Abstract
Introduction
It remains unclear if naturally occurring respiratory muscle (RM) work influences leg diffusive O2 transport during exercise in heart failure patients with reduced ejection fraction (HFrEF). In this retrospective study, we hypothesized that RM unloading during submaximal exercise will lead to increases in locomotor muscle O2 diffusion capacity (DMO2) contributing to the greater leg VO2.
Methods
Ten HFrEF patients and 10 healthy control matched participants performed two submaximal exercise bouts (i.e., with and without RM unloading). During exercise, leg blood flow was measured via constant infusion thermodilution. Intrathoracic pressure was measured via esophageal balloon. Radial arterial and femoral venous blood gases were measured and used to calculate leg arterial and venous content (CaO2 and CvO2, respectively), VO2, O2 delivery, and DMO2.
Results
From CTL to RM unloading, leg VO2, O2 delivery, and DMO2 were not different in healthy participants during submaximal exercise (all, p > .15). In HFrEF, leg VO2 (CTL: 0.7 ± 0.3 vs. RM unloading: 1.0 ± 0.4 L/min, p < .01), leg O2 delivery (CTL: 0.9 ± 0.4 vs. RM unloading: 1.4 ± 0.5 L/min, p < .01), and leg DMO2 (CTL: 31.5 ± 11.4 vs. RM unloading: 49.7 ± 18.6 ml min−1 mmHg−1) increased from CTL to RM unloading during submaximal exercise (all, p < .01), whereas CaO2‐CvO2 was not different (p = .51). The degree of RM unloading (i.e., % decrease in esophageal pressure‐time integral during inspiration) was related to the % increase in leg DMO2 with RM unloading (r = −.76, p = .01).
Conclusion
Our data suggest RM unloading leads to increased leg VO2 due to greater convective and diffusive O2 transport during submaximal exercise in HFrEF patients.
Keywords: leg blood flow, oxygen transport, respiratory muscle metaboreflex, work of breathing
Respiratory muscle work negatively influences convective O2 transport in heart failure patients. The present study investigated the impact of respiratory muscle work on leg O2 diffusive capacity during submaximal exercise. We found the degree of respiratory muscle unloading in heart failure patients is related to the improvement in leg O2 diffusive capacity during submaximal exercise.

1. INTRODUCTION
Heart failure patients with reduced ejection fraction (HFrEF) exhibit impaired exercise tolerance, which is a hallmark symptom of patients with HFrEF. The underlying pathophysiologic mechanisms responsible for this compromised exercise tolerance are multifactorial, but include impaired cardiac output and exaggerated sympathetically mediated vasoconstriction in the periphery, which limits locomotor muscle blood flow (QL; Poole, Hirai, Copp, & Musch, 2012). In addition, patients with HFrEF often present with pulmonary abnormalities including obstructive‐restrictive lung disorders, lower lung diffusion capacity, increased physiologic dead space, and ventilation/perfusion mismatch (Olson, Snyder, & Johnson, 2006; Poole et al., 2012; Smith & Olson, 2019). Importantly, HFrEF patients, compared to healthy adults, have an augmented ventilatory response (i.e., ↑ ventilatory equivalent for carbon dioxide slope), respiratory muscle work, and subsequent cardiac output distribution to the respiratory muscles (i.e., diaphragm) during exercise (Agostoni, Cattadori, Bianchi, & Wasserman, 2003; Cross, Sabapathy, Beck, Morris, & Johnson, 2012; Musch, 1993; Olson et al., 2010; Smith, Hageman, Harms, Poole, & Musch, 2017; Smith et al., 2020).
The high respiratory muscle work and blood flow coupled with the limited cardiac output reserve during exercise in these patients significantly impacts cardiac output distribution and consequently contributes to exercise intolerance (Borghi‐Silva et al., 2008; Musch, 1993; O'Donnell, D'Arsigny, Raj, Abdollah, & Webb, 1999; Olson et al., 2010; Smith et al., 2017). Specifically, Olson and colleagues found that unloading the naturally occurring respiratory muscle work during submaximal exercise in patients with HFrEF resulted in greater leg oxygen uptake (VO2). This respiratory muscle unloading‐induced increase in leg VO2 was due to greater QL, total cardiac output (QT), and QL as a percent of QT (%QL), as leg arteriovenous oxygen difference (CaO2‐CvO2) was not different compared to control (i.e., without respiratory muscle unloading; Olson et al., 2010). As such, these findings suggest that the respiratory muscle unloading‐induced increase in convective O2 transport was primarily responsible for these findings. However, HFrEF patients exhibit exaggerated sympathetically mediated vasoconstriction coupled with microvascular abnormalities that compromise diffusive O2 transport (Behnke, Delp, Poole, & Musch, 2007; Esposito, Mathieu‐Costello, Shabetai, Wagner, & Richardson, 2010; Poole, Copp, Hirai, & Musch, 2011; Poole et al., 2012; Richardson, Kindig, Musch, & Poole, 2003). Specifically, HFrEF is associated with impaired capillary hemodynamics at rest and during exercise constraining muscle O2 diffusing capacity (DMO2). Importantly, superfusion of sodium nitroprusside prior to electrically stimulated muscle contractions in HFrEF rats increased microvascular PO2 (i.e., driving pressure for O2 from blood to myocyte) during contractions to a similar level reported in the sham rats (Ferreira et al., 2006) suggesting that increases in QL can improve the microvascular abnormalities in HFrEF. As high respiratory muscle work contributes to sympathetically mediated vasoconstriction in HFrEF (Chiappa et al., 2008; Olson et al., 2010), it is plausible that partial alleviation of the sympathetically mediated vasoconstriction via respiratory muscle unloading may also improve DMO2. These proposed findings would have important clinical implications as they would suggest that interventions to “unload” the respiratory muscles (e.g., inspiratory muscle training) would improve both convective and diffusive O2 transport in patients with HFrEF.
As mentioned earlier, Olson et al. determined the influence of respiratory muscle unloading on convective O2 transport during submaximal exercise in patients with HFrEF; however, DMO2 was not investigated. Therefore, the purpose of this study was to retrospectively examine data from this previous study (Olson et al., 2010) and specifically quantify the influence of respiratory muscle work on DMO2 during submaximal exercise in patients with HFrEF. We hypothesized that respiratory muscle unloading during submaximal exercise would result in greater DMO2 compared to control in HFrEF patients. Further, we hypothesized that the degree of respiratory muscle unloading would be significantly related to the improvement in DMO2 during exercise.
2. METHODS
2.1. Participants
Ten HFrEF patients were recruited from the Mayo Clinic Heart Failure Service and the Cardiovascular Health Clinic and 10 healthy matched adult participants were recruited as previously described (Olson et al., 2010). Briefly, inclusion criteria for the HFrEF patients included diagnosis of ischemia or dilated cardiomyopathy with duration of >1 year of symptoms, stable HF symptoms (>3 months), left ventricular ejection fraction ≤35%, body mass index of <35 kg/m2, nonsmokers with a smoking history of <15 pack‐years, and no diagnosis of coexisting pulmonary disease or taking medications. All aspects of this study were approved by the Mayo Clinic Institutional Review Board and conformed to the standards set forth by the latest revision of the Declaration of Helsinki. All participants were informed about the experimental procedures and potential risk involved, and provided written and verbal informed consent.
2.2. Experimental design
As previously described (Olson et al., 2010), participants performed all protocols and measurements during two study visits. On the first study visit, participants were first familiarized with all experimental measurements and protocols and then completed an incremental cycle ergometry exercise test to volitional fatigue to determine peak oxygen uptake (VO2peak). On the second study visit, participants performed two steady‐state exercise sessions at 60% peak workload. The first exercise session consisted of 3 min of rest followed by 15 min of constant load cycling. During the first and third 5 min, the participants breathed normally under room air conditions. During the second 5 min, the respiratory muscles were unloaded with the assistance of a mechanical ventilator during inspiration. The first and second exercise session protocols were similar except the second 5 min of the second exercise session consisted of respiratory muscle loading via inspiratory resistance. The primary focus of this study was to determine if respiratory muscle unloading influences leg DMO2 during exercise in HFrEF patients. As such, only the data from the exercise session with respiratory muscle unloading are reported herein.
As previously described in depth (Olson et al., 2010), QL was measured via constant infusion thermodilution, intrathoracic pressure via esophageal balloon, arterial blood pressure via radial arterial catheter, arterial and femoral venous blood gases via radial arterial and femoral venous blood sampling, and QT via open‐circuit acetylene wash‐in technique.
2.3. Calculated variables
Radial arterial and femoral venous blood sampling occurred anaerobically over 10–15 s during control and unloading exercise for measurements of partial pressure of oxygen (PaO2 and PvO2), hemoglobin (Hb), and saturation of oxygen (SaO2 and SvO2; IL‐1620, Instrumentation Laboratories). Blood gases were analyzed in duplicate, averaged, and temperature corrected at a temperature of 37°C. Direct measures assessed via blood sampling were used to calculate leg arterial and venous content [CaO2 = (1.34 × Hb × SaO2) + (PaO2 × 0.0031) and CvO2 = (1.34 × Hb × SvO2) + (PvO2 × 0.0031)]. Leg VO2 was calculated as QL multiplied by leg CaO2‐CvO2. Leg O2 delivery was calculated as QL multiplied by CaO2. Leg O2 diffusion capacity (DMO2) was calculated via Fick's Law of Diffusion, VO2 = DMO2 × (PcapO2 − PmitO2), where PcapO2 and PmitO2 are mean capillary and mitochondrial PO2, respectively. During submaximal exercise (~50%–60% VO2peak), previous studies have found that PcapO2 is proportional to PvO2 and PmitO2 is ~1–3 mmHg (and thus was assumed to be zero; Honig, Gayeski, Clark, & Clark, 1991; Richardson, Noyszewski, Kendrick, & Leigh, 1995; Roca et al., 1985). As such, Fick's Law of Diffusion was simplified as VO2 = DO2 × PvO2 (Ade, Broxterman, Moore, & Barstow, 2017; Esposito et al., 2010). It should be noted that the previous studies examining myoglobin PO2 during exercise were conducted in healthy adults or animal models. It was assumed in this study that similar myoglobin PO2 levels are reached during submaximal exercise in HFrEF. Furthermore, we recognize that the simplification of Fick's Law of Diffusion and use of PvO2 will lead to higher DMO2 values compared to when PcapO2 is used because PcapO2 is systematically higher than PvO2 (Roca et al., 1985).
2.4. Statistical analyses
Values are reported as mean ± standard deviation (SD). Statistical analyses were performed using SigmaStat 2.0 (Jandel Scientific). Normality and equal variance were assessed using the Shapiro–Wilk and Levene tests, respectively, and nonparametric tests were used when appropriate. Cardiovascular variables were compared within (control vs. respiratory muscle unloading) and between groups (HFrEF vs. healthy participants) using mixed factorial analysis of variance and Tukey's post hoc test when appropriate. Relationships were determined via linear regression. Statistical significance was set at p < .05.
3. RESULTS
3.1. Participant characteristics
As previously described (Olson et al., 2010), the patients with HFrEF had a mean age of 54 ± 15 years, left ventricular ejection fraction of 31 ± 8%, VO2peak of 17 ± 5 ml kg−1 min−1 and ischemic (n = 5) and idiopathic (n = 5) etiologies. Medications for the HFrEF patients included angiotensin converting enzyme inhibitors (n = 5), beta blockers (n = 9), digitalis (n = 3), aspirin (n = 7), and diuretics (n = 6). Lastly, age, height, weight, and sex were not different between the healthy participants and HFrEF patients (Olson et al., 2010).
3.2. Leg O2 transport with RM unloading
For the healthy participants, leg VO2 (control: 1.5 ± 0.8 versus. RM unloading 1.5 ± 0.9 L/min), O2 delivery (control: 2.1 ± 1.2 vs. RM unloading 2.1 ± 1.2 L/min), and DMO2 (control: 64.5 ± 37.4 vs. RM unloading 68.5 ± 41.4 ml min−1 mmHg−1) were not different between control and respiratory muscle unloading (all, p > .15), whereas CaO2‐CvO2 was higher with respiratory muscle unloading (control: 14.0 ± 1.0 vs. RM unloading 14.4 ± 1.2 ml/dl; p = .03). Figure 1 shows leg VO2, CaO2‐CvO2, O2 delivery, and DMO2 during submaximal exercise with control and respiratory muscle unloading in HFrEF patients. With respiratory muscle unloading, leg VO2, O2 delivery, and DMO2 increased in all HFrEF patients by ~55%–60% compared to control (all, p < .01). Furthermore, the % change in Pes,insp TI with respiratory muscle unloading was negatively related to the % change in DMO2 with respiratory muscle unloading (compared to control) during submaximal exercise (r = −.76, p = .01; Figure 2). In contrast, leg CaO2‐CvO2 was not different with respiratory muscle unloading compared to control (p = .51).
FIGURE 1.
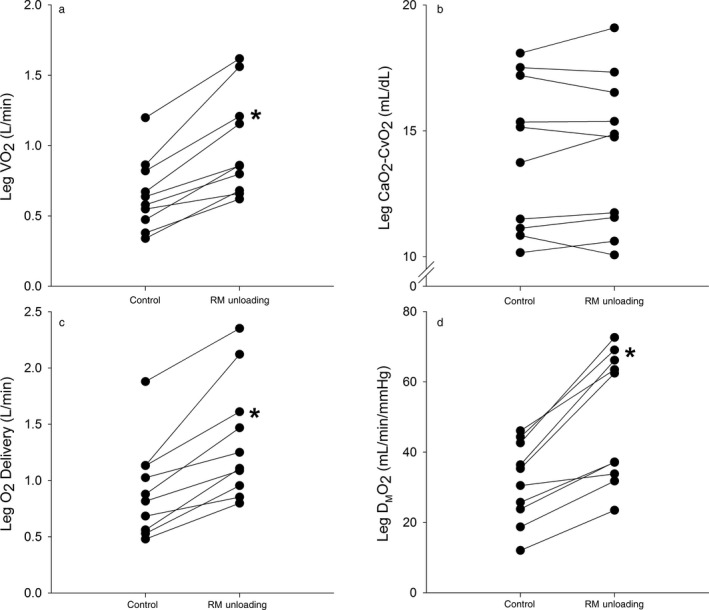
O2 delivery and utilization in heart failure patients with reduced ejection fraction during submaximal exercise. The individual responses with control and respiratory muscle (RM) unloading for leg VO2 (a), CaO2‐CvO2 (b), O2 delivery (c), and DMO2 (d). Leg VO2, O2 delivery, and DMO2 increased from control to RM unloading (all, p < .01), whereas CaO2‐CvO2 was not different (p = .51). * significantly different from control
FIGURE 2.
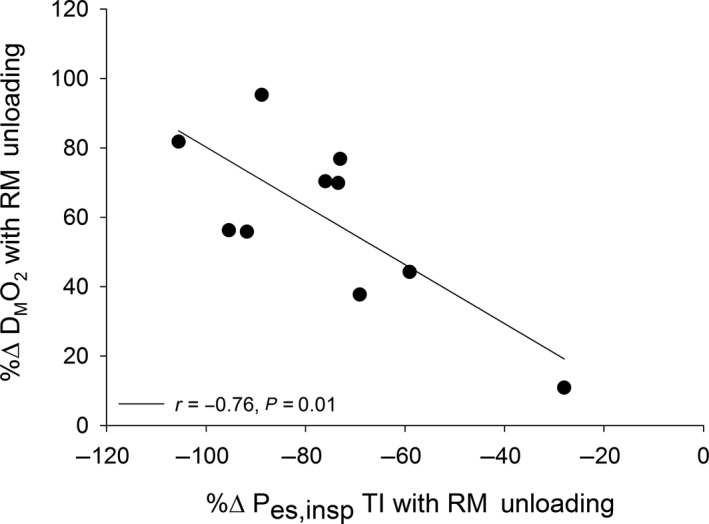
Relationship between intrathoracic pressure and leg DMO2 with respiratory muscle unloading. There was a negative relationship between the %Δ in inspiratory esophageal pressure time integral (Pes,inspTI) and %Δ in leg DMO2 with respiratory muscle (RM) unloading compared to control (r = −.76, p = .01)
4. DISCUSSION
4.1. Major findings
The purpose of this retrospective study was to determine if partially unloading the naturally occurring respiratory muscle work influenced locomotor muscle O2 diffusing capacity (DMO2) in addition to convective O2 transport during submaximal exercise in HFrEF patients. The principal novel finding of this study was that respiratory muscle unloading resulted in greater DMO2 during submaximal exercise in HFrEF, but not healthy participants. Second, the increase in DMO2 was significantly related to the degree of respiratory muscle unloading during submaximal exercise in the patients with HFrEF. These results indicate that the naturally occurring respiratory muscle work in HFrEF patients contributes to impaired leg VO2 during submaximal exercise via impairments in convective and diffusive O2 transport. Furthermore, these findings have important clinical implications as they suggest that interventions (e.g., inspiratory muscle training) aimed at ameliorating the respiratory muscle metaboreflex‐induced consequences on leg convective O2 transport will likely also improve diffusive O2 transport.
4.2. Respiratory muscle work and diffusive O2 transport
In this study, we found that unloading the naturally occurring respiratory muscle work increased DMO2 by ~60% during submaximal exercise in HFrEF patients. Furthermore, we found that the increase in DMO2 was associated with the degree of respiratory muscle unloading. These data in concert with those showing that respiratory muscle unloading leads to increases in QT, QL, and %QL (Olson et al., 2010) suggest the HFrEF‐induced respiratory muscle work during submaximal exercise impairs leg VO2 by altering both convective and diffusive O2 transport. Figure 3 illustrates the integration of convective and diffusive O2 transport in determining leg VO2 during submaximal exercise. As previously described (Ade et al., 2017; Poole et al., 2012; Wagner, 1991, 1996), the curve line represents convective O2 transport described with Fick Principal and the straight line represents DMO2 described with Fick's Law of Diffusion with the intersecting point representing leg VO2. If unloading the respiratory muscles increased leg VO2 only via increases in convective O2 transport, leg VO2 would have increased from A to B. However, respiratory muscle unloading also increased DMO2 revealing that the combined increases in convective and diffusive O2 transport led to greater increases in leg VO2 (from A to C). In contrast to DMO2, respiratory muscle unloading did not alter leg Ca‐CvO2 during submaximal exercise. As O2 utilization = 1 − (with β as the linear approximation to the slope of the O2 dissociation curve; Poole et al., 2012; Wagner, 1991), changes in both QL and DMO2 influence changes in leg Ca‐CvO2. Because QL and DMO2 increased to a similar extent (and thus the ratio between them was similar), it is then not surprising that leg Ca‐CvO2 was not different between conditions.
FIGURE 3.
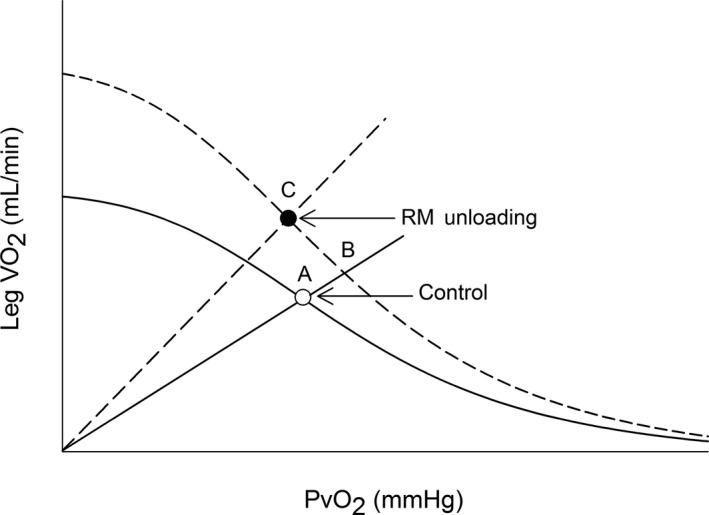
Illustration of the convective and diffusive components that integrate to determine VO2 with control and respiratory muscle unloading. This model integrates convective O2 (Fick Principal, curved lines) and diffusive O2 components (Fick's Law, straight lines from origin) to determine leg VO2 for control and respiratory muscle (RM) unloading. With RM unloading, the curved Fick Principle line is higher because of the increases in convective O2 delivery. Furthermore, there was greater leg diffusing O2 capacity (DMO2) with RM unloading compared to control (i.e., the slope of the straight Fick's Law line was greater with RM unloading than control). If RM unloading increased leg VO2 during submaximal exercise in HFrEF solely due to greater convective O2 delivery than leg VO2 would have moved from A to B. However, the greater DMO2 with RM unloading presented herein suggests that the RM unloading‐induced increase in leg VO2 was due to both greater convective and diffusive O2 transport (A to C)
The primary factors that determine diffusion of O2 across a membrane (as indicated in Fick's Law of Diffusion) include (a) physical properties of the gas, (b) membrane thickness, (c) surface area available for diffusion, and (d) the partial pressure gradient across the membrane with the latter two likely contributing to the findings presented herein. Specifically, Richardson et al. found that HFrEF rats exhibited a reduction in the percentage of capillaries that support red blood cells at rest and during electrically elicited contractions compared to control rats (Richardson et al., 2003). Furthermore, the HFrEF rats had blunted increases in capillary red blood cell flux and velocity compared to control rats with contractions (Richardson et al., 2003). Importantly, the lower capillary red blood cell velocity in HFrEF compared to control rats will reduce surface area available for capillary‐myocyte O2 exchange and thereby DMO2 (Poole et al., 2011). The pathophysiological mechanisms responsible for this impaired capillary hyperemic response in HFrEF are multifactorial with exaggerated sympathetically mediated vasoconstriction having a contributory role (Richardson et al., 2003). This disruption in capillary hemodynamics in HFrEF rats subsequently results in lower microvascular PO2 (i.e., driving pressure for O2 from blood to myocyte) during muscle contractions (Behnke et al., 2007; Ferreira et al., 2006). Crucially, superfusion of sodium nitroprusside prior to electrically stimulated muscle contractions increased microvascular PO2 at baseline and during contractions in the HFrEF rats to a similar level reported in the sham rats (Ferreira et al., 2006) suggesting that increases in muscle blood flow can ameliorate the pathophysiologic microvascular PO2 response in HFrEF. As previously described (Olson et al., 2010), unloading the respiratory muscles in HFrEF patients during submaximal exercise resulted in decreased leg vascular resistance facilitating greater QL and O2 transport. Taken together, attenuating the exaggerated sympathetically mediated vasoconstriction via respiratory muscle unloading likely increased red blood cell velocity improving both surface area for capillary‐myocyte O2 exchange and microvascular PO2 during submaximal exercise; however, future studies are necessary to confirm this hypothesis in human HF.
The improvements in convective and diffusive O2 transport with respiratory muscle unloading culminating in greater leg VO2 during submaximal exercise in patients with HFrEF have important clinical significant implications. Consistent with previous studies (Sullivan, Knight, Higginbotham, & Cobb, 1989; Zelis, Longhurst, Capone, & Mason, 1974), these findings indicate that leg VO2 is impaired during submaximal exercise in patients with HFrEF resulting in a greater reliance on anaerobic metabolism thereby precipitating fatigue development. In this study, the % change in leg VO2 was negatively related to the % change in blood pH with respiratory muscle unloading (compared to control; r = −.75, p = .02) suggesting the respiratory muscle unloading‐induced higher leg VO2 was related to higher oxidative metabolism thereby sparring anaerobic energy sources. These findings highlight the potential impact of interventions focused on improving respiratory muscle function (e.g., inspiratory muscle training) may have on exercise tolerance and exertional symptomology for patients with HFrEF.
5. CONCLUSIONS
During submaximal exercise, respiratory muscle unloading resulted in greater DMO2 HFrEF patients. On the basis of these data, we conclude that the respiratory muscle unloading‐induced increases in leg VO2 is due to greater convective and diffusive O2 transport. Future prospective studies are necessary to determine if clinically pertinent interventions aimed at unloading the respiratory muscles (e.g., inspiratory muscle training) also improves diffusive O2 transport in HFrEF patients.
CONFLICT OF INTEREST
There are no conflicts of interest to report.
AUTHOR CONTRIBUTIONS
JRS, TBC, MJJ, and TPO conceived and designed the research. TBC, MJJ, and TPO performed the experiments. JRS and JDB analyzed the data, interpreted the results of the experiment, and prepared figures. JRS drafted the manuscript. All authors edited, revised, and approved the final version of the manuscript.
ACKNOWLEDGMENT
We thank the participants who volunteered for this study.
Smith JR, Berg JD, Curry TB, Joyner MJ, Olson TP. Respiratory muscle work influences locomotor convective and diffusive oxygen transport in human heart failure during exercise. Physiol Rep. 2020;8:e14484 10.14814/phy2.14484
Funding information
This work was supported by the National Institutes of Health [HL126638 to TPO, HL139854 to MJJ] and American Heart Association [18POST3990251 to JRS].
REFERENCES
- Ade, C. J. , Broxterman, R. M. , Moore, A. D. , & Barstow, T. J. (2017). Decreases in maximal oxygen uptake following long‐duration spaceflight: Role of convective and diffusive O2 transport mechanisms. Journal of Applied Physiology, 122(4), 968–975. 10.1152/japplphysiol.00280.2016 [DOI] [PubMed] [Google Scholar]
- Agostoni, P. , Cattadori, G. , Bianchi, M. , & Wasserman, K. (2003). Exercise‐induced pulmonary edema in heart failure. Circulation, 108, 2666–2671. 10.1161/01.CIR.0000097115.61309.59 [DOI] [PubMed] [Google Scholar]
- Behnke, B. J. , Delp, M. D. , Poole, D. C. , & Musch, T. I. (2007). Aging potentiates the effect of congestive heart failure on muscle microvascular oxygenation. Journal of Applied Physiology, 103(5), 1757–1763. 10.1152/japplphysiol.00487.2007 [DOI] [PubMed] [Google Scholar]
- Borghi‐Silva, A. , Carrascosa, C. , Oliveira, C. C. , Barroco, A. C. , Berton, D. C. , Vilaca, D. , … Neder, J. A. (2008). Effects of respiratory muscle unloading on leg muscle oxygenation and blood volume during high‐intensity exercise in chronic heart failure. American Journal of Physiology. Heart and Circulatory Physiology, 294, H2465–H2472. 10.1152/ajpheart.91520.2007 [DOI] [PubMed] [Google Scholar]
- Chiappa, G. R. , Roseguini, B. T. , Vieira, P. J. , Alves, C. N. , Tavares, A. , Winkelmann, E. R. , … Ribeiro, J. P. (2008). Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. Journal of the American College of Cardiology, 51, 1663–1671. 10.1016/j.jacc.2007.12.045 [DOI] [PubMed] [Google Scholar]
- Cross, T. J. , Sabapathy, S. , Beck, K. C. , Morris, N. R. , & Johnson, B. D. (2012). The resistive and elastic work of breathing during exercise in patients with chronic heart failure. European Respiratory Journal, 39, 1449–1457. 10.1183/09031936.00125011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito, F. , Mathieu‐Costello, O. , Shabetai, R. , Wagner, P. D. , & Richardson, R. S. (2010). Limited maximal exercise capacity in patients with chronic heart failure: Partitioning the contributors. Journal of the American College of Cardiology, 55, 1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, L. F. , Hageman, K. S. , Hahn, S. A. , Williams, J. , Padilla, D. J. , Poole, D. C. , & Musch, T. I. (2006). Muscle microvascular oxygenation in chronic heart failure: Role of nitric oxide availability. Acta Psychologica, 188, 3–13. [DOI] [PubMed] [Google Scholar]
- Honig, C. R. , Gayeski, T. E. , Clark Jr., A. , & Clark, P. A. (1991). Arteriovenous oxygen diffusion shunt is negligible in resting and working gracilis muscles. American Journal of Physiology, 261, H2031–H2043. 10.1152/ajpheart.1991.261.6.H2031 [DOI] [PubMed] [Google Scholar]
- Musch, T. I. (1993). Elevated diaphragmatic blood flow during submaximal exercise in rats with chronic heart failure. American Journal of Physiology, 265, H1721–H1726. 10.1152/ajpheart.1993.265.5.H1721 [DOI] [PubMed] [Google Scholar]
- O'Donnell, D. E. , D'Arsigny, C. , Raj, S. , Abdollah, H. , & Webb, K. A. (1999). Ventilatory assistance improves exercise endurance in stable congestive heart failure. American Journal of Respiratory and Critical Care Medicine, 160, 1804–1811. 10.1164/ajrccm.160.6.9808134 [DOI] [PubMed] [Google Scholar]
- Olson, T. P. , Joyner, M. J. , Dietz, N. M. , Eisenach, J. H. , Curry, T. B. , & Johnson, B. D. (2010). Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. Journal of Physiology, 588, 2487–2501. 10.1113/jphysiol.2009.186056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, T. P. , Snyder, E. M. , & Johnson, B. D. (2006). Exercise‐disordered breathing in chronic heart failure. Exercise and Sport Sciences Reviews, 34, 194–201. 10.1249/01.jes.0000240022.30373.a2 [DOI] [PubMed] [Google Scholar]
- Poole, D. C. , Copp, S. W. , Hirai, D. M. , & Musch, T. I. (2011). Dynamics of muscle microcirculatory and blood‐myocyte O2 flux during contractions. Acta Psychologica, 202, 293–310. 10.1111/j.1748-1716.2010.02246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole, D. C. , Hirai, D. M. , Copp, S. W. , & Musch, T. I. (2012). Muscle oxygen transport and utilization in heart failure: Implications for exercise (in)tolerance. American Journal of Physiology. Heart and Circulatory Physiology, 302, H1050–H1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, R. S. , Noyszewski, E. A. , Kendrick, K. F. , & Leigh, J. S. & Wagner, P. D. (1995). Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. Journal of Clinical Investigation, 96, 1916–1926. 10.1172/JCI118237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, T. E. , Kindig, C. A. , Musch, T. I. , & Poole, D. C. (2003). Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions. Journal of Applied Physiology, 95(3), 1055–1062. 10.1152/japplphysiol.00308.2003 [DOI] [PubMed] [Google Scholar]
- Roca, J. , Hogan, M. C. , Story, D. , Bebout, D. E. , Haab, P. , Gonzalez, R. , … Wagner, P. D. (1989). Evidence for tissue diffusion limitation of VO2max in normal humans. Journal of Applied Physiology, 67(1), 291–299. 10.1152/jappl.1989.67.1.291 [DOI] [PubMed] [Google Scholar]
- Smith, J. R. , Hageman, K. S. , Harms, C. A. , Poole, D. C. , & Musch, T. I. (2017). Effect of chronic heart failure in older rats on respiratory muscle and hindlimb blood flow during submaximal exercise. Respiratory Physiology & Neurobiology, 243, 20–26. 10.1016/j.resp.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. R. , Hart, C. R. , Ramos, P. A. , Akinsanya, J. G. , Lanza, I. R. , Joyner, M. J. , … Olson, T. P. (2020). Metabo and mechanoreceptor expression in human heart failure: Relationships with the locomotor muscle afferent influence on exercise responses. Experimental Physiology, 105(5), 809–818. 10.1113/EP088353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. R. , & Olson, T. P. (2019). Ventilatory constraints influence physiological dead space in heart failure. Experimental Physiology, 104, 70–80. 10.1113/EP087183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, M. J. , Knight, J. D. , Higginbotham, M. B. , & Cobb, F. R. (1989). Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation, 80, 769–781. 10.1161/01.CIR.80.4.769 [DOI] [PubMed] [Google Scholar]
- Wagner, P. D. (1991). Central and peripheral aspects of oxygen transport and adaptations with exercise. Sports Medicine, 11, 133–142. 10.2165/00007256-199111030-00001 [DOI] [PubMed] [Google Scholar]
- Wagner, P. D. (1996). Determinants of maximal oxygen transport and utilization. Annual Review of Physiology, 58, 21–50. 10.1146/annurev.ph.58.030196.000321 [DOI] [PubMed] [Google Scholar]
- Zelis, R. , Longhurst, J. , Capone, R. J. , & Mason, D. T. (1974). A comparison of regional blood flow and oxygen utilization during dynamic forearm exercise in normal subjects and patients with congestive heart failure. Circulation, 50, 137–143. 10.1161/01.CIR.50.1.137 [DOI] [PubMed] [Google Scholar]


