Highlights
-
•
Climate change is likely to increase global incidence of mosquito-borne viruses.
-
•
Outbreaks of disease caused by Aedes-borne arboviruses have been more frequent and more intense in recent years.
-
•
Current evidence suggests that climate could be partially driving recent outbreaks across the world.
-
•
More longitudinal analyses are needed at the local level to better understand climate's impact on arbovirus transmission.
-
•
Recent studies in Cordoba, Argentina, could be useful for understanding arbovirus emergence globally.
Abstract
Climate change is leading to increases in global temperatures and erratic precipitation patterns, both of which are contributing to the expansion of mosquito-borne arboviruses and the populations of the mosquitos that vector them. Herein, we review recent evidence of emergence and expansion of arboviruses transmitted by Aedes mosquitos that has been driven in part by environmental changes. We present as a case study of recent work from Córdoba, Argentina, where dengue has been actively emerging in the past decade. We review recent empirical and modeling studies that aim to understand the impact of climate on future expansion of arboviruses, and we highlight gaps in empirical studies linking climate to arbovirus transmission at regional levels.
Current Opinion in Virology 2020, 40:41–47
This review comes from a themed issue on Viruses in a changing world
Edited by Kellie Ann Jurado and Sara Cherry
For a complete overview see the Issue and the Editorial
Available online 20th June 2020
https://doi.org/10.1016/j.coviro.2020.05.001
1879-6257/© 2020 Elsevier B.V. All rights reserved.
Introduction
Arboviruses transmitted by mosquitoes have been expanding their global distribution in recent years, and new viruses, such as chikungunya and Zika, have emerged and spread globally [1]. This expansion is thought to be attributed to a combination of increased urbanization, global travel, and environmental changes associated with increasing temperatures and changing precipitation patterns [2]. That the latter of these is driving arbovirus expansion is not unexpected: mosquitos are ectotherms that depend upon water sources to complete their life cycles, so temperature, precipitation, and humidity play critical roles in the mosquito-vectored arbovirus transmission cycle [3, 4, 5]. Global surface temperatures have increased by 0.8–1.2°C above pre-industrial levels, and precipitation data show uncharacteristically extreme variations in rainfall [6,7]. Because of this, the global landscape of mosquito-borne arboviruses is being transformed as suitability for mosquito populations and the viruses they transmit changes. Autochthonous transmission of mosquito-borne arboviruses is occurring more frequently in subtropical and temperate regions, occurring at higher elevations in tropical regions, and becoming increasingly more intense in endemic regions, leading to larger outbreaks [8••,9,10••]. In the present work, we investigate recent emergence and expansion of arboviruses transmitted by the anthropophilic mosquito species Aedes aegypti (and in some case the secondary vector Aedes albopictus) as a case study for environmentally driven arboviral expansion. We first describe known relationships among meteorological variables, mosquitoes, and arboviruses. We next describe the ongoing global expansion of dengue as it relates to climate and discuss the novel outbreaks of chikungunya and Zika virus that began in 2013 and 2015, respectively. We discuss recent arbovirus activity in the temperate Argentinian city of Córdoba, where the emergence of dengue began in 2009 and has been ongoing. Finally, we review recent work aimed at better understanding the impacts of climate and changes thereof on present and future arbovirus activity and highlight gaps in empirical and modeling studies that could help us better understand the future of arbovirus transmission under a changing climate.
Arboviruses, mosquitoes, and climate
Dengue (DENV serotypes 1–4), Zika (ZIKV), and chikungunya (CHIKV) viruses are transmitted to people primarily by the female Ae. aegypti mosquito [11]. Ae albopictus has been implicated as a secondary vector in the transmission of all three arboviruses as well [11]. These viruses cause a significant and growing burden of febrile illness in people worldwide. Severe clinical manifestations of dengue fever, although less common, can result in hemorrhage, shock, and death in some cases [12]. CHIKV infections are characterized by fever and severe arthralgias, with increasing evidence of fatal complications [13]. ZIKV was designated by the World Health Organization as a Public Health Emergency of International Concern in 2016 after congenital and neurological complications were associated with infections [14]. Co-infections are common, and differential diagnosis is difficult in the absence of molecular diagnostics (e.g., PCR) due to similar clinical presentation [15, 16, 17, 18, 19, 20, 21]. No specific therapeutic treatment is available for these arboviruses, and vector control remains the primary public health intervention to prevent and respond to epidemics. A DENV vaccine is available in some countries; however, it is recommended to be used only for seropositive individuals older than nine years [22].
Temperature plays an important role in the transmission of arboviruses, due to nonlinear effects on mosquito physiology, which affect rates of development and mortality in Aedes mosquitos [5,23,24]. Temperature also regulates viral incubation in the mosquito vector, with optimal warm temperatures reducing the extrinsic incubation period [25,26••]. Understanding the effects of rainfall on arbovirus transmission is more complex and depends on the local social-ecological context. The Ae. aegypti mosquito prefers to breed and lay eggs in containers that hold water, often in and around the home, where female mosquitoes blood feed on people. Increases in rainfall can increase vector populations due to an abundance of rain-filled containers around dwellings [27]. However, drought conditions and water scarcity can also increase vector populations if people begin to store water in containers around the home [28].
Numerous studies have looked at the links among temperature, Aedes populations, and arbovirus transmission. Mechanistic models based on mosquito thermal biology suggest that DENV transmission occurs optimally at 28.1°C in Ae. aegypti, and 26.4°C in Ae. albopictus [29]. Other studies have examined suitability of habitats for Aedes mosquito expansion and potential of regions to support arbovirus transmission under current climate conditions and predicted climate change scenarios [10••,30,31]. A recent study in Ecuador determined that Ae. aegypti will expand its range into higher elevations of the Andean foothills, estimated to affect 4215 km2 of new territory and 12 000 people under the most extreme scenario of climate change by 2050 [32••]. Similar elevational range expansions are to be expected throughout the tropical Andes, increasing the population at risk of arboviral infections. Although arboviral disease burden is expected to worsen globally, some of these models suggest that transmission of arboviruses may decrease in tropical regions due to temperatures that become too warm to sustain mosquito populations [10••,29]. It is also possible that local mosquito populations may evolve to be better adapted to changing climate conditions.
Expansion of DENV
DENV re-emerged in the Americas during the 1980s and 1990s, with all four serotypes (DENV1-4) co-circulating and causing major epidemics in urban areas throughout the region [33]. In the past two decades alone, dengue has expanded its range beyond subtropical and tropical regions of the globe, with autochthonous transmission and outbreaks occurring in parts of the United States, southern Europe, Uruguay, central Argentina, and Australia [34, 35, 36, 37, 38, 39, 40]. In the United States, outbreaks have occurred in Hawaii, southern Florida, and along the Mexico-Texas border [34,35,38,41]. In Europe, a large outbreak of dengue was reported in Madeira, Portugal in 2012–2013, with DENV likely originating from Venezuela [37,42]. Autochthonous transmission has also been reported for the first time in southern France and Croatia [36,39]. In South America, autochthonous dengue transmission was reported in subtropical regions of Argentina in 1997 and Uruguay in 2016 for the first time since Ae. aegypti was though to to be eliminated in the 1960s [40,43]. Today, Aedes populations are found throughout Europe, the temperate Southern Cone of South America, and the United States, and the threat of importation of Aedes populations, and potentially the arboviruses transmitted by the mosquito vectors, is rising with increases in global travel and as the climate becomes more suitable for transmission.
In endemic populations, dengue outbreaks are increasing in frequency and magnitude. In 2019, much of tropical and subtropical parts of the world experienced increases in dengue transmission that surpassed orders of magnitude. The Americas are currently reporting a resurgence of DENV, with 3 139 335 cases of dengue fever reported by the Pan American Health Organization (PAHO) in 2019 alone, the highest number of cases ever reported in a single year in the Americas [44]. Over two million cases were reported from Brazil alone; however, the highest incidence rates were reported from countries in Central America (Nicaragua, Belize, Honduras) [44]. This resurgence continues in 2020, with PAHO reporting over 1.6 million cases to date (EW 24). Argentina is currently experiencing its largest dengue epidemic on record, with more than 78,000 cases from EW 31 in 2019 to EW 20 in 2020, according to the National Epidemiological Reports of the Ministry of Health; approximately half of the cases are from the central temperate region [67,68].
Emergence of CHIKV and ZIKV
In 2013, CHIKV emerged in the Caribbean and spread rapidly throughout Central and South America [45]. Both Ae. aegypti and Ae. albopictus were implicated in the transmission of CHIKV [46]. The expansion of both vectors due to meteorological extremes is also a likely factor in the rapid spread of chikungunya, as a number of outbreaks followed extreme rainfall events [47,48]. Furthermore, the year 2014 was the first in a sequence of years that are now listed as the warmest years on record [49]. A large outbreak of chikungunya was reported in Italy, with over 300 cases reported, and autochthonous transmission was also reported in France [50,51]. In the Americas, over 1.1 million cases were reported in 2014 [52].
Beginning in 2015, ZIKV emerged and spread with extreme intensity throughout the Americas. Between 2015–2016 laboratory-confirmed and suspected cases were reported from 87 countries and territories worldwide, with over 750 000 cases reported from the Americas alone [53,54]. Autochthonous ZIKV transmission was reported in southern Florida, France, and Argentina—regions at the edge of the historical range of arbovirus transmission [53].
Case study of Córdoba, Argentina
A region of notable Ae. aegypti and DENV emergence is the temperate Southern Cone of South America. Early cases of dengue fever were reported in Argentina in 1916, and no cases were reported for 80 years [43]. Ae aegypti was eliminated from all of Argentina in 1963 following aggressive vector control campaigns in response to the yellow fever pandemic; however, the vector became re-established in its historic range and has spread into new southern latitudes. By 1986, the vector was detected in the northeast subtropical region, and in 1997 the first autochthonous cases were confirmed in northwestern Argentina [43].
Ae aegypti was detected in the city of Córdoba, Argentina, for the first time in 1995. The city has a population of about 1.4 million and is located in the central region of the country (31.4°S, 64.2°W). Córdoba has a temperate climate with warm rainy summers (November–March), and cool, dry winters (June–September). Of note, the Ministry of Health has not detected vector activity during the winter months, thus suggesting that local arbovirus transmission requires annual importation of the virus, likely from people traveling to endemic regions [55••].
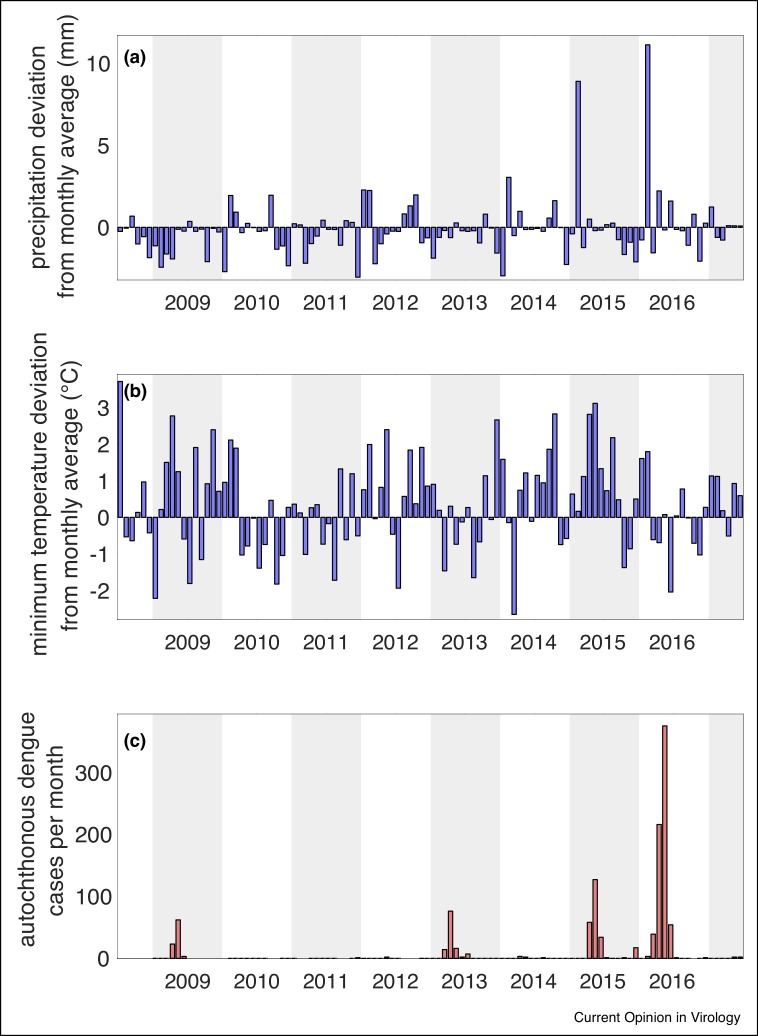
In 2009, Córdoba experienced its first dengue epidemic on record, with 130 total cases reported [55••]. In 2013, 2015, and 2016, Córdoba experienced epidemics of increasing magnitude, with 822 cases of dengue reported in 2016 (Figure 1 ). The city is currently experiencing the largest dengue outbreak on record, with more than 7,500 cases reported from EW 31 in 2019 to EW 24 in 2020 [68]. In 2014 and 2016 imported cases of CHIKV and ZIKV, respectively, were first reported in Córdoba, although no vector-borne autochthonous transmission has been reported for either virus. Imported DENV cases were noted from the northern provinces of Argentina and neighboring dengue endemic countries (Brazil, Bolivia, and Paraguay) [55••].
Figure 1.
Meteorological anomalies and autochthonous dengue transmission data from Córdoba, Argentina for the period 01 July 2008 –31 June 2017. (a) Deviation of mean monthly precipitation (anomalies) from the thirty-year monthly precipitation average for a given month. (b) Deviation of mean monthly minimum temperature (anomalies) from the thirty-year monthly minimum temperature average for a given month. (c) Number of new autochthonous dengue cases reported each month. All thirty-year averages and monthly anomalies were calculated from 1987–2017 using daily climate data from the Observatory meteorological station (31.42° S, 64.20° W) provided by the National Meteorological Service of Argentina. Dengue cases were extracted from weekly epidemological bulletins provided by the Argentina Health Secretary.
Following the first outbreak, a robust entomological surveillance study was initiated in 2009, allowing for monitoring of vector dynamics during the first 10 years of arbovirus emergence [56••]. A recent analysis of Ae. aegypti from this surveillance study, dengue cases and local climate in Córdoba revealed significant increases in Ae. aegypti population size between 2010 and 2017. Oviposition and larval abundance were significantly positively correlated with mean temperature in the same month. Monthly anomalies in minimum temperature, calculated using a thirty-year base period (1987–2017), indicate that this period of dengue emergence has been characterized by warmer than average temperatures, with 60% of months (65 of 108) warmer than the long term average of a given month (Figure 1b). Each of the outbreaks has been preceded by extreme meteorological events (Figure 1). Outbreaks in 2015 and 2016 followed extreme precipitation events where monthly precipitation reached a peak of 8.90 and 11.13 mm, respectively, above monthly averages (Figure 1a,c). Outbreaks in 2013 and 2016 followed closely after periods in which the monthly mean minimum temperature was as much as 3°C higher than monthly averages, and the 2009 and 2015 outbreaks occurred during periods in which the monthly mean temperature was 2–3°C higher than monthly averages (Figure 1b,c). The current 2020 epidemic is an order of magnitude greater than outbreaks previously experienced. However, it is hypothesized that the epidemic has grown due to the increased time that people are spending in their homes, exposed to Ae. aegypti, due to mandatory quarantine during the COVID-19 pandemic. Climate and other non-climate co-variates that may have increased the likelihood of the current epidemic also need to be explored.
Ongoing research in Córdoba continues to assess the role of climate in mosquito-borne disease emergence at a local level over a long-term period. Although meteorological anomolies have been associated with each of the dengue outbreaks in the last decade, it is difficult to find significant relationships between climate and dengue transmission in a period of initial emergence with four outbreaks across ten years. It is, however, possible with the available data to begin exploring the effects of climare variability and extreme climate events on arbovirus transmission, and, as we have done here, use the results of this exploration to inform future studies aimed at finding signficant links between climate and arbovirus transmission. Córdoba is among a short list of places where an actively emerging arbovirus can be carefully studied and may provide important insights to understanding the emergence of arboviruses globally.
Conclusions
With concerns of continued global expansion of arboviruses driven by climate change, much effort has been placed on developing a better understanding of which regions of the world are at risk today and in the future. Many mathematical and statistical studies have investigated the potential for dengue and related arboviruses to emerge in naive populations as a result of global rises in surface temperatures [57]. Studies have emphasized the connection between climate and dengue and predicted the potential for expansion of both the vectors and the arboviruses into regions at the margins of transmission that are currently deemed unsuitable for arbovirus transmission [10••,58,59•,60]. Region-specific studies have predicted the potential for greater suitability for arbovirus transmission in some major U.S. cities [61•], rural and southern regions of Brazil [62], and central and western regions of China [63], among others.
Although multiple studies have been conducted at the global scale, there are a dearth of local longitudinal studies in zones of current and future arbovirus emergence. The recent work investigating the impacts of climate on dengue in Córdoba is among the few longitudinal studies that aim to connect dengue emergence with temperature and precipitation in a temperate region. While global analyses are informative, it is local analyses that underscore the evidence that climate and arbovirus transmission are closely linked. Studies aimed at detecting arbovirus emergence and investigating links between transmission and climate are vital to our ability to understand, predict, and respond to emergence events [64,65]. In a recent study, health sector stakeholders from the Caribbean have highlighted the urgent need for more local studies linking climate and arboviral disease—without this information, they are limited in their ability to take informed actions to adapt to a changing climate [66•].
Additional empirical, modeling, and statistical studies are imperative for developing a better understanding of the links between climate and arbovirus transmission, particularly in regions at the margin of arbovirus emergence. In addition, resources are needed to strengthen entomological and epidemiological surveillance by the public health sector in regions of emergence, and the establishment of sentinel surveillance sites in zones of likely future emergence.
Conflict of interest
M.A. Robert, A.M. Stewart-Ibarra, and E.L. Estallo report no conflict of interest associated with this work.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgement
This work was supported in part by grants to Anna M. Stewart-Ibarra and Michael A. Robert from the U.S. Embassy in Argentina administered through the Fulbright Commission. ELE is member of the Consejo de Investigaciones Cientificas y Tecnológicas (CONICET) from Argentina.
References
- 1.Brady O.J., Hay S.I. The global expansion of dengue: how Aedes aegypti mosquitoes enabled the first pandemic arbovirus. Ann Rev Entomol. 2020;65:191–208. doi: 10.1146/annurev-ento-011019-024918. [DOI] [PubMed] [Google Scholar]
- 2.Young P.R. Arboviruses: a family on the move. In: Hilgenfeld R., Vasudevan S.G., editors. Dengue and Zika: Control and Antiviral Treatment Strategies. Springer; 2018. pp. 1–10. [Google Scholar]
- 3.Costa E.A.P. de A., Santos E.M. de M., Correia J.C., Albuquerque C.M.R. de. Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae) Rev Bras entomol. 2010;54:488–493. [Google Scholar]
- 4.Alto B.W., Juliano S.A. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): implications for range expansion. J Med Entomol. 2001;38:646–656. doi: 10.1603/0022-2585-38.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady O.J., Johansson M.A., Guerra C.A., Bhatt S., Golding N., Pigott D.M., Delatte H., Grech M.G., Leisnham P.T., Maciel-de-Freitas R. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasites Vectors. 2013;6:351. doi: 10.1186/1756-3305-6-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trenberth K. Changes in precipitation with climate change. Clim Res. 2011;47:123–138. [Google Scholar]
- 7.Dore M.H.I. Climate change and changes in global precipitation patterns: what do we know? Environ Int. 2005;31:1167–1181. doi: 10.1016/j.envint.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 8••.Kamal M., Kenawy M.A., Rady M.H., Khaled A.S., Samy A.M. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS One. 2018;13 doi: 10.1371/journal.pone.0210122. [DOI] [PMC free article] [PubMed] [Google Scholar]; A detailed assessment of the current distributions of Aedes mosquitoes and predictions of how those distributions might change under climate change scenarios.
- 9.Kraemer M.U.G., Reiner R.C., Brady O.J., Messina J.P., Gilbert M., Pigott D.M., Yi D., Johnson K., Earl L., Marczak L.B. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4:854–863. doi: 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Ryan S.J., Carlson C.J., Mordecai E.A., Johnson L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Neglect Trop D. 2019;13 doi: 10.1371/journal.pntd.0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]; Analyzes the potential climate-driven expansion and reduction of suitable regions for Ae. aegypti and Ae. albopictus and the viruses they transmit.
- 11.2017. Surveillance and Control of Aedes aegypti and Aedes albopictus in the United States. [Google Scholar]
- 12.World Health Organization, editor. Dengue: Guidelines for Diagnosis, Treatment, Prevention, and Control. TDR: World Health Organization; 2009. Special Programme for Research and Training in Tropical Diseases. [PubMed] [Google Scholar]
- 13.Lima Neto A.S., Sousa G.S., Nascimento O.J., Castro M.C. Chikungunya-attributable deaths: a neglected outcome of a neglected disease. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome. [date unknown],.
- 15.Vogels C.B.F., Rückert C., Cavany S.M., Perkins T.A., Ebel G.D., Grubaugh N.D. Arbovirus coinfection and co-transmission: a neglected public health concern? PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villamil-Gómez W.E., González-Camargo O., Rodriguez-Ayubi J., Zapata-Serpa D., Rodriguez-Morales A.J. Dengue, chikungunya and Zika co-infection in a patient from Colombia. J Infect Public Health. 2016;9:684–686. doi: 10.1016/j.jiph.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Göertz G.P., Vogels C.B.F., Geertsema C., Koenraadt C.J.M., Pijlman G.P. Mosquito co-infection with Zika and chikungunya virus allows simultaneous transmission without affecting vector competence of Aedes aegypti. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chahar H.S., Bharaj P., Dar L., Guleria R., Kabra S.K., Broor S. Co-infections with chikungunya virus and dengue virus in Delhi, India. Emerg Infect Dis. 2009;15:1077–1080. doi: 10.3201/eid1507.080638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuya-Kanamori L., Liang S., Milinovich G., Soares Magalhaes R.J., Clements A.C.A., Hu W., Brasil P., Frentiu F.D., Dunning R., Yakob L. Co-distribution and co-infection of chikungunya and dengue viruses. BMC Infect Dis. 2016;16:84. doi: 10.1186/s12879-016-1417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Coupanec A., Tchankouo-Nguetcheu S., Roux P., Khun H., Huerre M., Morales-Vargas R., Enguehard M., Lavillette D., Missé D., Choumet V. Co-infection of mosquitoes with chikungunya and dengue viruses reveals modulation of the replication of both viruses in midguts and salivary glands of Aedes aegypti mosquitoes. Int J Mol Sci. 2017;18:1708. doi: 10.3390/ijms18081708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson C.J., Mendenhall E. Preparing for emerging infections means expecting new syndemics. Lancet. 2019;394:297. doi: 10.1016/S0140-6736(19)31237-1. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization, WHO . 2018. WHO | Revised SAGE Recommendation on Use of Dengue Vaccine.https://www.who.int/immunization/diseases/dengue/revised_SAGE_recommendations_dengue_vaccines_apr2018/en/ Accessed 25 Feb 2020. Available From: [Google Scholar]
- 23.Rueda L.M., Patel K.J., Axtell R.C., Stinner R.E. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 1990;27:892–898. doi: 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- 24.Samuel G.H., Adelman Z.N., Myles K.M. Temperature-dependent effects on the replication and transmission of arthropod-borne viruses in their insect hosts. Curr Opin Insect Sci. 2016;16:108–113. doi: 10.1016/j.cois.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan M., Johansson M.A. The incubation periods of dengue viruses. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Tesla B., Demakovsky L.R., Mordecai E.A., Ryan S.J., Bonds M.H., Ngonghala C.N., Brindley M.A., Murdock C.C. Temperature drives Zika virus transmission: evidence from empirical and mathematical models. Proc R Soc B Biol Sci. 2018;285 doi: 10.1098/rspb.2018.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study utilizes data describing temperature-dependent traits of vectors and virus transmission to better understand suitable temperatures for Aedes-borne arboviruses.
- 27.Lowe R., Gasparrini A., Van Meerbeeck C.J., Lippi C.A., Mahon R., Trotman A.R., Rollock L., Hinds A.Q.J., Ryan S.J., Stewart-Ibarra A.M. Nonlinear and delayed impacts of climate on dengue risk in Barbados: a modelling study. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart Ibarra A.M., Ryan S.J., Beltrán E., Mejía R., Silva M., Muñoz Á. Dengue vector dynamics (Aedes aegypti) influenced by climate and social factors in Ecuador: implications for targeted control. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mordecai E.A., Cohen J.M., Evans M.V., Gudapati P., Johnson L.R., Lippi C.A., Miazgowicz K., Murdock C.C., Rohr J.R., Ryan S.J. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Neglect Trop D. 2017;11 doi: 10.1371/journal.pntd.0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brady O.J., Golding N., Pigott D.M., Kraemer M.U.G., Messina J.P., Reiner R.C., Jr., Scott T.W., Smith D.L., Gething P.W., Hay S.I. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit Vectors. 2014;7:338. doi: 10.1186/1756-3305-7-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butterworth M.K., Morin C.W., Comrie A.C. An analysis of the potential impact of climate change on dengue transmission in the southeastern United States. Environ Health Perspect. 2017;125:579–585. doi: 10.1289/EHP218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Lippi C.A., Stewart-Ibarra A.M., Loor M.E.F.B., Zambrano J.E.D., Lopez N.A.E., Blackburn J.K., Ryan S.J. Geographic shifts in Aedes aegypti habitat suitability in Ecuador using larval surveillance data and ecological niche modeling: implications of climate change for public health vector control. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007322. [DOI] [PMC free article] [PubMed] [Google Scholar]; An ecological niche modeling study of current distributions of Ae. aegypti in Ecuador and predictions of changes in distribution under climate change scenarios.
- 33.Pinheiro F. 1997. Re-Emergence of Dengue and Emergence of Dengue Haemorrhagic Fever in the Americas. [PubMed] [Google Scholar]
- 34.Rey J.R. Dengue in Florida (USA) Insects. 2014;5:991–1000. doi: 10.3390/insects5040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radke E.G., Gregory C.J., Kintziger K.W., Sauber-Schatz E.K., Hunsperger E., Gallagher G.R., Barber J.M., Biggerstaff B.J., Stanek D.R., Tomashek K.M. dengue outbreak in Key West, Florida, USA, 2009. Emerg Infect Dis. 2012;18 doi: 10.3201/eid1801.110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gjenero-Margan I., Komparak S., Martić R., Đuričić S., Betica-Radić L., Okmadžić J., Vilibić-Čavlek T., Babić-Erceg A., Turković B., Avšić-Županc T. Autochthonous dengue fever in Croatia, August– September 2010. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- 37.Sousa C.A., Clairouin M., Seixas G., Viveiros B., Novo M.T., Silva A.C., Escoval M.T., Economopoulou A. Ongoing outbreak of dengue type 1 in the Autonomous Region of Madeira, Portugal: preliminary report. Eurosurveillance. 2012;17 doi: 10.2807/ese.17.49.20333-en. [DOI] [PubMed] [Google Scholar]
- 38.Effler P.V., Pang L., Kitsutani P., Vorndam V., Nakata M., Ayers T., Elm J., Tom T., Reiter P., Rigau-Perez J.G. Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis. 2005;11:742–749. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jourdain F., Leparc-Goffart I., Charlet F., Ollier L., Mantey K., Mollet T., Fournier J.P., Torrents R., Leitmeyer K., Hilairet P. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010;15:19676. [PubMed] [Google Scholar]
- 40.El Observador . El Observador; 2020. MSP confirmó nuevo caso de dengue autóctono en Uruguay.https://www.elobservador.com.uy/nota/msp-confirmo-nuevo-caso-de-dengue-autoctono-en-uruguay-20203520370 Date Accessed: 08 March 2020. Available from: [Google Scholar]
- 41.Brunkard J.M., López J.L.R., Ramirez J., Cifuentes E., Rothenberg S.J., Hunsperger E.A., Moore C.G., Brussolo R.M., Villarreal N.A., Haddad B.M. Dengue fever seroprevalence and risk factors, Texas–Mexico Border, 2004. Emerg Infect Dis. 2007;13:1477–1483. doi: 10.3201/eid1310.061586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franco L., Pagan I., Serre Del Cor N., Schunk M., Neumayr A., Molero F., Potente A., Hatz C., Wilder-Smith A., Sánchez-Seco M.P. Molecular epidemiology suggests Venezuela as the origin of the dengue outbreak in Madeira, Portugal in 2012–2013. Clin Microbiol Infect. 2015;21:713.e5–713.e8. doi: 10.1016/j.cmi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Avilés G., Rangeón G., Vorndam V., Briones A., Baroni P., Enria D., Sabattini M.S. Dengue reemergence in Argentina. Emerg Infect Dis. 1999;5 doi: 10.3201/eid0504.990424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan American Health Organization, World Health Organization . 2020. Epidemiological Update: Dengue. [Google Scholar]
- 45.Weaver S.C. Arrival of chikungunya virus in the new world: prospects for spread and impact on public health. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coffey L.L., Failloux A.-B., Weaver S.C. Chikungunya virus–vector interactions. Viruses. 2014;6:4628–4663. doi: 10.3390/v6114628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epstein P.R. Chikungunya fever resurgence and global warming. Am J Trop Med Hyg. 2007;76:403–404. [PubMed] [Google Scholar]
- 48.Roiz D., Boussès P., Simard F., Paupy C., Fontenille D. Autochthonous chikungunya transmission and extreme climate events in southern France. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arguez A., Hurley S., Inamdar A., Mahoney L., Sanchez-Lugo A., Yang L. Should we expect each year in the next decade (2019-2028) to be ranked among the top 10 warmest years globally? Bull Am Meteorol Soc. 2020;101:E655–E663. doi: 10.1175/BAMS-D-19-0215.1. [DOI] [Google Scholar]
- 50.Rezza G., Nicoletti L., Angelini R., Romi R., Finarelli A., Panning M., Cordioli P., Fortuna C., Boros S., Magurano F. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 51.Tomasello D., Schlagenhauf P. Chikungunya and dengue autochthonous cases in Europe, 2007–2012. Travel Med Infect Dis. 2013;11:274–284. doi: 10.1016/j.tmaid.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Yactayo S., Staples J.E., Millot V., Cibrelus L., Ramon-Pardo P. Epidemiology of Chikungunya in the Americas. J Infect Dis. 2016;214:S441–S445. doi: 10.1093/infdis/jiw390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Word Health Organization . 2019. Zika Epidemiology Update. [Google Scholar]
- 54.Hills S.L., Fischer M., Petersen L.R. Epidemiology of Zika virus infection. J Infect Dis. 2017;216:S868–S874. doi: 10.1093/infdis/jix434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.Robert M.A., Tinunin D.T., Benitez E.M., Ludueña-Almeida F.F., Romero M., Stewart-Ibarra A.M., Estallo E.L. Arbovirus emergence in the temperate city of Córdoba, Argentina, 2009–2018. Sci Data. 2019;6:1–6. doi: 10.1038/s41597-019-0295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive description of the emergence of dengue in Córdoba, Argentina.
- 56••.Estallo E.L., Sippy R., Stewart-Ibarra A.M., Grech M.G., Benitez E.M., Ludueña-Almeida F.F., Ainete M., Frias-Cespedes M., Robert M., Romero M.M. A decade of arbovirus emergence in the temperate southern cone of South America: dengue, Aedes aegypti and climate dynamics in Córdoba, Argentina. bioRxiv. 2020 doi: 10.1101/2020.01.16.908814. [pre-print] Available from: https://www.biorxiv.org/content/10.1101/2020.01.16.908814v1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ten-year longitudinal study in Córdoba, Argentina, that links climate variables to recent emergence of dengue.
- 57.Naish S., Dale P., Mackenzie J.S., McBride J., Mengersen K., Tong S. Climate change and dengue: a critical and systematic review of quantitative modelling approaches. BMC Infect Dis. 2014;14:167. doi: 10.1186/1471-2334-14-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ebi K.L., Nealon J. Dengue in a changing climate. Environ Res. 2016;151:115–123. doi: 10.1016/j.envres.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 59•.Liu-Helmersson J., Brännström Å, Sewe M.O., Semenza J.C., Rocklöv J. Estimating past, present, and future trends in the global distribution and abundance of the arbovirus vector Aedes aegypti under climate change scenarios. Front Public Health. 2019;7 doi: 10.3389/fpubh.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]; Estimates that global abundance of Ae. aegypti could increase by 20–30% in the next century due to climate change.
- 60.Campbell L.P., Luther C., Moo-Llanes D., Ramsey J.M., Danis-Lozano R., Peterson A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Robert M.A., Christofferson R.C., Weber P.D., Wearing H.J. Temperature impacts on dengue emergence in the United States: investigating the role of seasonality and climate change. Epidemics. 2019;28 doi: 10.1016/j.epidem.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; A mathematical modeling analysis of the influence of temperature (and climate change) on the potential for dengue emergence in major U.S. cities.
- 62.Barcellos C., Lowe R. Expansion of the dengue transmission area in Brazil: the role of climate and cities. Trop Med Int Health. 2014;19:159–168. doi: 10.1111/tmi.12227. [DOI] [PubMed] [Google Scholar]
- 63.Liu B., Gao X., Ma J., Jiao Z., Xiao J., Hayat M.A., Wang H. Modeling the present and future distribution of arbovirus vectors Aedes aegypti and Aedes albopictus under climate change scenarios in Mainland China. Sci Total Environ. 2019;664:203–214. doi: 10.1016/j.scitotenv.2019.01.301. [DOI] [PubMed] [Google Scholar]
- 64.Ebi K.L., Ogden N.H., Semenza J.C., Woodward A. Detecting and attributing health burdens to climate change. Environ Health Perspect. 2017;125 doi: 10.1289/EHP1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LaBeaud Ad, Bashir F., King C.H. Measuring the burden of arboviral diseases: the spectrum of morbidity and mortality from four prevalent infections. Popul Health Metrics. 2011;9:1. doi: 10.1186/1478-7954-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Stewart-Ibarra A.M., Romero M., Hinds A.Q.J., Lowe R., Mahon R., Meerbeeck C.J.V., Rollock L., Hilaire M.G.-S., Ville S.S., Ryan S.J. Co-developing climate services for public health: stakeholder needs and perceptions for the prevention and control of Aedes-transmitted diseases in the Caribbean. PLoS Neglect Trop D. 2019;13 doi: 10.1371/journal.pntd.0007772. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies needs of public health stakeholders for the development of successful programs to control arbovirus spread made worse by climate change.
- 67.Pan-American Health Organization (PAHO). Dengue, https://www.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en.html (2020).
- 68.Ministerio de Salud y Desarrolo Social. Boletines epidemiologicos, https://www.argentina.gob.ar/sites/default/files/biv_497_edicionsemanal.pdf (2020).