.9 +− 9.0 | <.001 | 64.3 +− 9.5 | 63.9 +− 9.4 | 0.19 |
| Women | 14668 (54.1) | 1227 (64.3) | <.001 | - | - | -- | -- | ||
| African American | 11053 (40.8) | 855 (44.8) | 0.0005 | 4281 (34.4) | 263 (38.7) | 0.02 | 6772 (46.2) | 592 (48.2) | 0.16 |
| Less than high school education | 3206 (11.8) | 351 (18.4) | <.001 | 1362 (10.9) | 109 (16.1) | <.001 | 1844 (12.6) | 242 (19.8) | <.001 |
| Income <$35,000 | 11095 (46.6) | 1051 (63.5) | <.001 | 4237 (37.5) | 329 (54.2) | <.001 | 6858 (54.8) | 722 (68.8) | <.001 |
| No health insurance | 1784 (6.6) | 120 (6.3) | 0.63 | 676 (5.4) | 41 (6.0) | 0.50 | 1108 (7.6) | 79 (6.4) | 0.15 |
| Married | 16231 (59.9) | 951 (49.9) | <.001 | 9662 (77.6) | 488 (71.8) | 0.0005 | 6569 (44.8) | 463 (37.7) | <.001 |
| Region of residence | <.001 | 0.07 | 0.004 | ||||||
| Stroke Belt | 9350 (34.5) | 694 (36.4) | 4225 (33.9) | 246 (36.2) | 5125 (34.9) | 448 (36.5) | |||
| Stroke Buckle | 5568 (20.5) | 451 (23.6) | 2301 (18.5) | 141 (20.7) | 3267 (22.3) | 310 (25.3) | |||
| Non-belt | 12200 (45.0) | 762 (40.0) | 5924 (47.6) | 293 (43.1) | 6276 (42.8) | 469 (38.2) | |||
| Pain | |||||||||
| Pain, any body part (moderate to severe) | 5268 (19.4) | 1134 (59.5) | <.001 | 1988 (16.0) | 364 (53.6) | <.001 | 3280 (22.4) | 770 (62.8) | <.001 |
| Comorbidities | |||||||||
| Diabetes | 5558 (21.3) | 554 (30.4) | <.001 | 2710 (22.4) | 199 (30.5) | <.001 | 2848 (20.3) | 355 (30.4) | <.001 |
| Coronary Heart Disease | 4627 (17.1) | 429 (22.5) | <.001 | 2903 (23.3) | 189 (27.8) | 0.007 | 1724 (11.8) | 240 (19.6) | <.001 |
| COPD | 2293 (8.5) | 327 (17.1) | <.001 | 964 (7.7) | 97 (14.3) | <.001 | 1329 (9.1) | 230 (18.7) | <.001 |
| History of stroke, PAD or aortic aneurism | 2306 (8.5) | 254 (13.3) | <.001 | 1286 (10.3) | 98 (14.4) | 0.007 | 1020 (7.0) | 156 (12.7) | <.001 |
| Left Ventricular Hypertrophy | 2614 (9.8) | 195 (10.4) | 0.38 | 1135 (9.3) | 65 (9.7) | 0.72 | 1479 (10.3) | 130 (10.8) | 0.53 |
| Atrial fibrillation | 2235 (8.4) | 225 (12.2) | <.001 | 1060 (8.7) | 78 (11.8) | 0.007 | 1175 (8.2) | 147 (12.5) | <.001 |
| History of Transient ischemic attack | 966 (3.8) | 95 (5.5) | 0.004 | 418 (3.6) | 30 (4.9) | 0.10 | 548 (4.0) | 65 (5.9) | 0.002 |
| Heart failure symptoms | 3966 (14.6) | 571 (29.9) | <.001 | 1554 (12.5) | 162 (23.8) | <.001 | 2412 (16.4) | 409 (33.3) | <.001 |
| History of deep venous thrombosis | 1326 (4.9) | 169 (9.0) | <.001 | 513 (4.1) | 56 (8.3) | <.001 | 813 (5.6) | 113 (9.3) | <.001 |
| History of pulmonary embolism | 313 (1.2) | 44 (2.3) | <.001 | 130 (1.0) | 16 (2.4) | 0.002 | 183 (1.2) | 28 (2.3) | 0.002 |
| History of pacemaker use | 438 (1.6) | 50 (2.6) | 0.001 | 286 (2.3) | 25 (3.7) | 0.02 | 152 (1.0) | 25 (2.0) | 0.001 |
| Any falls in a past year | 4124 (15.2) | 539 (28.4) | <.001 | 1476 (11.9) | 144 (21.2) | <.001 | 2648 (18.1) | 395 (32.4) | <.001 |
| Psychological and self-reported health | |||||||||
| Depressive symptoms (CES-D=>4) | 2657 (9.9) | 463 (24.4) | <.001 | 846 (6.8) | 114 (16.9) | <.001 | 1811 (12.4) | 349 (28.6) | <.001 |
| Cognitive score, mean, SD | 5.6 ± 0.7 | 5.6 ± 0.7 | 0.09 | 5.6 +− 0.8 | 5.5 +− 0.8 | 0.01 | 5.7 +− 0.7 | 5.6 +− 0.6 | 0.49 |
| Physical component score of SF-12, mean, SD | 47.3 ± 9.9 | 35.9 ± 11.9 | <.001 | 48.3±9.3 | 37.8±11.8 | <.001 | 46.5±10.4 | 34.9±11.9 | <.001 |
| Perceived stress score, mean, SD | 3.1 ± 2.9 | 4.3 ± 3.4 | <.001 | 2.6±2.7 | 3.6±3.2 | <.001 | 3.5±3.0 | 4.6±3.5 | <.001 |
| Physiological parameters | |||||||||
| Body mass index, kg/m2, mean, SD | 29.2 ± 6.1 | 31.0 ± 7.2 | <.001 | 28.5 +− 5.0 | 29.8 +− 5.8 | <.001 | 29.8 +− 6.8 | 31.6 +− 7.9 | <.001 |
| Systolic blood pressure, mmHg | 127.5 ± 16.5 | 128.5 ± 17.7 | 0.01 | 128.9 +− 16.1 | 128.5 +− 16.9 | 0.54 | 126.3 +− 16.8 | 128.5 +− 18.1 | <.001 |
| Total cholesterol mg/dL, mean, SD | 192.2 ± 39.9 | 189.6 ± 42.4 | 0.009 | 182.9 +− 38.2 | 179.7 +− 40.0 | 0.04 | 200.2 +− 39.7 | 195.3 +− 42.7 | 0.0001 |
| High-Density Lipoprotein, mg/dL, mean, SD | 51.9 ± 16.1 | 50.9 ± 16.5 | 0.01 | 45.5 +− 13.7 | 43.7 +− 14.1 | 0.00 | 57.3 +− 16.1 | 54.9 +− 16.3 | <.001 |
| Triglycerides, mg/dL, mean, SD | 131.3 ± 86.8 | 145.1 ± 86.5 | <.001 | 137.2 +− 98.2 | 146.9 +− 85.8 | 0.01 | 126.2 +− 75.3 | 144.1 +− 86.9 | <.001 |
| QT Interval, corrected for heart rate, ms, mean, SD | 407.2 ± 23.6 | 409.5 ± 23.8 | <.001 | 403.8 +− 24.6 | 405.3 +− 25.1 | 0.10 | 410.1 +− 22.3 | 411.9 +− 22.7 | 0.009 |
| Albumin-to-creatinine ratio, mg/g, median, [25th–75th%] | 7.4[4.6–15.9] | 8.0[4.9–17.6] | 0.0006 | 6.8[4.2–16.8] | 7.3[4.3–18.5] | 0.15 | 7.8[5.0–15.4] | 8.3[5.2–17.3] | 0.008 |
| C reactive protein, mg/L, median, [25th – 75th %] | 2.1[0.9–4.8] | 3.6[1.5–8.1] | <.001 | 1.7[0.8–3.7] | 2.9[1.1–6.5] | <.001 | 2.7[1.1–5.9] | 4.2[1.6–8.7] | <.001 |
| Medication Use | |||||||||
| Medication non-adherence | 7207 (29.4) | 616 (33.3) | 0.0004 | 3076 (27.8) | 233 (35.2) | <.001 | 4131 (30.7) | 383 (32.2) | 0.27 |
| Benzodiazepines | 1147(4.2) | 337(17.7) | <.001 | 427(3.4) | 111(16.3) | <.001 | 720(4.9) | 226(18.4) | <.001 |
| Antihypertensive | 13693 (51.1) | 1182 (62.8) | <.001 | 6022 (48.7) | 400 (59.3) | <.001 | 7671 (53.1) | 782 (64.7) | <.001 |
| Statins | 8900 (33.1) | 702 (37.2) | 0.0003 | 4439 (36.0) | 251 (37.3) | 0.49 | 4461 (30.7) | 451 (37.2) | <.001 |
| Aspirin | 11795 (43.5) | 786 (41.2) | 0.05 | 6301 (50.6) | 314 (46.2) | 0.03 | 5494 (37.5) | 472 (38.5) | 0.49 |
| NSAIDs | 3578 (13.2) | 514 (27.2) | <.001 | 1312 (10.6) | 160 (23.6) | <.001 | 2266 (15.5) | 354 (29.2) | <.001 |
| Insulin | 1445 (5.6) | 172 (9.6) | <.001 | 704 (5.9) | 55 (8.6) | 0.005 | 741 (5.3) | 117 (10.2) | <.001 |
| Number of antibiotic prescriptions in a past year, mean, SD | 1.1 ± 9.7 | 2.1 ± 15.1 | <.001 | 1.1 +− 10.9 | 1.7 +− 14.5 | 0.17 | 1.1 +− 8.6 | 2.3 +− 15.4 | <.001 |
| Health Behaviors | |||||||||
| Smoking, pack/years, mean, SD | 13.2 ± 22.8 | 17.1 ± 25.2 | <.001 | 18.1± 26.4 | 24.3 ±29.2 | <.001 | 9.0 +− 18.1 | 13.2 +− 21.7 | <.001 |
| Alcohol drinks per week, mean, SD | 2.2 ± 6.5 | 1.3 ± 4.7 | <.001 | 3.4 +− 8.3 | 2.5 +− 6.9 | 0.004 | 1.2 +− 4.0 | 0.7 +− 2.6 | <.001 |
| Physical Inactivity | 8848 (33.1) | 898 (47.9) | <.001 | 3338 (27.2) | 275 (41.2) | <.001 | 5510 (38.1) | 623 (51.6) | |
| Adherence to Mediterranean diet, mean score, SD | 4.4 ± 1.7 | 4.1 ± 1.7 | <.001 | 4.5 +− 1.7 | 4.3 +− 1.7 | 0.008 | 4.3 +− 1.7 | 4.0 +− 1.6 | <.001 |
Abbreviations: COPD-chronic obstructive pulmonary disease. NSAIDs – nonsteroidal anti-inflammatory drugs, PAD –periphery artery disease, PO-prescription opioid, SD-standard deviation, SF- Short Form
Figure 1.
Prescription opioid (PO) use by number of prescriptions and type of medication. Percent shown are proportions from any PO overall, among women and among men. “2 or more Rx” indicates percentage of individuals having two or more PO at the same time. Long acting PO include methadone, fentanyl, and extended release preparations of oxycodone, morphine, and hydromorphone.
Characteristics of persons with opioids prescription in the REGARDS study
Table 1 presents the baseline sample characteristics according to presence or absence of PO at baseline. Nearly two thirds of persons with PO were women (64%), 45% were African American, and their mean age was 64 (SD+9) years. More than half of persons with PO reported moderate to severe pain in the past 4 weeks (Table 1).Persons with PO had greater prevalence of comorbid medical conditions: 30% had diabetes and 23% had baseline CHD. Persons with PO reported poorer daily physical functioning, more pack-years of smoking, 24% of them had clinically significant depressive symptoms and a half - were physically inactive. The differences between persons with PO and those without PO were similar after stratification by gender (Table 1). Differences between two groups were attenuated after propensity score matching in all included characteristics, demonstrating that the sample balance was achieved fully (Supplement eTable 1).
All-cause mortality
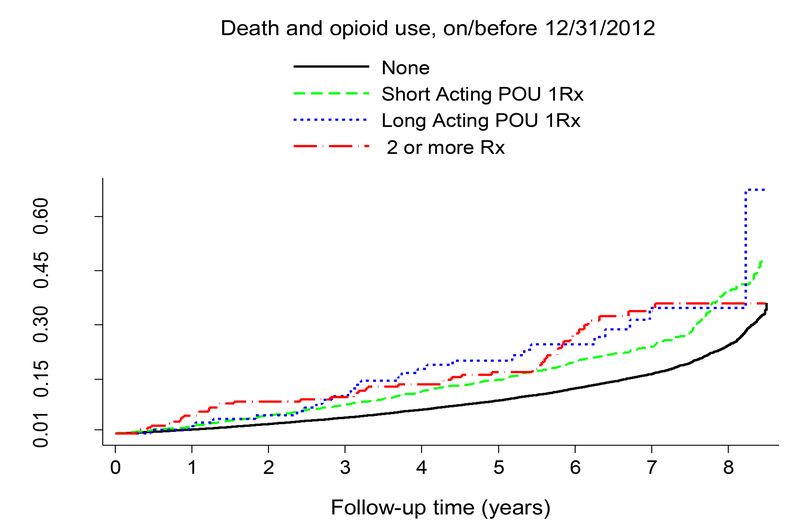
Overall, 4428 deaths occurred in the cohort, with 413 deaths (21.7%) among persons with PO and 4015 deaths (14.8%) among those without PO. In the total sample, unadjusted long-term all-cause mortality risk was elevated among persons with PO compared to those without as illustrated in Figure 2 (log-rank p<.001). Figure 3 presents the Kaplan-Meier plots for cumulative mortality for the 4 mutually exclusive groups: persons with 1 short-acting PO, persons with 1 long-acting PO, persons with of 2 or more PO, and individuals without PO.. After 3 years of follow-up, persons receiving long-acting PO formulations had more deaths than other groups (log-rank p<.001).
Figure 2:
Figure 2 presents Kaplan-Meier plots of the cumulative incidence of all-cause mortality over the follow-up separately for persons with prescription opioid receipt and non-users. The unadjusted mortality rate among persons with PO is statistically significantly higher than among non-users, log-rank test p-value <.001.
Figure 3:
Figure 3 presents Kaplan-Meier plots of the cumulative incidence of all-cause mortality over the follow-up separately for persons with 2 medications or more, persons prescribed long-acting opioids (1 medication) and persons prescribed short-acting opioids (1 medication) and non-users. Long acting PO include methadone, fentanyl, extended release preparations of oxycodone, morphine, and hydromorphone. The unadjusted mortality rates among persons with PO are statistically significantly higher than among non-users, log-rank test p-value <.001.
Table 2 presents results of the propensity-matched analyses. The hazard for all-cause mortality was 15% greater for persons with PO compared to those without (HR 1.15 [95% CI 1.04–1.28]), with a significant interaction between PO use and gender (interaction p=0.0008). In the gender-specific analysis, compared to women who did not have PO, women with PO had greater hazard for death (HR 1.21 [95% CI 1.04–1.48]) (Table 2). There was no significant association between all-cause mortality and PO use among men.
Table 2.
Propensity-matched analysis of the association of prescription opioids (PO) with mortality, overall and by gender, through 12/31/2012
| Overall (n=25,095) | Men (n=13,130) | Women (n=15,895) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Causes of death | Deaths of persons with PO/ Total Deaths, n | HR(95%CI) | P-value | Interaction p for Gender-PO* | Deaths of persons with PO/ Total Deaths, n | HR(95%CI) | P-value | Deaths of persons with PO/ Total Deaths, n | HR(95% CI) | P-value |
| All Causes | 413/4428 | 168/2572 | 245/1856 | |||||||
| Model 1 | 1.15 (1.04–1.28) | 0.008 | 0.92(0.77–1.10) | 0.34 | 1.21 (1.04–1.40) | 0.01 | ||||
| Model 2 | 1.12 (1.00–1.25) | 0.045 | 0.0008 | 0.88(0.73–1.06) | 0.18 | 1.18 (1.02–1.37) | 0.03 | |||
| Cardiovascular: | 138/1491 | 45/829 | 93/617 | |||||||
| Model 1 | 1.09 (0.91–1.32) | 0.35 | 0.69 (0.49–0.97) | 0.03 | 1.43 (1.12–1.84) | 0.004 | ||||
| Model 2 | 1.05 (0.87–1.28) | 0.52 | <.0001 | 0.68 (0.47–0.99) | 0.04 | 1.40 (1.09–1.80) | 0.008 | |||
| a. Sudden Death | 39/428 | 12/261 | 27/140 | |||||||
| Model 1 | 1.15 (0.81–1.63) | 0.42 | 0.61 (0.31–1.19) | 0.15 | 2.02 (1.29–3.15) | 0.002 | ||||
| Model 2 | 1.08 (0.76–1.54) | 0.65 | 0.0004 | 0.64 (0.33–1.25) | 0.19 | 1.91 (1.22–2.98) | 0.005 | |||
| b. Non-Sudden CVD | 99/1063 | 33/568 | 66/477 | |||||||
| Model 1 | 1.07 (0.85–1.32) | 0.54 | 0.72 (0.48–1.09) | 0.12 | 1.29 (0.96–1.72) | 0.09 | ||||
| Model 2 | 1.47 (1.10–1.96) | 0.01 | 0.0009 | 0.70 (0.45–1.10) | 0.12 | 1.27 (0.94–1.71) | 0.11 | |||
| Cancer | 102/1206 | 50/718 | 52/488 | |||||||
| Model 1 | 1.36 (1.10–1.69) | 0.005 | 1.01 (0.71–1.44) | 0.94 | 1.23 (0.89–1.69) | 0.30 | ||||
| Model 2 | 1.30 (1.04–1.61) | 0.02 | 0.92 | 0.97 (0.68–1.38) | 0.87 | 1.17 (0.85–1.61) | 0.33 | |||
| COPD | 30/237 | 14/129 | 16/108 | |||||||
| Model 1 | 1.07 (0.7–1.62) | 0.75 | 1.29 (0.66–2.50) | 0.46 | 0.71 (0.40–1.27) | 0.24 | ||||
| Model 2 | 0.98 (0.64–1.49) | 0.92 | 0.75 | 1.22 (0.63–2.35) | 0.56 | 0.63 (0.34–1.15) | 0.13 | |||
| Infection | 51/477 | 30/287 | 21/190 | |||||||
| Model 1 | 1.25 (0.92–1.7) | 0.16 | 1.26 (0.80–1.98) | 0.31 | 0.87 (0.53–1.43) | 0.58 | ||||
| Model 2 | 1.23 (0.90–1.68) | 0.20 | 0.06 | 1.17 (0.74–1.84) | 0.50 | 0.88 (0.53–1.47) | 0.63 | |||
| Accidents | 17/159 | 6/100 | 11/59 | |||||||
| Model 1 | 1.35 (0.79–2.32) | 0.27 | 1.02 (0.37–2.83) | 0.96 | 2.18 (1.03–4.60) | 0.04 | ||||
| Model 2 | 1.31 (0.76–2.26) | 0.32 | 0.12 | 0.83 (0.27–2.55) | 0.75 | 2.16 (1.02–4.60) | 0.04 | |||
Model 1 - Cox Proportional Hazards Model fitted within matched REGARDS participants with PO and non-users. Model 2 - model 1 adjusted for baseline use of benzodiazepines.
p-value for the interaction term between gender and PO from overall model 2.
Bolded results are statistically significant. Abbreviations: COPD-chronic obstructive pulmonary disease, CVD – cardiovascular, HR- hazard ratio, CI- confidence interval, PO-prescription opioids
Cause-specific mortality
Overall, there were 1491 CVD deaths (of which 428 were sudden deaths), 1206 cancer deaths, 477 deaths from infection, 237 deaths from chronic lung disease, and 159 deaths due to accidents (Table 2). Among POU, there were 138 CVD deaths (33% of deaths in POU; of these 39 [9%] were sudden deaths), 102 (25%) cancer deaths, 51 (12%) deaths from infection, 30 (7%) deaths from chronic lung disease, and 17 (4%) accidental deaths (9 deaths related to medical complications of surgery or other medical procedures, 5 falls and their complications, 1 suicide, 1 homicide, and 1 methadone overdose). Among persons with PO none of the accidental deaths were related to motor vehicle crashes (MVC), whereas among those without opioid prescription 23(16%) of accidental deaths were attributable to MVC.
We observed a significant interaction between POU and gender in the models of all-cause, sudden, non-sudden CVD, and all CVD deaths (interaction p-values 0.0008, 0.0004, 0.0009 and <0.0001, respectively). Women who had PO were 2 times as likely as matched women without PO to experience sudden death, 1.4 times as likely to die from all CVD causes, but no more likely to die of non-sudden CVD causes. Women with PO had increased risk of accidental death (HR 2.18 [95% CI 1.03–4.60]) (Table 2).
Effect of benzodiazepine/PO co-prescription
In the REGARDS total sample, 1147 (4.2%) individuals without PO and 337 (17.7%) persons with PO were prescribed benzodiazepines (p<0.001) (Table 1). As seen in model 2 in Table 2, adjustment for benzodiazepine use did not change the association between PO use and all-cause mortality. The overall benzodiazepine-adjusted HR of death for PO use was 1.12 [95%CI 1.00–1.25, p=0.045]. However, the benzodiazepine-adjusted HR for non-sudden CVD death became significant (HR 1.47 [95% CI 1.10–1.96]). The previously observed associations between PO use and mortality among women remained significant after adjustment for benzodiazepine use for sudden death (HR 1.91 [95% CI 1.22–2.98]), CVD death (HR 1.40 [95% CI 1.09–1.80]) and accidental death (HR 2.16 [95% CI 1.02–4.60]).
Discussion
There are several important findings of this study. First, after applying weighted propensity score matching on a broad set of potential confounders, prescription opioid use at baseline was significantly associated with 12% higher risk of all-cause (non-overdose) mortality over 6 years’ follow-up in this sample of community-dwelling older adults. Second, after stratification by gender, higher mortality risk was observed only among women with PO but not among men with PO, in comparisons that carefully matched women and men without PO. Third, in terms of specific causes of death, women with PO were at greater risk of death from CVD, sudden death (a subset of CVD) and accidental causes. This pattern was not observed among men with baseline opioid prescription. Fourth, benzodiazepine co-prescribing did not change the PO-mortality associations in most comparisons, except for increasing the risk of death from non-sudden CVD causes in the overall sample of persons with PO. The associations observed here were independent of an extremely wide range of potential social, demographic and medical characteristics, all of which were measured prospectively. Such data cannot prove causality but they hint at possible and non-causal associations that may yet emerge over future study.
POU was common in REGARDS, similar to previous estimates,10,11 and women were more likely to have opioid prescriptions than men. Consistent with previous population-based research, compared with individuals not treated with PO, persons with opioid prescriptions in our study were more likely to have low socio-economic status42,43, a longer history of cigarette smoking44, greater chronic pain in spite of PO42,44,45, lower physical functioning,42,46 and greater comorbidity burden.42,44,47
Again, consistent with prior population-based studies, 23,48our study found a moderately increased long-term risk of non-overdose mortality among persons who had opioid prescriptions. A study of a cohort of 13,127 Danish community dwelling adults found increased risk of death and hospital inpatient admission among persons with chronic non-cancer pain treated with opioids:. 23 As for specific causes of death, there was no statistically significant association between POU and CVD or cancer mortality in the Danish cohort, but persons with PO had higher risks of injuries and poisoning resulting in acute hospitalizations23. The difference in the magnitude of the POU-mortality association between our study and the Danish study may stem from several factors, including the limited number of confounding variables available in that study, differences in characteristics between Danes and black and white Americans, and the lack of a propensity score or similar approach to overcoming confounding by indication in the Danish study.
In the Veterans Aging Cohort Study, persons with PO were at 25% increased risk of death,49 which was greater than our finding of mortality risk for baseline POU in the REGARDS study. It is noteworthy that even after propensity score matching in the study conducted by Weisberg et al, veterans treated with PO significantly differed from non-users in the rates of smoking and comorbid conditions.49 This suggests that Weisberg’s study may not have achieved balance between POU and non-users on baseline characteristics, and raises the possibility of suboptimal minimization of confounding by indication. Excess mortality among persons with PO in some previous epidemiological studies may be related to the burden of worse socio-economic factors and/or comorbidity that could not be accounted for in those analyses50.
For our primary analysis we combined use of all available PO. However, unadjusted analysis showed a greater magnitude of association between long-acting opioids and death, hinting at their potentially more deleterious effect compared to short-acting formulations. This could be seen as aligning with a Veterans Health Administration study in which patients receiving long-acting opioids had higher risk of overdose than those receiving short-acting opioids (HR 2.33 [95% CI 1.26–4.32]),51 provided we acknowledge that our cohort’s deaths did not feature overdose significantly. A possible differential risk of all-cause mortality associated with individual opioid medications warrants further investigation. Furthermore, consistent with previous reports44,52 concurrent benzodiazepine prescribing was common. It generally did not change the association between POU and mortality, except for increasing association of POU with non-sudden CVD death.
A novel finding from this study is a gender difference in the risk for both all-cause and cause-specific mortality among persons with PO. Compared to propensity-matched women who did not have PO, women with PO were at 20% increased risk of all-cause mortality, driven by higher risks for cardiovascular deaths, sudden deaths and fatal accidents. This was not observed in men with PO. The increased risk of sudden death in PO using women may be explained by increased incidence of arrhythmias among persons PO53,54 and oxycodone- and methadone-related QT interval prolongation.55,56 Elevated CVD mortality among patients treated with PO has been previously reported24,57 but to our knowledge no prior reports have described increased CVD mortality in women with PO, compared to similar men. Previous data from the REGARDS cohort demonstrated increased risk of CHD in women but not men with PO.27 The underlying mechanism why women may be more susceptible to the deleterious effects of PO is unknown yet, however previous data from studies of morphine effects during anesthesia showed some important sex differences in morphine pharmacodynamics.58 In the study of healthy volunteers, morphine had a greater potency among women and was associated with higher rate of respiratory depression in women, compared to men58. A more recent pharmacogenetic study of long-term treatment with PO has demonstrated that women experienced more of opioid-related adverse effects, such as nausea, headaches, insomnia, loss of appetite, weight change, depression and dizziness than men, which was attributed to genetic polymorphism between men and women59. Additionally, a study of postoperative opioid-related adverse effects in children found greater incidence of respiratory depression and nausea among girls than among boys.60 Perhaps, similar genetic, neuro-hormonal and/or pharmacodynamic sex differences could explain why women with PO were more likely to die than similar men, but these mechanisms yet remain to be more investigated and understood in the future research.
This study contributes to the understanding of long-term risks of PO in the treatment of chronic non-cancer pain. Among our study’s notable strengths are the large national sample of community-dwellers; long follow-up; availability of many physiologic and patient-reported characteristics; expert-adjudicated mortality outcomes; and the propensity score matched analysis. Limitations include the observational design with restricted opportunity for drawing causal inferences. Although we conducted a propensity matched analyses that achieved balance between users and non-users, there is still a possibility of residual confounding. Importantly, receipt of opioid prescriptions was only measured once at baseline and we did not have data on duration of opioid use, and whether it continued up to death. Additionally REGARDS medication data did not include dose or information on whether use was concordant with physician recommendations. Similarly we had very little information on opioid diversion or misuse.
Conclusion
In this national prospective cohort study of community dwelling adults, women but not men with baseline PO were at increased risk of death, unrelated to overdose. Over a 6-year period, women with PO had elevated risk for all-cause mortality, sudden death, CVD death, and fatal accidents compared to matched women non-users. Opioids were not associated with increased risk of death among men. Extra caution when prescribing opioid therapy for women with non-cancer chronic pain may be warranted until these findings can be confirmed.
Supplementary Material
Acknowledgement
This research project was supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. This research was funded in part by an AGENCY FOR HEALTHCARE RESEARCH AND QUALITY (AHRQ) training grant to the University of Alabama at Birmingham (2T32HS013852-16). Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Additional support was provided by grants R01 HL08477 and K24 HL111154 from the National Heart, Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org The REGARDS study invites collaboration. More information can be found on the REGARDS web-site: http://www.regardsstudy.org/researchers.
References
- 1.Sawyer P, Bodner EV, Ritchie CS, Allman RM. Pain and pain medication use in community-dwelling older adults. The American journal of geriatric pharmacotherapy. 2006;4(4):316–324. [DOI] [PubMed] [Google Scholar]
- 2.Przekop P, Haviland MG, Oda K, Morton KR. Prevalence and correlates of pain interference in older adults: why treating the whole body and mind is necessary. Journal of bodywork and movement therapies. 2015;19(2):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laslett LL, Quinn SJ, Winzenberg TM, Sanderson K, Cicuttini F, Jones G. A prospective study of the impact of musculoskeletal pain and radiographic osteoarthritis on health related quality of life in community dwelling older people. BMC musculoskeletal disorders. 2012;13:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003–2009 in persons with knee osteoarthritis. Arthritis care & research. 2014;66(10):1489–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(2 Suppl):S63–88. [PubMed] [Google Scholar]
- 6.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401–435. [PubMed] [Google Scholar]
- 7.Schwetz TA, Calder T, Rosenthal E, Kattakuzhy S, Fauci AS. Opioids and Infectious Diseases: A Converging Public Health Crisis. The Journal of Infectious Diseases. 2019;220(3):346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. The New England journal of medicine. 2015;372(3):241–248. [DOI] [PubMed] [Google Scholar]
- 9.Guy GP Jr., Zhang K, Bohm MK, et al. Vital Signs: Changes in Opioid Prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017;66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA. 2016;315(15):1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138(3):507–513. [DOI] [PubMed] [Google Scholar]
- 12.(CDC) CfDCaP. Adult Use of Prescription Opioid Pain Medications, Utah, 2008. MMWR Morbidity and Mortality Weekly Report 2010;59(06):4. [PubMed] [Google Scholar]
- 13.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. [DOI] [PubMed] [Google Scholar]
- 14.McCabe SE, West BT, Boyd CJ. Medical use, medical misuse, and nonmedical use of prescription opioids: results from a longitudinal study. Pain. 2013;154(5):708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Visconti AJ, Santos GM, Lemos NP, Burke C, Coffin PO. Opioid Overdose Deaths in the City and County of San Francisco: Prevalence, Distribution, and Disparities. J Urban Health. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerda M, Ransome Y, Keyes KM, et al. Prescription opioid mortality trends in New York City, 1990–2006: examining the emergence of an epidemic. Drug Alcohol Depend. 2013;132(1–2):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid MC, Bennett DA, Chen WG, et al. Improving the pharmacologic management of pain in older adults: identifying the research gaps and methods to address them. Pain Med. 2011;12(9):1336–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitz A, Moore AA, Papaleontiou M, Granieri E, Turner BJ, Reid MC. Primary care providers’ perspective on prescribing opioids to older adults with chronic non-cancer pain: a qualitative study. BMC geriatrics. 2011;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamison RN, Sheehan KA, Scanlan E, Matthews M, Ross EL. Beliefs and attitudes about opioid prescribing and chronic pain management: survey of primary care providers. J Opioid Manag 2014;10(6):375–382. [DOI] [PubMed] [Google Scholar]
- 20.Liberman JS, Samuels LR, Goggins K, Kripalani S, Roumie CL, Vics. Opioid Prescriptions at Hospital Discharge Are Associated With More Postdischarge Healthcare Utilization. Journal of the American Heart Association. 2019;8(3):e010664–e010664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Prescription of Long-Acting Opioids and Mortality in Patients With Chronic Noncancer Pain. JAMA. 2016;315(22):2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abnett D, Jones N, White S, Hansen J, Park N. Wallace & Gromit : plots in space. London: Titan; 2007. [Google Scholar]
- 23.Ekholm O, Kurita GP, Hojsted J, Juel K, Sjogren P. Chronic pain, opioid prescriptions, and mortality in Denmark: A population-based cohort study. Pain. 2014;155(12):2486–2490. [DOI] [PubMed] [Google Scholar]
- 24.Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med 2010;170(22):1979–1986. [DOI] [PubMed] [Google Scholar]
- 25.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010;170(22):1968–1976. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Setoguchi S, Cabral H, Jick S. Opioid use for noncancer pain and risk of myocardial infarction amongst adults. J Intern Med 2013;273(5):511–526. [DOI] [PubMed] [Google Scholar]
- 27.Khodneva Y, Muntner P, Kertesz S, Kissela B, Safford MM. Prescription Opioid Use and Risk of Coronary Heart Disease, Stroke, and Cardiovascular Death among Adults from a Prospective Cohort (REGARDS Study). Pain Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med 2011;155(5):325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan MD, Ballantyne JC. What are we treating with long-term opioid therapy? Arch Intern Med 2012;172(5):433–434. [DOI] [PubMed] [Google Scholar]
- 30.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. [DOI] [PubMed] [Google Scholar]
- 31.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barkin RL, Barkin SJ, Barkin DS. Propoxyphene (dextropropoxyphene): a critical review of a weak opioid analgesic that should remain in antiquity. American journal of therapeutics. 2006;13(6):534–542. [DOI] [PubMed] [Google Scholar]
- 33.Stuart EA. Matching methods for causal inference: A review and a look forward. Statistical science : a review journal of the Institute of Mathematical Statistics. 2010;25(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949;37(2):161–186. [DOI] [PubMed] [Google Scholar]
- 35.Radloff LS, Rae DS. Susceptibility and precipitating factors in depression: sex differences and similarities. J Abnorm Psychol 1979;88(2):174–181. [DOI] [PubMed] [Google Scholar]
- 36.Stewart AL, Napoles-Springer A. Health-related quality-of-life assessments in diverse population groups in the United States. Med Care 2000;38(9 Suppl):II102–124. [PubMed] [Google Scholar]
- 37.Soliman EZ, Howard G, Cushman M, et al. Prolongation of QTc and risk of stroke: The REGARDS (REasons for Geographic and Racial Differences in Stroke) study. Journal of the American College of Cardiology. 2012;59(16):1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24(1):67–74. [DOI] [PubMed] [Google Scholar]
- 39.Redmond N, Richman J, Gamboa CM, et al. Perceived stress is associated with incident coronary heart disease and all-cause mortality in low- but not high-income participants in the Reasons for Geographic And Racial Differences in Stroke study. Journal of the American Heart Association. 2013;2(6):e000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Statistics in medicine. 1991;10(4):585–598. [DOI] [PubMed] [Google Scholar]
- 41.Mitra R, Reiter JP. A comparison of two methods of estimating propensity scores after multiple imputation. Statistical methods in medical research. 2012. [DOI] [PubMed] [Google Scholar]
- 42.Eriksen J, Sjogren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1–2):172–179. [DOI] [PubMed] [Google Scholar]
- 43.Svendsen K, Fredheim OM, Romundstad P, Borchgrevink PC, Skurtveit S. Persistent opioid use and socio-economic factors: a population-based study in Norway. Acta Anaesthesiol Scand 2014;58(4):437–445. [DOI] [PubMed] [Google Scholar]
- 44.Dobscha SK, Morasco BJ, Duckart JP, Macey T, Deyo RA. Correlates of prescription opioid initiation and long-term opioid use in veterans with persistent pain. Clin J Pain. 2013;29(2):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fredheim OM, Mahic M, Skurtveit S, Dale O, Romundstad P, Borchgrevink PC. Chronic pain and use of opioids: a population-based pharmacoepidemiological study from the Norwegian prescription database and the Nord-Trondelag health study. Pain. 2014;155(7):1213–1221. [DOI] [PubMed] [Google Scholar]
- 46.Ashworth J, Green DJ, Dunn KM, Jordan KP. Opioid use among low back pain patients in primary care: Is opioid prescription associated with disability at 6-month follow-up? Pain. 2013;154(7):1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mellbye A, Karlstad O, Skurtveit S, Borchgrevink PC, Fredheim OM. Co-morbidity in persistent opioid users with chronic non-malignant pain in Norway. European journal of pain. 2014;18(8):1083–1093. [DOI] [PubMed] [Google Scholar]
- 48.Sjogren P, Gronbaek M, Peuckmann V, Ekholm O. A population-based cohort study on chronic pain: the role of opioids. Clin J Pain. 2010;26(9):763–769. [DOI] [PubMed] [Google Scholar]
- 49.Weisberg DF, Gordon KS, Barry DT, et al. Long-term Prescription of Opioids and/or Benzodiazepines and Mortality Among HIV-Infected and Uninfected Patients. Journal of acquired immune deficiency syndromes. 2015;69(2):223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose AJ, McBain R, Schuler MS, et al. Effect of Age on Opioid Prescribing, Overdose, and Mortality in Massachusetts, 2011 to 2015. J Am Geriatr Soc 2019;67(1):128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA internal medicine. 2015;175(4):608–615. [DOI] [PubMed] [Google Scholar]
- 52.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qureshi WT, O’Neal WT, Khodneva Y, et al. Association Between Opioid Use and Atrial Fibrillation: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. JAMA internal medicine. 2015;175(6):1058–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winton JC, Twilla JD. Sudden cardiac arrest in a patient on chronic methadone after the addition of azithromycin. Am J Med Sci. 2013;345(2):160–162. [DOI] [PubMed] [Google Scholar]
- 55.Fanoe S, Jensen GB, Sjogren P, Korsgaard MP, Grunnet M. Oxycodone is associated with dose-dependent QTc prolongation in patients and low-affinity inhibiting of hERG activity in vitro. Br J Clin Pharmacol. 2009;67(2):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van den Beuken-van Everdingen MH, Geurts JW, Patijn J Prolonged QT interval by methadone: relevance for daily practice? A prospective study in patients with cancer and noncancer pain. J Opioid Manag 2013;9(4):263–267. [DOI] [PubMed] [Google Scholar]
- 57.Khodneva Y, Muntner P, Kertesz S, Safford MM. Prescription opioid use and risk of cardiovascular disease among older adults from a community sample. Drug & Alcohol Dependence.140:e103. [Google Scholar]
- 58.Sarton E, Olofsen E, Romberg R, et al. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology. 2000;93(5):1245–1254; discussion 1246A. [DOI] [PubMed] [Google Scholar]
- 59.Planelles B, Margarit C, Inda MD, et al. Gender based differences, pharmacogenetics and adverse events in chronic pain management. Pharmacogenomics J. 2019. [DOI] [PubMed] [Google Scholar]
- 60.Sadhasivam S, Chidambaran V, Olbrecht VA, et al. Opioid-related adverse effects in children undergoing surgery: unequal burden on younger girls with higher doses of opioids. Pain Med 2015;16(5):985–997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.