Abstract
Plants generate a plethora of secondary compounds (toxins) that potently influence the breadth of the breeding niches of animals, including Drosophila. Capsaicin is an alkaloid irritant from hot chili peppers, and can act as a deterrent to affect animal behaviors, such as egg laying choice. However, the mechanism underlying this ovipositional avoidance remains unknown. Here, we report that Drosophila females exhibit a robust ovipositional aversion to capsaicin. First, we found that females were robustly repelled from laying eggs on capsaicin-containing sites. Second, genetic manipulations show that the ovipositional aversion to capsaicin is mediated by activation of nociceptive neurons expressing the painless gene. Finally, we found that capsaicin compromised the health and lifespan of flies through intestinal dysplasia and oxidative innate immunity. Overall, our study suggests that egg-laying sensation converts capsaicin into an aversive behavior for female Drosophila, mirroring an adaptation to facilitate the survival and fitness of both parents and offspring.
Subject terms: Social behaviour, Stress and resilience
Introduction
The environment contains a variety of threats to inhabitants, and many plants generate toxins that influence the breadth of the breeding niche of animals1. Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is a major pungent component in chili peppers. It exerts potent effects on numerous physiological processes in animals2. Accumulating evidence suggests that capsaicin causes a sensation of burning pain through chemoreceptors and nociceptors3,4. Accordingly, it acts as an irritant for many species ranging from insects to mammals. Extensive studies have shown that capsaicin affects foraging, food-averse migratory behavior, and social behavior in insects5–7. For instance, capsaicin inhibits the foraging of the beetle (Tenebrio molitor)8. Any inhabitants must use elaborate defensive mechanisms to protect against capsaicin, because overcoming this antagonism is critical for survival. Notably, several studies have reported that capsaicin acts as a repellent that affects the egg-laying decisions of several insects8,9. However, this behavior and the mechanism underlying how capsaicin causes contact- or ingestion-dependent pathogenesis in flies remain to be thoroughly investigated in an ecological context. The vinegar fly, Drosophila melanogaster, mainly breeds on decaying fruits and continuously explores favorite substrates prior to depositing each egg. The egg-laying behavior is an innate behavior for Drosophila propagation10,11, making D. melanogaster as a feasible model for investigating the effect of capsaicin on this behavior. Female egg-laying involves a complex assessment of cues regarding the opportunities and threats in the surroundings11. Given that detecting danger is a primary task for their survival and reproduction, Drosophila females are sensitive to potentially toxic substrates, such as pathogens12,13, wasps14, and alkaline substances15. Nevertheless, whether Drosophila is repelled from oviposition by the capsaicin remains unknown.
The adult fly gut, similar to the mammalian gut, is a plastic and functionally compartmentalized organ lined by epithelia16,17. The intestinal epithelia form a gut barrier that allows the gut to digest and absorb nutrients but restricts host contact with various xenobiotics. Concurrently, the gut tolerates a variety of stresses and is susceptible to acute and chronic toxicants. Stress results in intestinal epithelium impairment and intestinal barrier dysfunction in mammals and Drosophila. Thus, capsaicin has been postulated to accelerate the onset of intestinal barrier defects and shorten the lifespan18. To maintain intestine integrity, intestinal epithelia undergo stress-induced turnover throughout the lifespan. In the adult midgut, intestinal stem cells are the sole dividing cells, and they generate two differentiated intestinal cell types: enteroendocrine cells and enterocytes18. This regeneration makes the Drosophila intestine a promising model for deciphering stress-related alterations in innate immune signaling and regenerative capacity. Studies have found that dual oxidase (Duox)-mediated production of reactive oxygen species (ROS) is a primary immune mechanism underlying intestinal homeostasis19,20. However, the mechanism through which immune activation leads to capsaicin-related pathologies and lifespan impairment is not fully understood.
In the current study, we expected that capsaicin would induce an ovipositional avoidance of Drosophila melanogaster. To pursue this hypothesis, we used this model organism to explore the roles of capsaicin in oviposition decisions and examined how capsaicin affects the lifespan of Drosophila, providing insight into an adaptation to facilitate the survival and propagation of flies.
Results
Egg-laying avoidance to capsaicin
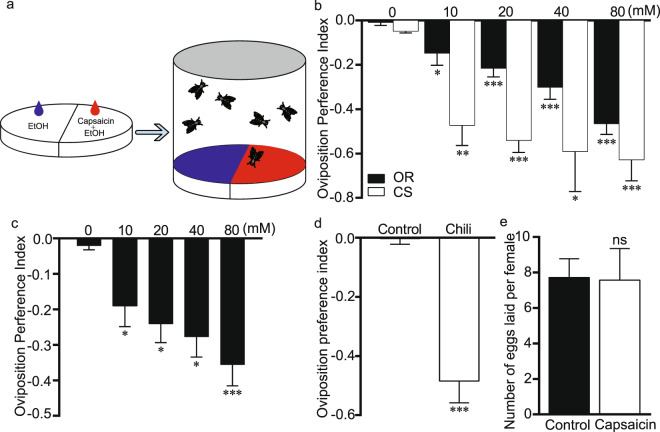
To investigate how gravid females respond to capsaicin, we evaluated their egg-laying preferences by using a two-choice apparatus10,21 (Fig. 1a). Interestingly, we found that Oregon R (OR) females used as wild-type flies significantly displayed ovipositional avoidance of capsaicin in a dose-dependent manner (Fig. 1b). For instance, OR females laid approximately 35% of their eggs on food halves with capsaicin, and the oviposition index (OI) was −0.46 (Fig. 1b). This result suggested that females were prone to avoiding laying eggs in response to capsaicin. In addition, another wild-type Canton S fly similarly showed oviposition repellence to 80 mM capsaicin, with an OI of −0.63 (Fig. 1b), thus suggesting that genetic variation did not account for this oviposition preference. Since two strains of Drosophila similarly responded to capsaicin, a representative Oregon R was used in our later experiments. Moreover, another closely related Drosophila species, D. yakuba, exhibited an aversion to laying eggs on capsaicin-containing substances (Fig. 1c), implicating that Drosophila has evolved a conserved capability of discriminate capsaicin prior to selecting oviposition sites. Interestingly, we also observed that Thailand chili repelled oviposition in OR females (Fig. 1d). Notably, capsaicin did not inhibit egg laying in general, because OR flies laid comparable numbers of eggs in the absence or presence of 40 mM capsaicin (Fig. 1e). This result suggests that the robustly negative oviposition preference for capsaicin is not simply attributable to a capsaicin-induced decrease in egg laying but instead represents an active choice made by female flies to avoid a repulsive substrate21. Together, our results demonstrated that capsaicin triggered ovipositional avoidance in Drosophila.
Figure 1.
Drosophila ovipositional aversion to capsaicin. (a) Schematic of the two-choice assay used to assess egg-laying preferences of Drosophila. Female flies were allowed to lay eggs in a dish in which one half of the food contained capsaicin, and the other contained ethanol. The numbers of eggs were counted on each half, and the oviposition preference index was calculated. (b) The ovipositional aversion to capsaicin substrate by wild-type Oregon R (OR) and Canton S (CS) flies. (c) The ovipositional aversion to capsaicin substrate by D. yakuba. (d) The quantification of ovipositional aversion to chili juice. (e) Capsaicin did not hinder the number of egg-laying in the whole-forced substrate. The average number of eggs laid by female was calculated. The one-sample t-test was used to assess the mean deviation of each column from 0 mM. n = 8–15, 3 replicates. Mean ± S.E.M.; symbols: NS p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001.
Positional and feeding avoidance of capsaicin
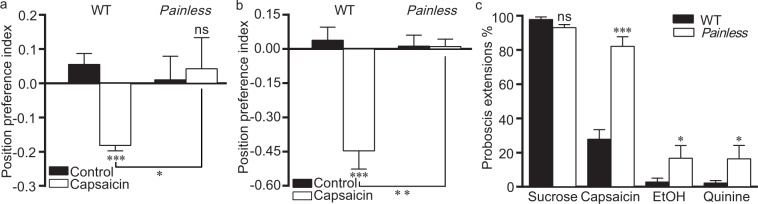
Given that females continue to seek preferred sites for oviposition after one egg laying event, their oviposition preference might be influenced by the positioning and feeding propensity for capsaicin. In tracking the positions of OR flies during oviposition, we found that females were robustly repelled from capsaicin, with a positional index of −0.18 (Fig. 2a). Additionally, the larvae of OR females were repelled from capsaicin on the surfaces of agar plates (Fig. 2b). The consistency of ovipositional and positional repellence suggests that capsaicin acts as a deterrent that naturally repels Drosophila. We further sought to explore the feeding preferences for capsaicin by using proboscis extension assays. The results showed that OR females showed aversive behavior to capsaicin (Fig. 2c). Concomitantly, feeding aversion was observed for quinine and ethanol treatment compared with H2O mock treatment (Fig. 2c). Together, these results demonstrated that capsaicin is a stimulant that simultaneously triggers ovipositional, positional, and feeding repellence.
Figure 2.
Positional and feeding avoidance of capsaicin by Drosophila. (a,b) The positional aversion of adults to capsaicin. (a) Wild-type adult female flies were averse to the capsaicin substrate, whereas Painless mutants showed neutral responses. The one-sample t-test was used to assess the mean deviation of each column from 0; 20 mM capsaicin was used. n = 150. (b) The positional aversion of wild-type larvae to capsaicin. Larvae were presented with a two-choice agar plate; 20 mM capsaicin was used, n = 10. (c) The feeding aversion to capsaicin. Wild-type flies showed less robust proboscis extension to capsaicin, EtOH and quinine compared with sucrose. Inactivation of pain sensation led to loss of aversion to capsaicin. The independent samples t-test was used to assess the mean deviation; n = 7. Mean ± S.E.M.; symbols: NS p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001.
The nociceptive system mediates ovipositional avoidance in response to capsaicin
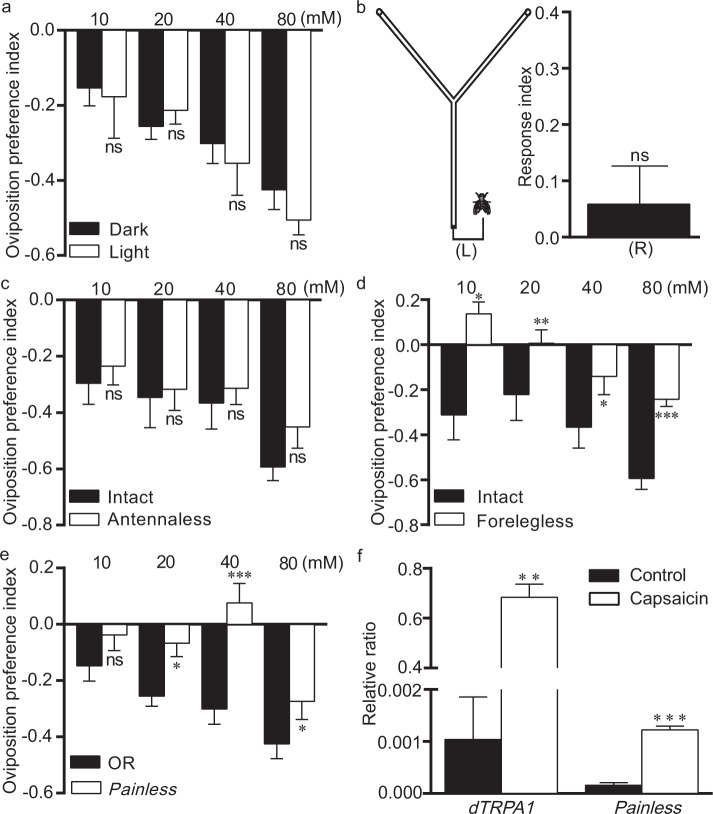
Drosophila ranks egg-laying sites via sensory modalities22, including vision23, gustation24, olfaction14, and nociception25. Next, we attempted to identify potential sensory modalities responsible for the ovipositional aversion to capsaicin. First, we tested the role of vision by allowing flies to lay eggs in darkness. The OR flies were still repelled from oviposition in food halves with capsaicin, and the OI in darkness did not significantly differ from that in light (Fig. 3a). Hence, it is unlikely that vision makes a critical contribution to the ovipositional avoidance to capsaicin. Given that capsaicin has volatile properties, we next examined whether olfaction might be required for this ovipositional avoidance by using the Y-maze assay14 (Fig. 3b, L). However, the OR flies displayed no bias to capsaicin, with a response index of 0.05 (Fig. 3b, R), indicating that the olfactory system was not required for the ovipositional repellence from capsaicin. To verify these results, we impaired olfaction by surgically dissecting the primary olfactory organs, the third antennal segments. Indeed, antennaectomized OR females still displayed egg-laying aversion to capsaicin (Fig. 3c). Thus, olfaction is not essential for the ovipositional aversion to capsaicin. Capsaicin elicits a burning pain by activating specific receptors (nociceptor) on sensory nerve endings. Because a subset of sensory neurons are embedded within the fly forelegs, we surgically removed the foreleg tips of OR flies. In those flies, the avoidance to capsaicin was markedly lower than that in intact OR flies (Fig. 3d), indicating that pain-sensing neurons were necessary for this avoidance. The forelegs also contain gustatory bristles that guide flies to select hospitable zones to deposit eggs. To rule out the potential roles of gustation, we next sought to identify the nociceptive receptors that regulate egg-laying aversion to capsaicin. We found that painless mutants showed lower avoidance to capsaicin at concentrations below 40 mM than wild-type flies (Fig. 3e). Moreover, the positional and feeding avoidance of painless mutants was completely impaired (Fig. 2a–c). Collectively, our results suggest that nociception is required for ovipositional aversion to capsaicin.
Figure 3.
The pain-sensing system mediates ovipositional aversion to capsaicin. (a) Vision is dispensable in the ovipositional repellence of capsaicin. For vision, wild-type flies in darkness and light were used. n = 6–15. (b) Olfaction is not required for ovipositional aversion to capsaicin. Schematic drawing of the Y maze olfactory assay used for olfaction assays with capsaicin (L) and the response index for capsaicin in the Y maze olfactory assay (R). (c) Olfaction is not required for ovipositional aversion to capsaicin. Wild-type females with surgically removed antennae were used, and the oviposition index was evaluated. n = 6. (d) Gustation/nociception is required for ovipositional aversion to capsaicin. The forelegs of flies were surgically ablated, and the oviposition index was quantified. n = 6. (e) Nociception mediated the ovipositional avoidance of capsaicin. (f) Capsaicin activated the expression of pain-associated genes in wild-type flies. n = 6–15. The independent samples t-test was used to assess the mean deviation within conditions. Mean ± S.E.M.; symbols: NS p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001.
To verify that capsaicin triggered the transcription of pain-associated genes, we further quantified the transcriptional level of painless and dTRPA126 (a nonselective cation channel) in capsaicin-treated guts of OR flies through quantitative PCR. As anticipated, our data showed that levels of both painless and dTRPA1 post capsaicin treatment were higher than those in the control group (Fig. 3f). Therefore, we concluded that capsaicin activates the nociceptive modality that mediates the ovipositional avoidance in response to capsaicin in Drosophila.
Capsaicin hinders the growth of Drosophila progeny
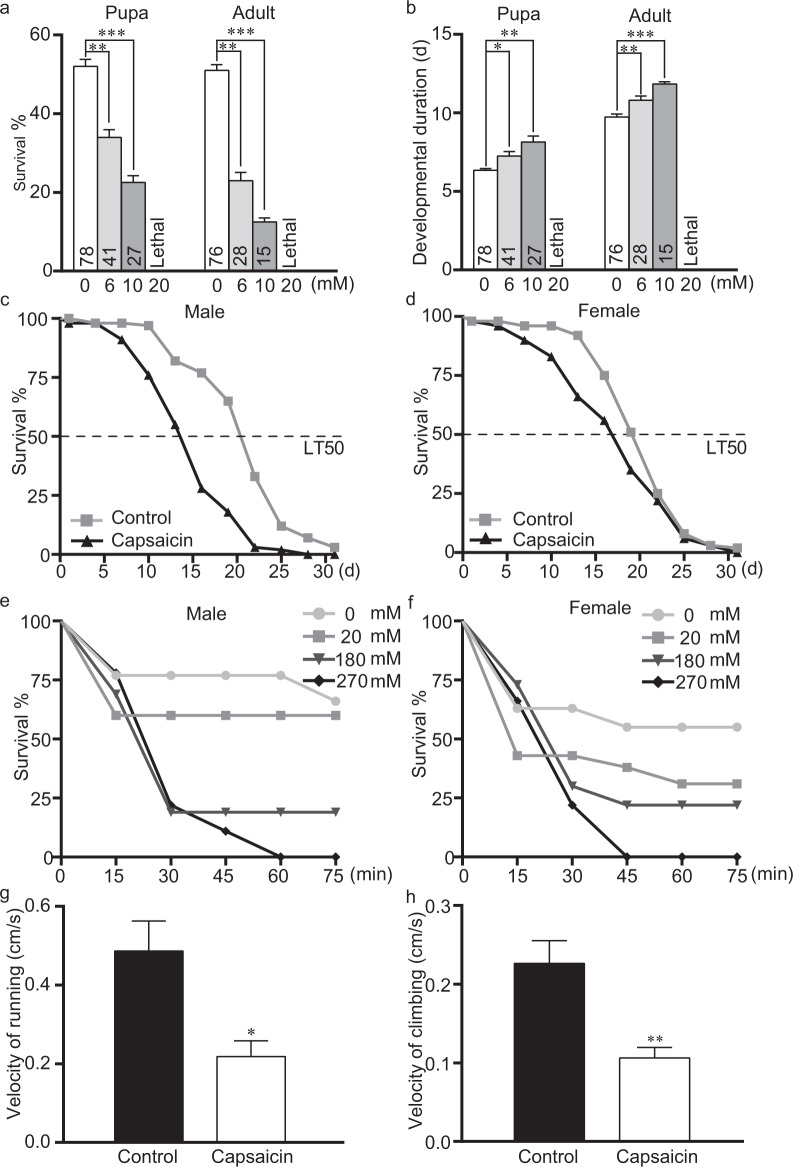
In nature, Drosophila populations deposit eggs in suitable sites to enhance the survival of their offspring10. To investigate how capsaicin affects the development of progeny, we treated eggs with different doses of capsaicin. The survival rates of pupae and adults were lower after treatment with capsaicin (Fig. 4a), whereas the animals survived normally in control food. For instance, the survival percentages of pupa and adult formation from eggs declined to 22% and 12% in food with 20 mM capsaicin, whereas the corresponding percentages were 52% and 51% in control food. Furthermore, the pupation and eclosion durations of flies were prolonged with capsaicin treatment compared with the control treatment (Fig. 4b). For example, the duration of formation of pupa and adults from eggs was 6.3 and 9.7 days in control food, respectively. Nevertheless, the corresponding durations were prolonged to 8.1 and 11.8 days in food containing 10 mM capsaicin. Overall, our data support that capsaicin diminishes the fitness of progeny, thus reflecting a strategy of ovipositional avoidance in wild flies.
Figure 4.
Capsaicin negatively affects Drosophila health and lifespan. (a) Capsaicin impaired the survival of progenies. Fly eggs were transferred to fly food with different doses of capsaicin, and the percentage of progenies that pupated and eclosed was assessed, respectively. 3 replicates. (b) The timing of pupa formation and emergence of adult progenies. The numbers of pupa formation and adult emergence were recorded each day post egg laying. 3 replicates. (c,d) The lifespan of flies was shortened by oral capsaicin. 80 mM capsaicin was used. n = 20, 4 replicates. (e,f) The lifespans of flies were decreased by contact capsaicin. n = 5, 4 replicates. (g,h) Capsaicin decreased the locomotion of flies. Flies were treated with capsaicin, and the velocity of climbing and running was assayed. 80 mM was used. n = 5, 4 replicates. The independent samples t-test was used to assess the mean deviation of each column. Mean ± S.E.M.; symbols: NS p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001.
Capsaicin decreases the lifespan and climbing behavior of adult flies
Capsaicin widely repels insects8 and therefore may directly exert adverse effects on adult OR flies. Indeed, the survival of these flies was dramatically less than that of control flies (Fig. 4c,d), indicating that capsaicin was toxic to flies. Notably, male flies were more susceptible to capsaicin than females, partially explained by the fact that bodyweight of males is lighter than that of females. Capsaicin also serves as a contact insecticide that has adverse effects on insect survival. Indeed, abdomen-contacted capsaicin caused greater lethality in females (Fig. 4e,f). Coordinated locomotion is required for fundamental activities of life. As expected, the results showed that capsaicin impaired running and climbing behaviors (Fig. 4g,h). Collectively, our results demonstrated that capsaicin decreased the fitness of both Drosophila parents and progenies.
Capsaicin disrupts intestinal integrity in Drosophila
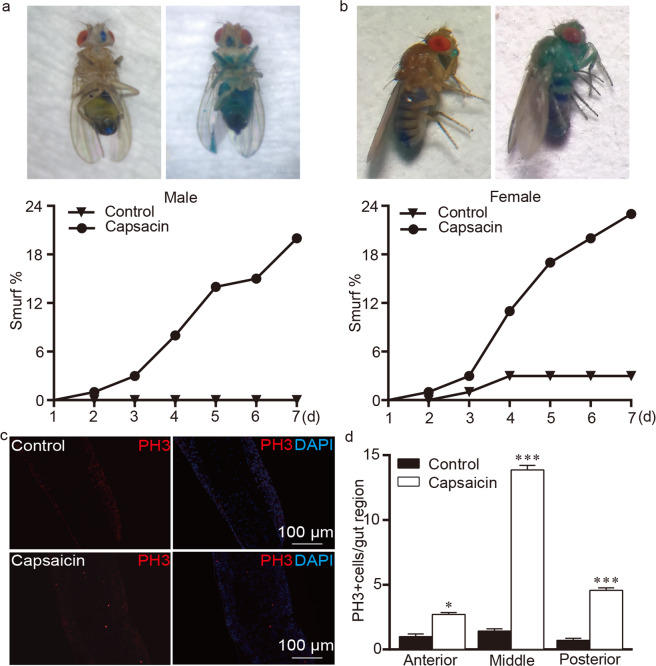
Wild-type flies may ingest capsaicin-containing food that adversely affects their digestive tracts. Using an unabsorbable blue dye, we assayed for loss of intestinal barrier function, as previously described18,27. We found that the percentage of loss of the intestinal barrier was higher in capsaicin-treated flies than in control flies (Fig. 5a,b), suggesting that capsaicin caused dye leakage into the hemolymph and consequently all tissue. Intestinal stem cells generate progeny to replenish cell loss induced by acute injury; therefore, we further assessed the number of dividing cells with an anti-phosphorylated histone 3 (PH3) antibody to determine mitotic activity28. The number of PH3-positive cells was significantly greater in flies treated with capsaicin than in control flies (Fig. 5c,d). Overall, our data suggested that capsaicin disrupts intestinal integrity of OR flies.
Figure 5.
Capsaicin disrupts the integrity of intestines. (a,b) The representative images of “smurf” flies (top) and the percentage of “smurf” in flies (below). “Smurf” flies displayed intestinal barrier dysfunction by blue dye permeation throughout the body. 80 mM capsaicin was used. n = 12. (c) Capsaicin stimulated the proliferation of intestinal stem cells in the midgut. Representative images in which mitosis is indicated by red staining with PH3, and nuclei are stained blue with DAPI. (d) Quantification of PH-3 positive cells in the gut regions of control and capsaicin exposed flies. The anterior gut, midgut, and posterior gut are parts of the gut regions. 80 mM capsaicin was used. n = 8, three replicates. The independent samples t-test was used to assess the mean deviation of each column. Values represent mean ± S.E.M. **P < 0.01; ***P < 0.001.
Capsaicin triggers oxidative innate immunity defense
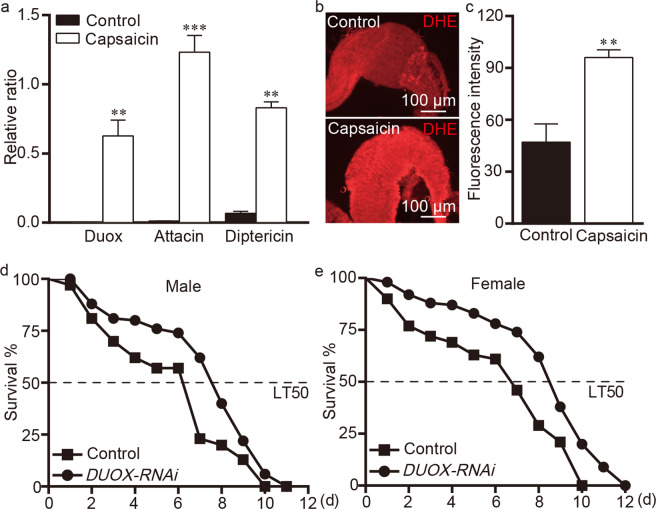
The expression of the antimicrobial peptides attacin and diptericin was elevated in the guts of OR flies after capsaicin treatment (Fig. 6a). Dual oxidase (Duox) is a member of the ROS-producing nicotinamide adenine dinucleotide phosphate (NADPH) oxidases29. As expected, the expression of Duox was significantly higher in the midgut in capsaicin-treated flies than control flies (Fig. 6a). Moreover, the ROS levels were higher in capsaicin-treated OR flies than control OR flies (Fig. 6b,c). To validate the role of ROS in mediating the response to capsaicin, we knocked down Duox in flies with the GAL4/UAS system20. The Duox silencing markedly decreased capsaicin’s toxicity to flies (Fig. 6d,e). Taken together, these results suggest that capsaicin triggers oxidative innate immunity in the midgut.
Figure 6.
Capsaicin triggers oxidative innate immunity. (a) Capsaicin activated the expression of target genes associated with oxidative innate immunity. DUOX, Attacin, and Diptericin mRNA levels for capsaicin-associated wild-type flies. 80 mM capsaicin was used. (b,c) Regulation of ROS activity in the gut. ROS activity was measured with DHE. 80 mM capsaicin was used. n = 6, three replicates. (d,e) Survival of Duox-silenced adult flies challenged with capsaicin. 80 mM was used. n = 150; The independent samples t-test was used to assess the mean deviation of each column. Values represent mean ± S.E.M. **P < 0.01; ***P < 0.001.
Discussion
Egg laying selection is used to detect aversion toward compounds that are toxic to both larvae and adults. In this work, we showed that Drosophila females exhibit a vigorous ovipositional aversion to capsaicin through the nociceptive neurons expressing the painless gene. In addition, capsaicin decreases the fitness of both offspring and parents through intestinal dysplasia and hyperactivation of oxidative innate immunity. Overall, our findings regarding capsaicin avoidance suggest an adaptation promoting the survival and fitness of both parents and offspring30,31, providing insight into the resource requirements and ecological behaviors of Drosophila species.
Because of their limited mobility, larvae are vulnerable to predators and toxicants. Female flies must select preferred oviposition sites to increase the survival of their offspring11. As such, selecting an appropriate site for oviposition has become an innate behavior in Drosophila females. For herbivorous insects, Drosophila feed on fruits and engage in a persistent battle with host plants32. However, many plants use a chemical defense system to generate many secondary metabolites, including capsaicin4. For example, capsaicin has been shown to hinder feeding by many invertebrates32. Recent studies, including our work, suggest a general theme in which Drosophila is robustly repelled from many toxicants, including harmful molds12. Ovipositional deterrents have not previously been tested for the onion fly with host models6. Flies at all times must balance the benefits and threats of progeny fitness and their survival, because survival and reproduction strategies are used in the context of systemic ecology10. Interestingly, a previous study has found a feeding preference for capsaicin when wild-type flies are given a choice between capsaicin-laced sucrose and sucrose alone4. The difference of these results may be explained by the differences in capsaicin concentrations. Because capsaicin affects thermoregulation of insects through TRPA receptors, we speculate that low concentration of capsaicin could provoke excitement, but high concentration would activate the defense reaction. Indeed, capsaicin in high concentrations has known insecticidal properties6,8, potentially resulting in fly feeding avoidance of capsaicin. Further exploration of the subtle interactions involved will be of great interest. Of note, it is unlikely that the ovipositional avoidance of capsaicin stems simply from the feeding and positioning avoidance of capsaicin (Fig. 2). The oviposition preference does not, in fact, constantly correspond to a positional preference, because Drosophila balances multiple environment cues, even competing behavioral drives before behavioral output22. For example, acetic acid is an ovipositional stimulant that flies otherwise avoid21. Our results showed that up to 200 mM capsaicin efficiently decreased the survival of flies in a contact toxicity bioassay (Fig. 4e,f). Therefore, it is postulated that female adults can endure capsaicin in sites with concentrations of capsaicin (20–80 mM) when they are laying eggs. In addition, female flies laid comparable numbers of eggs in the absence or presence of 40 mM capsaicin (Fig. 1e), suggesting that low concentrations of capsaicin do little harm to adult females. Overall, our findings suggest that female flies make an independent decision to avoid ovipositing in response to capsaicin.
Nociception is caused by physical (heat, cold, and pressure) or chemical (acid, irritants, and inflammatory mediators) stimuli33. Nociceptive sensory neurons detect these stimuli and activate neural circuits that elicit stereotyped escape responses34. Our findings showed that the ovipositional aversion toward capsaicin is mediated by the activation of the nociceptive neurons expressing the painless gene (Fig. 3). In Drosophila, the painless gene—an evolutionary homolog of the mammalian isothiocyanate receptor TRPA1/ANKTM14—is required for the foraging avoidance of isothiocyanates, because isothiocyanates from wasabi and capsaicin can give rise to a burning sensation25. Painful or threatening experiences trigger escape responses that are guided by nociceptive neuronal circuitry3. Efficient and rapid escape behavior in reaction to threatening sensory stimuli is vital for defense and ultimately survival. Drosophila larvae must forage for food while avoiding noxious stimuli and predators, such as parasitoid wasps14. Capsaicin is derived from the genus Capsicum, its receptor, transient receptor potential vanilloid subfamily member 1 (TRPV1), is localized to many human organs34, including the brain, intestine, liver, pancreas, lung, and kidney. Nociception is the encoding of a noxious stimulus and its transduction into electric signals. Noxious stimuli are detected by nerve endings found throughout the body and originating from the PSNs. The activation of TRPV1 induces cation (Ca2+) influx, which further triggers voltage-gated sodium channels and generates an action potential26.
Animals across multiple species must navigate a complicated and ever-changing environment for survival and propagation. For instance, oxidative stress induces immune activation in both Drosophila and humans35. Capsaicin ingested from the environment transiently interacts with intestine epithelia by passing through the alimentary flowing stream. Consequently, capsaicin results in intestinal epithelium impairment and intestinal barrier dysfunction in adult flies (Fig. 5). Intestinal barrier dysfunction is a good predictor of stress-induced mortality. To adapt to acute stress in the gut, animals have evolved to modulate their innate immunity to achieve intestinal homeostasis35. Although immunity is critical for host fitness in response to stress, the specific signaling pathways through which capsaicin triggers immunity are not yet entirely clear. This study was based on the well-characterized DUOX-dependent intestine immunity in flies. We found that capsaicin resulted in DUOX-dependent intestinal immune activation (Fig. 6). DUOX, a member of the NADPH oxidase family, functions as the first line of defense against intestinal stress by producing ROS. DUOX-dependent ROS are considered to be involved directly or indirectly in epithelial turnover through activation of intestinal stem cells in the presence of stress36. In lines with previous findings37, capsaicin triggers the immune pathway via de novo production of AMPs. Thus, capsaicin-induced DUOX-activating signaling exerts a pronounced effect on the lifespan and health of Drosophila.
Using Drosophila, we investigated an ecological phenomenon whereby capsaicin repels Drosophila females from oviposition. Consistent with this finding, capsaicin decreases the fitness of Drosophila through intestinal dysplasia. Future research will facilitate more comprehensive understanding of the relationship between the molecular pathology and ecological behaviors of Drosophila.
Materials and Methods
Fly husbandry and stocks
All fly stocks were raised at 25 °C, 60% humidity in a 12/12 h light/dark cycle on standard cornmeal-yeast-sucrose food unless otherwise noted10. Oregon R and Canton S strains were used as wild-type flies. NP3084 and DUOX mutants were a gift from Prof. Gao Guanjun (Shanghai Technology University)20. Painless mutants and D. yakuba were from the Core Facility of Drosophila Resource and Technology, Shanghai Institute of Biochemistry and Cell Biology, CAS, China.
Oviposition preference assays
The two-choice apparatus was assembled with a transparent 80-mm column with a 60-mm Petri dish at the bottom10,21. The two-choice dishes were generated by evenly dividing food into halves with a razor blade. In brief, capsaicin (MACKLIN) was dissolved in absolute alcohol, and diluted capsaicin-containing solutions (10 mM, 20 mM, 40 mM, and 80 mM) were added to the surfaces of the egg-laying substrate. The two-choice dishes were placed in air for 3 h to volatilize the alcohol. For each test, 25 newly eclosing females were collected and mated for 3 days in the presence of yeast paste. Flies were gently transferred into assay cages without CO2 anesthesia and were allowed to lay eggs for 8 h in darkness. To assess oviposition preference, we counted the number of eggs on each half and determined the OI as follows: [OI = (number of eggs laid on experimental food – number of eggs laid on control food)/total number of eggs laid]. For one-forced assays, 25 mated females were transferred to cages totally with capsaicin-containing substrate, where they were allowed to lay eggs for 12 h. Flies were removed, and laid eggs were counted. Egg laying was calculated by dividing the number of eggs by the total number of live females.
Position preference assays
The two-choice apparatus was the same as that used in oviposition preference assays10,21. For positional preference assays, 200 flies were transferred to cages. The number of flies on each food half was counted at 10-min intervals for 2 h via a camera. The number of flies was summed and averaged, and a position index (PI) was calculated as follows: [PI = (number of flies on experimental food – number of flies on control food)/total number of flies on food].
For surgeries, females were anesthetized with CO2 on a pad, and the antennae and tarsi were removed with fine forceps. Flies were allowed to recover for 48 h before testing.
Feeding preference assays
To assay feeding preferences, each compound was dissolved in 5% sucrose solution as previously described14. Briefly, files were collected on the day of eclosion and kept in standard corn meal food for 3–7 days at 25 °C. Before the feeding assays, females were starved for 24 h at 25 °C in vials with a water-saturated Whatman filter paper. Flies were anaesthetized by chilling on ice, mounted by their backs/wings on a microscope slide using double-sided Scotch tape and allowed to recover for 1 h at room temperature. Taste solutions were delivered with a 10 μl pipette to legs for up to 20 s, and the time of proboscis extension was examined. The feeding index (FI) was calculated as follows: FI = the total time of proboscis extension/20 s ×100%.
Contact toxicity bioassay
The contact toxicity bioassay was performed as previously described38. Briefly, files were collected on the day of eclosion and kept in standard corn meal food for 5 days at 25 °C. Flies were anaesthetized with CO2, mounted by their wings on a microscope slide using double-sided Scotch tape and allowed to recover for 30 min at room temperature. Each compound was dissolved in 75% EtOH solution, and 10 μL solutions with different concentrations of capsaicin were evenly spread on their abdomens (n = 5, 4 replicates). Flies without leg movements were designed as dead flies every 15 minutes, and the survival rate was calculated.
Survival ratio assays
In feeding experiments39, flies were starved at 25 °C for 4 h before transfer to cages containing a filter paper with 1 ml capsaicin–sucrose. Capsaicin feeding was performed at 25 °C. For viability analysis, we used 20 flies per vial, and the experiments were repeated four times. Flies were transferred to new cages with new capsaicin–sucrose filter paper discs every 24 h and counted every day. The percentage of survival was calculated.
Dye penetration test (smurf)
The intestinal barrier dysfunction assay was conducted on flies with starved at 25 °C for 5 h. The feeding experiment was performed as described above. Twenty flies were used in every vial and kept at 25 °C. Flies were first exposed to capsaicin–sucrose filter paper for 5 d and then transferred to new cages with filter paper mixed with 2.5% Erioglaucine (FD&C Blue #1)18 and 5% sucrose. For the dye penetration test, 20 wild-type flies were fed on filter paper soaked with capsaicin-sucrose solution and Erioglaucine. A fly was counted as a Smurf when dye coloration was observed outside the digestive tract. Smurf % = number of smurf flies/(number of non-Smurf flies + number of smurf flies) × 100%.
Development timing assays
The embryos were inoculated into fly food with 0 mM, 6 mM, 10 mM, or 20 mM capsaicin (n = 20–35, 5 replicates). The number of embryos was counted with a stereo microscope. The numbers of pupae and emerging adults were counted everyday following embryo incubation. Developmental timing was calculated with the following formula: T = (T1 × N1 + T2 × N2 + … + Tm × Nm)/(N1 + N2 + … + Nm), where T is the developmental timing; Tm is the number of days to form pupae and adults after egg laying; and Nm is the number of pupae and adults on the mth day12.
Climbing and running assays
Polystyrene vials were prepared by placing a Kimwipe moistened with 5% sucrose at the bottom. Capsaicin solution (80 mM) was delivered to the vials everyday. Flies were treated for 5 d and then transferred to a glass graduated cylinder or a serological pipette (5 ml). Locomotion assays were conducted as described with the following modifications40. To analyze climbing ability, all flies were synchronized to be tapped down to the bottom of the cylinder. Climbing was recorded using a video camera, and climbing distances at the 6th second were calculated. To analyze running velocity, 5 flies was gently pounded down to the end of a serological pipette in a dark room. This apparatus was horizontal and perpendicular to the light source 15 cm away. Running was recorded using a video camera, and the time to pass the line at the other end of the serological pipette was calculated.
Real time-PCR analysis
Flies were challenged with 80 mM capsaicin for 48 h, and the guts were dissected and transferred to cold PBS buffer. A total of 40 guts were homogenized with TRIzol reagent (Invitrogen), and RNA was extracted as previously described12. RNA was reverse transcribed with an oligo-dT primer. The primer sequences are available upon request. Expression of these genes was assessed with a Bio-Rad CFX instrument. The ΔCt method was used to analyze data with rp49 as the reference gene. The relative expression value was calculated with the following formula: △Ct = Ct (target gene) - Ct (reference gene), and the relative expression was equal to 2−△△Ct.
Immunofluorescence staining
The immunofluorescence assay was performed as described in previous studies28. The flies were fed with 5% sucrose solution with or without 80 mM capsaicin for 5 d. Guts were dissected in PBS and fixed with 4% paraformaldehyde for 30 min at room temperature. Samples were blocked with blocking solution (PBS with 0.3% Triton X-100, 0.2% goat serum and 0.1% fetal calf serum) for 30 min. Guts were incubated in phospho-histone 3 (EMD Millipore, 1:1000 dilution) overnight at 4°C, washed in PBS supplemented with 0.3% Triton X-100, and incubated in PBS with anti-rabbit secondary antibodies (Invitrogen, 1:1000 dilution) and DAPI (Invitrogen, 1:1000 dilution) for 2 h at room temperature. Samples were mounted with Vectshield and observed under a fluorescence microscope (Leica DM4000).
In vivo detection of reactive oxygen species
ROS production in intestinal epithelial cells was measured with the intracellular ROS-sensitive fluorescent dye dihydroethidium19. After capsaicin feeding for 48 h, the midguts of flies were dissected in PBS and incubated in a 5 μM concentration of the intracellular ROS-sensitive fluorescent dye dihydroethidium (hydroethidine) (Invitrogen) for 30 min at room temperature in the dark. Then, the midguts were washed three times with PBS, and the tissues were immediately immobilized with 4% paraformaldehyde for 10 min. Next, the tissues were washed three times with PBS. Subsequently, the gut samples were transferred to a glass slide in a drop of PBS for epifluorescence examination and observed under any fluorescence microscope (Leica DM4000). The intensity of immunofluorescence was quantified in ImageJ software.
Statistical analysis
All analysis was performed in GraphPad Prism statistical software. Specific statistical tests are noted for individual experiments. Significance testing was conducted via a Student’s t-test. Figures show mean ± standard error of the mean (S.E.M.). * equals P < 0.05, ** equals P < 0.01 and *** equals P < 0.00141,42.
Acknowledgements
We would like to thank all members of Liu Wei’s laboratory for helpful discussions. This work was supported by the National Natural Science Foundation of China (31501175), grants from Shanxi Medical University Fenyang College (2018C02 and 2019D09), and the Shanxi Undergraduate Training Program for Innovation and Entrepreneurship (2018810).
Author contributions
W.L., E.T. and Y.L. designed all experiments. Y.L., P.B., L.W., R.K., L.C. and M.Z. performed experiments. W.L. and Y.L. wrote the main manuscript text and prepared all figures. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yaoxing Li, Peng Bai and Longsheng Wei.
Contributor Information
Longsheng Wei, Email: wls6ghxy@163.com.
Wei Liu, Email: liuwei@sxmu.edu.cn.
References
- 1.Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nature Reviews Neuroscience. 2015;16:290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- 2.McCarty, M. F., DiNicolantonio, J. J. & O’Keefe, J. H. Capsaicin may have important potential for promoting vascular and metabolic health: Table 1. Open Heart2, 10.1136/openhrt-2015-000262 (2015). [DOI] [PMC free article] [PubMed]
- 3.Caterina MJ, Schumacher MA. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 4.Al-Anzi B, Tracey WD, Jr., Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Antonious GF, Meyer JE, Snyder JC. Toxicity and repellency of hot pepper extracts to spider mite, Tetranychus urticae Koch. J Environ Sci Health B. 2006;41:1383–1391. doi: 10.1080/0360123060096419. [DOI] [PubMed] [Google Scholar]
- 6.Cowles RS, Keller JE, Miller JR. Pungent spices, ground red pepper, and synthetic capsaicin as onion fly ovipositional deterrents. Journal of Chemical Ecology. 1989;15:719–730. doi: 10.1007/BF01014714. [DOI] [PubMed] [Google Scholar]
- 7.Lale NES. Oviposition-deterrent and repellent effects of products from dry chilli pepper fruits, Capsicum species on Callosobruchus maculatus. Postharcest Biology and Technology. 1992;1:343–348. doi: 10.1016/0925-5214(92)90036-O. [DOI] [Google Scholar]
- 8.Olszewska J, Tegowska E. Opposite effect of capsaicin and capsazepine on behavioral thermoregulation in insects. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2011;197:1021–1026. doi: 10.1007/s00359-011-0657-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spurr, E. B. & Mcgregor, P. G. Potential invertebrate antifeedants for toxic baits used for vertebrate pest control: A literature review. Science for Conservation (2003).
- 10.Liu W, et al. Enterococci Mediate the Oviposition Preference of Drosophila melanogaster through Sucrose Catabolism. Sci Rep. 2017;7:13420. doi: 10.1038/s41598-017-13705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang CH, Belawat P, Hafen E, Jan LY, Jan YN. Drosophila Egg-Laying Site Selection as a System to Study Simple Decision-Making Processes. Science. 2008;319:1679–1683. doi: 10.1126/science.1151842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su W, Liu J, Bai P, Ma B, Liu W. Pathogenic fungi-induced susceptibility is mitigated by mutual Lactobacillus plantarum in the Drosophila melanogaster model. BMC Microbiol. 2019;19:302. doi: 10.1186/s12866-019-1686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stensmyr MC, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Dweck HK, et al. Olfactory preference for egg laying on citrus substrates in Drosophila. Curr Biol. 2013;23:2472–2480. doi: 10.1016/j.cub.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Li G, Liu J, Liu J, Xu XZ. TMC-1 Mediates Alkaline Sensation in C. elegans through Nociceptive Neurons. Neuron. 2016;91:146–154. doi: 10.1016/j.neuron.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemaitre B, Miguel-Aliaga I. The digestive tract of Drosophila melanogaster. Annu Rev Genet. 2013;47:377–404. doi: 10.1146/annurev-genet-111212-133343. [DOI] [PubMed] [Google Scholar]
- 17.Miguel-Aliaga I, Jasper H, Lemaitre B. Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster. Genetics. 2018;210:357–396. doi: 10.1534/genetics.118.300224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci USA. 2012;109:21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei G, et al. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc Natl Acad Sci USA. 2017;114:5994–5999. doi: 10.1073/pnas.1703546114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao X, et al. A Mesh-Duox pathway regulates homeostasis in the insect gut. Nat Microbiol. 2017;2:17020. doi: 10.1038/nmicrobiol.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph RM, Devineni AV, King IFG, Heberlein U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. PNAS. 2009;106:11352–11357. doi: 10.1073/pnas.0901419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karageorgi M, et al. Evolution of Multiple Sensory Systems Drives Novel Egg-Laying Behavior in the Fruit Pest Drosophila suzukii. Curr Biol. 2017;27:847–853. doi: 10.1016/j.cub.2017.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solar ED, Guijón AM, Walker L. Choice of Colored Substrates for Oviposition inDrosophila Melanogaster. Bolletino di zoologia. 2009;41:253–260. doi: 10.1080/11250007409430120. [DOI] [Google Scholar]
- 24.Amrein H, Thorne N. Gustatory perception and behavior in Drosophila melanogaster. Curr Biol. 2005;15:R673–684. doi: 10.1016/j.cub.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Jordt SE, Julius D. Molecular Basis for Species-Specific Sensitivity to “Hot” Chili Peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 26.Bandell M, et al. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/S0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 27.Clark RI, et al. Distinct Shifts in Microbiota Composition during Drosophila Aging Impair Intestinal Function and Drive Mortality. Cell Rep. 2015;12:1656–1667. doi: 10.1016/j.celrep.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, Jiang F, Bi X, Zhang YQ. Drosophila FMRP participates in the DNA damage response by regulating G2/M cell cycle checkpoint and apoptosis. Hum Mol Genet. 2012;21:4655–4668. doi: 10.1093/hmg/dds307. [DOI] [PubMed] [Google Scholar]
- 29.Buchon N, Silverman N, Cherry S. Immunity in Drosophila melanogaster–from microbial recognition to whole-organism physiology. Nat Rev Immunol. 2014;14:796–810. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibáñez-Álamo JD, Soler M. Male and female Blackbirds (Turdus merula) respond similarly to the risk of nest predation. Journal of Ornithology. 2016;158:533–539. doi: 10.1007/s10336-016-1403-x. [DOI] [Google Scholar]
- 31.Aranha MM, Vasconcelos ML. Deciphering Drosophila female innate behaviors. Curr Opin Neurobiol. 2018;52:139–148. doi: 10.1016/j.conb.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Richmond RC, Gerking JL. Oviposition site preference in Drosophila. Behavior Genetics. 1979;9:233–241. doi: 10.1007/BF01071304. [DOI] [PubMed] [Google Scholar]
- 33.Caterina MJ, et al. Impaired Nociception and Pain Sensation in Mice Lacking the Capsaicin Receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 34.Baliki MN, Apkarian AV. Nociception, Pain, Negative Moods, and Behavior Selection. Neuron. 2015;87:474–491. doi: 10.1016/j.neuron.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan SJ, Abidi SNF, Skinner A, Tian Y, Smith-Bolton RK. The Drosophila Duox maturation factor is a key component of a positive feedback loop that sustains regeneration signaling. PLoS Genet. 2017;13:e1006937. doi: 10.1371/journal.pgen.1006937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu SC, Cao ZS, Chang KM, Juang JL. Intestinal microbial dysbiosis aggravates the progression of Alzheimer’s disease in Drosophila. Nat Commun. 2017;8:24. doi: 10.1038/s41467-017-00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi XJ, Pang X, Cao JQ, Du SS. Comparative analysis on bioactivity against three stored insects of Ligusticum pteridophyllum Franch. rhizomes essential oil and supercritical fluid (SFE-CO2) extract. Environ Sci Pollut Res Int. 2020;27:15584–15591. doi: 10.1007/s11356-020-08043-5. [DOI] [PubMed] [Google Scholar]
- 39.Pan, J. C. Research on the function of rgn gene in Drosophila gut and innate immunity Doctor thesis, Northeast forestry university (2014).
- 40.Madabattula, S. T. et al. Quantitative Analysis of Climbing Defects in a Drosophila Model of Neurodegenerative Disorders. J Vis Exp, e52741, 10.3791/52741 (2015). [DOI] [PMC free article] [PubMed]
- 41.Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, Edgar BA, Boutros M. ATF3 acts as a rheostat to control JNK signalling during intestinal regeneration. Nat Commun. 2017;8:14289. doi: 10.1038/ncomms14289. [DOI] [PMC free article] [PubMed] [Google Scholar]