Abstract
Introduction
Osteoarthritis (OA) is an age-related chronic degenerative disease. Accumulation of advanced glycation end products (AGEs) induces degradation of the articular extracellular matrix (ECM) and is considered a critical step toward the development and progression of OA. GPR40 is a well-known free fatty acid receptor, which possesses pleiotropic effects in different types of diseases. However, the biological function of GPR40 in OA is indistinct. The purpose of the present study was to determine the impact of the GPR40 agonist GW9508 on AGEs-treated chondrocytes.
Materials and Methods
Cultures of human SW1353 chondrocytes were stimulated with GW9508, followed by exposure to 100 µg/mL AGEs. Gene and protein expression of TNF-α, IL-6, MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 were measured by real-time PCR and ELISA analysis. The levels of type II collagen, aggrecan, and nuclear NF-κB p65 were measured by Western blot analysis. A luciferase assay measured the transcriptional activity of NF-κB.
Results
The results show that treatment with AGEs decreased the expression of GPR40 in human SW1353 chondrocytes. Treatment with GW9508 plays a beneficial role in protecting type II Collagen and aggrecan from degeneration by attenuating the expression of MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5. Additionally, GW9508 reduces the appearance of pro-inflammatory cytokines and suppresses NF-κB activation in AGEs-induced chondrocytes. Notably, co-treatment with GW1100, a specific antagonist of GPR40, abolishes the beneficial role of GW9508 against AGEs, implying that GPR40 mediates these effects of GW9508.
Conclusion
Our results suggest that GPR40 is a novel therapeutic target for OA and that GPR40 agonists, including GW9508, may have therapeutic potential in preventing and slowing the progression of OA.
Keywords: osteoarthritis, GPR40, GW9508, MMPs, ADAMTS, NF-κB
Introduction
Osteoarthritis (OA) is a worldwide chronic degenerative joint disease. It is characterized by inflammation, excessive degeneration of the articular cartilage, apoptosis of chondrocytes, and structural changes within the joint associated with aging. Although OA is a significant global disease that has rapidly become more prevalent in recent decades, the physiopathology of OA is still not clear.1 Previous studies have confirmed that OA has a strong effect on the degeneration of the cartilage extracellular matrix (ECM).2
Furthermore, decreased chondrocyte populations have been found in osteoarthritic cartilage, indicating that chondrocyte apoptosis may contribute to the structural changes in OA.3 There is presently considerable evidence suggesting that the accumulation of advanced glycation end products (AGEs) is responsible for the development and progression of OA.4,5 Recent research has proven that stimulation with AGEs leads to the release of pro-inflammatory cytokines, including tumor necrosis factor α (TNF-α)6 and interleukin-6 (IL-6)7 via the nuclear factor-κB (NF-κB) signaling pathway. AGEs have also been proven to upregulate the expressions of matrix metalloproteinases (MMPs)8 and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)9 in chondrocytes, which play pivotal roles in the degradation of type II collagen and aggrecan. Blockage of AGE-induced degradation of articular ECM and inflammatory response in chondrocytes has become an important therapeutic strategy for the treatment of OA.
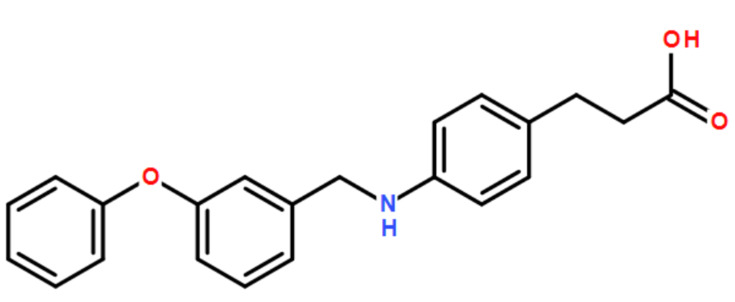
GTP-binding protein coupling receptor 40 (GPRP40), which belongs to the free fatty acid receptor family,10 is a cell surface receptor that widely exists in immune cells, splenocytes, and brain cells.11 GPR40 couples mainly with a G protein α-subunit of the Gq family during insulin secretion.12 Previous research has confirmed that the activation of GPR40 can increase the secretion of insulin.13,14 Owing to this function, many GPR40 agonists have the potential for extensive applications in type II diabetes mellitus (T2DM).15,16 In contrast, little research has been done to investigate the physiological effects of GPR40 in other fields. GW9508 (3-[4-({[3-(phenyloxy) phenyl]methyl}amino)phenyl] propanoic acid) is a selective agonist for GPR40. It was originally identified from a high-throughput screen of the GlaxoSmithKline chemical collection. The molecular structure of GW9508 is shown in Figure 1. GW9508 has been shown in either functional or binding assays to be at least 100-fold selective for GPR40 against 220 other GPCRs, 60 kinases, 63 proteases, seven integrins and 20 nuclear receptors.17 GW9508 has displayed a diversity of pharmacological functions in previous studies. For example, treatment with GW9508 enhances the intracellular Ca2+ concentrations via GPR40 receptor in a dose-dependent manner. In current researches, GW9508 has been demonstrated to potentiate glucose-stimulated insulin secretion.18 Also, activation of GPR40 by GW9508 also alleviated epileptic activity in model in vitro.19 The purpose of this study is to investigate whether the agonism of GPR40 with GW9508 has a beneficial effect against AGE-induced damages in human SW1353 chondrocytes.
Figure 1.
Molecular structure of GW9508.
Materials and Methods
Cell Culture and Treatment
Human SW1353 chondrosarcoma cell line (ATCC, USA) was maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA), 2 mM L-glutamine (Thermo Fisher Scientific, USA), and 1% antibiotics (penicillin/streptomycin) (Gibco, USA). Our experimental protocols were approved by the ethics committee of Yangzhou University. Briefly, the cells were seeded onto 6-well plates at a density of 105 cells/cm2 and cultured in a monolayer for 48 h at a temperature of 37 °C in a 95% O2/5% CO2 incubator. For all experiments, the cells were pretreated with 25 or 50 µM GW9508 (Purity: ≥98%; Cayman Chemical, USA) followed by exposure to 100 µg/mL AGEs. For the GPR40 inhibition experiment, cells were treated with the GPR40 antagonist GW1100 (10 µM) (Purity: ≥98%; Cayman Chemical, USA).
Real-Time Polymerase Chain Reaction (PCR)
The total RNA was extracted from chondrocytes using an RNeasy Micro Kit from Qiagen (Qiagen, USA) following the manufacturer’s instructions. A Nanodrop spectrophotometer (Thermo Fisher Scientific, USA) was used to assess the quality and quantity of the extracted RNA. Then, 1 μg RNA was used to synthesize the corresponding cDNA using iScript™ Reverse Transcription Supermix (Bio-Rad Laboratories, USA) for RT-qPCR. SYBR-based real-time PCR was performed on the ABI7500 platform (Applied Biosystems, USA) to detect the total mRNA transcripts of human GPR40. The relative expression of GPR40 was normalized to that of GAPDH using the 2−∆∆ CT method and is presented as the fold-change. The following primers were used in this study: MMP-3: forward, 5ʹ-CCTCTATGGACCTCCCACAGAATC-3ʹ, reverse, 5ʹ-GGTGCTGACTGCATCGAAGGACAAA-3ʹ; MMP-13: forward, 5ʹ- CTGGCCTGCTGGCTCATGCTT-3ʹ, reverse, 5ʹ-CCTCAGAAAGAGCAGCATCGATATG-3ʹ; ADAMTS-4: forward, 5ʹ-ACACTGAGGACTGCCCAAC-3ʹ, reverse, 5ʹ-GGTGAGTTTGCACTGGTCCT-3ʹ; ADAMTS-5: forward, 5ʹ-GCAGAACATCGACCAACTCTACTC-3ʹ, reverse, 5ʹ-CCAGCAATGCCCACCGAAC-3ʹ; TNF-α, forward,5ʹ-GTCACTCATTGCTGAGCCTCT-3ʹ,reverse, 5ʹ-AGCTTCTTCCCACCCACAAG-3ʹ; IL-6, forward, 5ʹ-AGGGCTCTTCGGGAAATGTA-3ʹ, reverse,5ʹ-TGCCCAGTGGACAGGTTTC-3ʹ; GAPDH: forward, 5ʹ-ACTGGCGTCTTCACCACCAT-3ʹ; reverse, 5ʹ-AAGGCCATGCCAGTGAGCTT-3ʹ.
Western Blot Analysis
To determine the protein expression of target genes, SW1353 chondrocytes were lysed using RIPA buffer (Cell Signaling Technology, USA) supplemented with Protease and Phosphatase Inhibitor Cocktail (100X) (1:100) (Roche, Switzerland). Nuclear protein was extracted from SW1353 chondrocytes using the nuclear and cytoplasmic extraction kit (#78835, Thermo fisher scientific, USA) following the manufacturer’s instructions. The buffer of CER I:CER II: NER reagents were maintained at 200:11:100 µL to remove the cytoplasm and generate nuclei fractions. Then, 20 μg cell lysates or nuclear extracts were immobilized using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated protein mixture was transferred to PVDF membranes (Bio-Rad Laboratories, USA) and blotted against the appropriate specific antibodies and corresponding secondary antibody. Pierce™ ECL Plus Western blotting substrate (Thermo Fisher Scientific, USA) was used to visualize the immunoblots.
Enzyme-Linked Immunosorbent Assay (ELISA)
To determine the protein levels of IL-6 and MCP-1 secreted by SW1353 chondrocytes, ELISA analysis was performed. Briefly, the cell culture media was collected, and the protein secretion levels of the target genes were measured using ELISA kits (R&D Systems, USA) following the manufacturer’s instructions. Then, 96-plate reader spectrometry (Tecan, USA) was employed for data collection, and a standardized 4-PL curve was used to obtain the absolute values.
Luciferase Assay
To determine the transcriptional activity of NF-κB, the promoter-luciferase activity of NF-κB was detected. Briefly, NF-κB promoter-luciferase (Clontech, USA) and β-galactosidase plasmids were transfected into human SW1353 cells. At 12 h post-transfection, the cells were treated with 25 or 50 µM GW9508, followed by exposure to 100 ng/mL AGEs. The resulting luciferase and β-galactosidase activities were detected using a Secrete-PairTM Dual luminescence assay kit (Gene Copoeia, USA) on an Infinite F500 Multimode Reader luminometer (TECAN, USA). The NF-κB luciferase activity was normalized to that of β-galactosidase.
Statistical Analysis
The experimental results are displayed as means ± S.E.M (standard error of the mean). Differences between groups were defined using ANOVA. Differences were considered significant when the value of P was less than 0.05.
Results
AGEs Reduced the Expression of GPR40 in Human SW1353 Chondrocytes
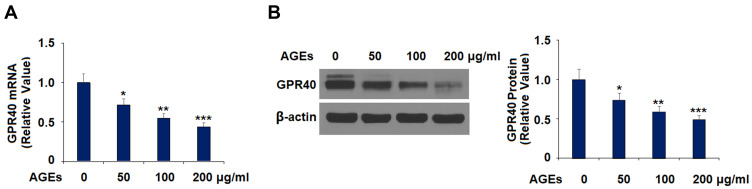
First, we measured the effect of AGEs on the expression of GPR40. Cells were treated with AGEs (50, 100, and 200 μg/mL) for 24 h. The results show that the three concentrations of AGEs inhibited the expression of GPR40 significantly at both the mRNA (Figure 2A) and protein (Figure 2B) levels dose-dependently. These results suggest that GPR40 might be involved in AGE-induced impairment of chondrocytes.
Figure 2.
Advanced glycation end-products (AGEs) reduced the expression of GPR40 in human SW1353 chondrocytes. Cells were treated with 50, 100, and 200 μg/mL AGEs for 24 h. (A) mRNA of GPR40; (B) Protein of GPR40 (*, **, ***, P<0.01, 0.001, 0.0001 vs. control group).
The GPR40 Agonist GW9508 Reduced AGE-Induced Expression and Secretion of Pro-Inflammatory Cytokines
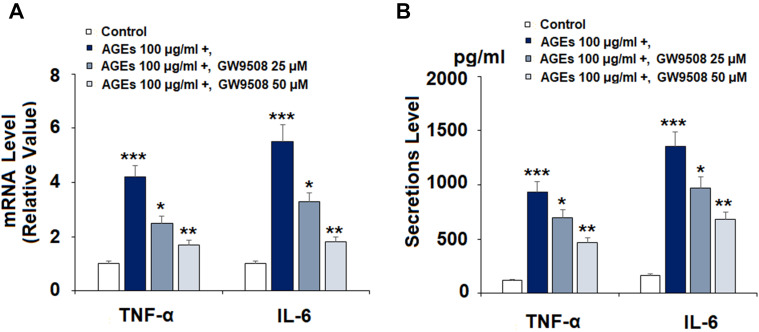
Due to the known involvement of pro-inflammatory cytokines in the progression and development of OA, we investigated the anti-inflammatory effects of GW9508 on AGE-induced TNF-α and IL-6 expression. Cells were treated with AGEs for 24 h with or without pretreatment with GW9508 (25 and 50 μM). The results in Figure 3A show that AGEs cause a significant increase in the mRNA levels of both cytokines to 4.2- and 5.5-fold, respectively. However, the two doses of GW9508 reduced the expression of TNF-α to 2.5- and 1.7-fold. Also, the two doses suppressed IL-6 expression to 3.3- and 1.8- fold. We further studied the secretions of TNF-α and IL-6 with ELISA analysis. The results in Figure 3B show that GW9508 significantly decreased the AGE-induced protein secretion of TNF-α and IL-6.
Figure 3.
The GPR40 agonist GW9508 reduced AGE-induced expression and secretion of pro-inflammatory cytokines in human SW1353 chondrocytes. Cells were treated with AGEs (100 μg/mL) in the presence or absence of GW9508 (25 and 50 μM) for 24 h. (A) mRNA of TNF-α and IL-6 as measured by real-time PCR; (B) Secretions of TNF-α and IL-6 as measured by ELISA (***, P<0.0001 vs. control group; *, **, P<0.01, 0.001 vs. AGE treatment group).
GW9508 Inhibited the Expression and Secretion of MMP-3 and MMP-13 Induced by AGEs
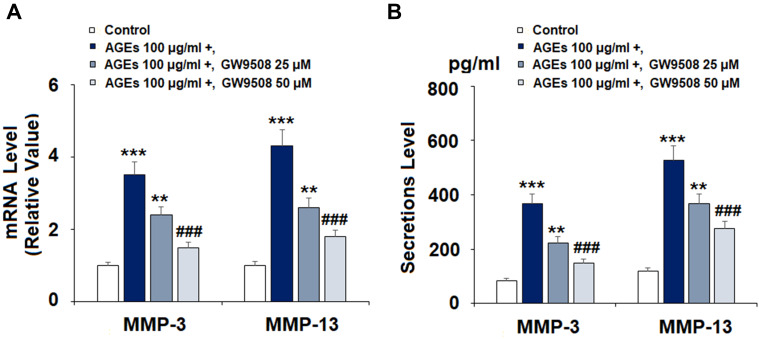
As the expression of MMPs has a strong effect on the degradation of type II collagen, we measured the effect of GW9508 on the expression of MMP-3 and MMP-13. Cells were exposed to 100 μg/mL AGEs after pretreatment with 25 and 50 μM GW9508 for 24 h. The results in Figure 4A reveal that the two doses of GW9508 reduced the mRNA level of MMP-3 to 2.4- and 1.5-fold, which was increased to 3.5-fold by exposure to AGEs alone. In regards to MMP-13, AGE-treatment increased the mRNA level of MMP-13 to 4.3-fold, while it was reduced to 2.6- and 1.8-fold by the two concentrations of GW9508, respectively. Concordantly, the data in Figure 4B shows that the expression of MMP-3 and MMP-13 at the protein levels are significantly suppressed by GW9508.
Figure 4.
GW9508 inhibited AGE-induced expression and secretion of MMP-3 and MMP-13 in human SW1353 chondrocytes. Cells were treated with AGEs (100 μg/mL) in the presence or absence of GW9508 (25, 50 μM) for 24 h. (A) mRNA of MMP-3 and MMP-13; (B) Secretions of MMP-3 and MMP-13 (***, P<0.0001 vs. control group; **, ###, P<0.001, 0.0001 vs. AGE treatment group).
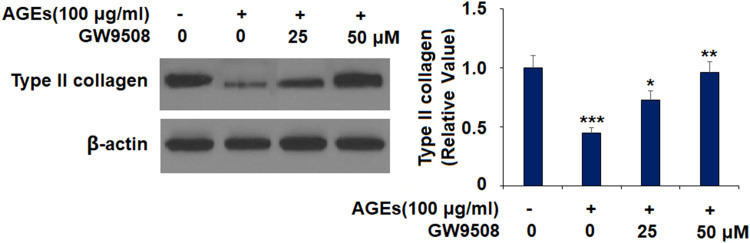
GW9508 Ameliorated the Degradation of Type II Collagen Induced by AGEs
We further tested whether GW9508 ameliorated the degradation of type II collagen. As shown in Figure 5, AGEs reduced the protein level of type II collagen to 0.45-fold, while the 25 μM dose of GW9508 increased it to 0.73-fold. Moreover, 50 μM GW9508 increased the protein level of type II collagen to 0.97-fold, which is near the baseline.
Figure 5.
GW9508 ameliorated AGE-induced degradation of type II collagen in human SW1353 chondrocytes. Cells were treated with AGEs (100 μg/mL) in the presence or absence of GW9508 (25 and 50 μM) for 24 h. Protein levels of type II collagen were determined (***, P<0.0001 vs. control group; *, **, P<0.01, 0.001 vs. AGE treatment group).
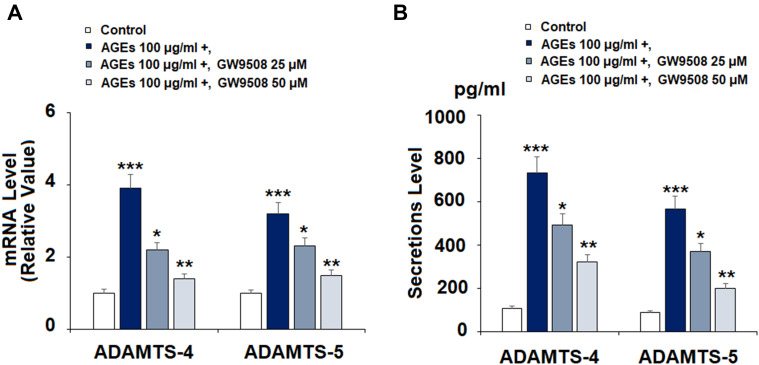
GW9508 Suppressed the Expression and Secretion of ADAMTS Induced by AGEs
Next, we determined the effects of GW9508 on AGE-induced expression and secretion of ADAMTS-4 and ADAMTS-5. The results in Figure 6A show that the two doses of GW9508 significantly reduced the mRNA levels of ADAMTS-4 and ADAMTS-5 in a dose-dependent manner as compared to treatment with AGEs alone. Furthermore, GW9508 remarkably decreased the expression of ADAMTS-4 and ADAMTS-5 at the protein levels, as shown in Figure 6B.
Figure 6.
GW9508 inhibited AGE-induced expression and secretion of ADAMTS-4 and ADAMTS-5 in human SW1353 chondrocytes. Cells were treated with AGEs (100 μg/mL) in the presence or absence of GW9508 (25 and 50 μM) for 24 h. (A) mRNA of ADAMTS-4 and ADAMTS-5; (B) Secretions of ADAMTS-4 and ADAMTS-5 (***, P<0.0001 vs. control group; *, **, P<0.01, 0.001 vs. AGE treatment group).
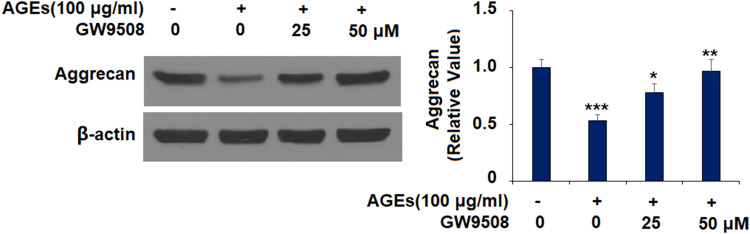
GW9508 Inhibited the Degradation of Aggrecan Stimulated by AGEs
The results in Figure 7 show that the protein level of aggrecan decreased by more than half upon exposure to AGEs alone. However, 25 μM GW9508 increased aggrecan protein expression to 0.78-fold, while 50 μM GW9508 increased the protein level of aggrecan to 0.97-fold, almost back to baseline. This suggests that GW9508 has a strong protective effect on aggrecan.
Figure 7.
GW9508 inhibited AGE-induced degradation of aggrecan in human SW1353 chondrocytes. Cells were treated with AGEs (100 μg/mL) in the presence or absence of GW9508 (25 and 50 μM) for 24 h. Protein levels of aggrecan were measured by Western blot analysis (***, P<0.0001 vs. control group; *, **, P<0.01, 0.001 vs. AGE treatment group).
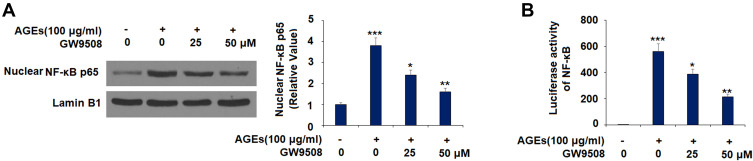
GW9508 Inhibited AGE-Induced Activation of NF-κB
To explore the underlying mechanism, we tested the effect of GW9508 on NF-κB activation. The results in Figure 8A show that the nuclear translocation of NF-κB p65 was increased to 3.8-fold by exposure to AGEs alone, while this level was reduced by the two concentrations of GW9508 to 2.4- and 1.6-fold, respectively. We further measured the luciferase activity of NF-κB and found that GW9508 remarkably decreases the NF-κB activity as compared to the upregulation induced by AGEs, which has been shown in Figure 8B.
Figure 8.
GW9508 inhibits AGE-induced activation of NF-κB. Cells were treated with AGEs (100 μg/mL) in the presence or absence of GW9508 (25 and 50 μM) for 6 h. (A) Nuclear translocation of NF-κB p65; (B) Luciferase activity of NF-κB (***, P<0.0001 vs. control group; *, **, P<0.01, 0.001 vs. AGE treatment group).
The Effects of GW9508 are Dependent on GPR40
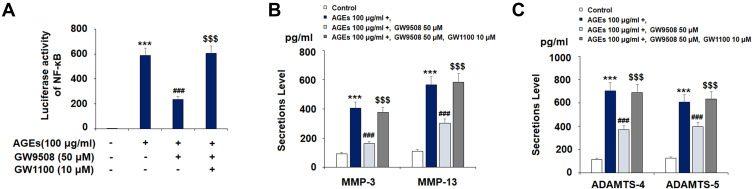
Lastly, we investigated whether or not the protective effects of GW9508 are dependent on GPR40. GW1100, a type of GPR40 antagonist, has a strong effect on weakening GPR40 activity.20–22 We selected the dose of GW1100 (10 μM) for our ex-vivo experiments based on a study that demonstrated GW1100 to be a selective GPR40 receptor antagonist at up to 10 μM.17 As shown in Figure 9, in the presence of GW1100, GW9508 had lesser effects on the inhibition of NF-κB (Figure 9A), MMP-3, MMP-13 (Figure 9B), ADAMTS-4, and ADAMTS-5 (Figure 9C) when compared with the absence of GW1100. These results suggest that the protective effects of GW9508 against AGE-induced chondrocyte damages are mediated by GPR40.
Figure 9.
The effects of GW9508 are dependent on GPR40. Cells were treated with AGEs (100 μg/mL) in the presence or absence of GW9508 (50 μM) or the GPR40 antagonist GW1100 (10 μM). (A) Luciferase activity of NF-κB; (B) Secretions of MMP-3 and MMP-13; (C) Secretions of ADAMTS-4 and ADAMTS-5 (***, P<0.0001 vs. control group; ###, P<0.0001 vs. AGE treatment group; $$$, P<0.0001 vs. AGE+ GW9508 group).
Discussion
As OA has become one of the major diseases impacting the health of the global population, there have been extensive studies seeking out safe and effective therapies to prevent or slow the progression of OA. In the physiopathology of OA, pro-inflammatory cytokines and cellular signaling pathways act as a master regulator in the development of OA and maintain a chronic inflammatory state.23 Furthermore, MMPs and ADAMTSs have been found to facilitate ECM degradation and chondrocyte apoptosis.9,24 This has led to them being widely explored as therapeutic targets for OA. However, the underlying mechanisms regulating these cytokines and enzymes are intricate. Also, accumulation of AGEs in tissues has been reported as playing a vital role in the development of OA via activation of the receptor for AGEs (RAGE), which is expressed on the surface of chondrocytes.25 GPR40 takes part in many physiological processes. As activation of GPR40 can increase insulin secretion, several GPR40 agonists are widely used for the treatment of T2DM. A previous study demonstrated that overexpression of GPR40 suppresses the inflammatory response in TNF-α-induced keratinocytes.26 However, little is known about whether GPR40 activation protects cartilage tissues in OA. To determine the effects of GPR40 on OA, human SW1353 chondrocytes stimulated by AGEs were employed to imitate the microenvironment of OA. To our knowledge, we are the first to demonstrate the anti-inflammatory effect of GW9508 by increasing GPR40 activation in AGE-induced human SW1353 chondrocytes.
Overexpression of pro-inflammatory cytokines plays a pivotal role in the pathogenesis of OA. TNF-α and IL-6 can stimulate chondrocytes to release nitric oxide (NO) and prostaglandin E2 (PGE2), which result in enhancing MMP production and activity.27 TNF-α also increases ADAMTS-4 and ADAMTS-5 transcription. Both MMPs and ADAMTS have a strong ability to promote the degeneration of the cartilage ECM. Moreover, evidence has confirmed that TNF-α significantly and directly inhibits the synthesis of type II collagen and aggrecan.28 Importantly, our results show that GW9508 remarkably inhibited AGE-induced expression of cytokines, such as TNF-α and IL-6. This finding suggests that GW9508 could be a potential strategy for managing the inflammatory response in OA.
Degeneration of the ECM is a significant target of OA treatment. AGE dosing resulted in a considerable increase in the degeneration of type II collagen and aggrecan. AGEs also increased the expression of catabolic enzymes such as MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5, which contribute to the hydrolysis of type II collagen and aggrecan. As the synthesis of type II collagen is very slow, it is unlikely that the excessive degeneration of type II collagen can be reversed.29 Thus, it is meaningful to down-regulate the expression of MMPs in OA therapies. Our results indicate that GW9508 significantly decreased the expression of MMP-3 and MMP-13, resulting in a protective effect for type II collagen against stimulation with AGEs. Moreover, we confirmed that GW9508 protects aggrecan from degradation by preventing the upregulation of ADAMTS-4 and ADAMTS-5 induced by AGEs. These results suggest that GW9508 has a strong protective effect on type II collagen and aggrecan against AGE-induced degeneration.
The NF-κB signaling pathway is believed to play an essential role in numerous diseases, including OA.30 Studies have suggested inhibiting NF-κB as an anti-inflammatory strategy. Activation of NF-κB has been shown to induce the expression of various pro-inflammatory cytokines and chemokines, which can increase the expression of MMPs and ADAMTS, thereby degrading type II collagen and aggrecan. In our present study, we tested the effects of GW9508 on the translocation of NF-κB p65 and luciferase activity of NF-κB. The results show that GW9508 reduces NF-κB activation. Notably, when GPR40 is inhibited by its antagonist GW1100, the protective effects of GW9508 in preventing NF-κB activation and its downstream catabolic events are abolished, suggesting the involvement of GPR40 in this process. To further elucidate the physiological function of GPR40 in chondrocytes and the pathological development of OA, another GPR40 agonist TAK-875 was used. The activity of TAK-875 in targeting GPR40 has been demonstrated in both disease models31 and clinical trials.32 Interestingly, we found that treatment with TAK-875 could also prevent AGEs- induced degradation of Type II collagen and aggrecan (data not shown), confirming the robust protective effect of GPR40 against AGEs-induced damage in chondrocytes and suggesting that might it become a promising therapeutic target for the treatment of OA.
Subchondral bone is the bedrock of a joint on which sits the articular cartilage, acts as the mechanical support of the collective linking to the diaphyseal bone. Increasing evidence shows that aberrant subchondral bone remodeling is associated with articular cartilage degeneration.33–35 Cytokines and growth factors produced by subchondral bone tissue are considered to stimulate cartilage degeneration. In patients with OA, microfractures, and neovascularisation are observed in the subchondral bone and at the osteochondral junction, resulting in a reparative response with new blood vessel formation and inflammation.36,37 Also, both osteoblast and osteoclast activity increase in OA by 96% and 190%, respectively.38 Osteoblasts from the subchondral bone increase the expression of MMP-13, which decreases the production of aggrecan. Furthermore, osteoblasts also express high levels of transforming growth factor β1 (TGFβ-1) and prostaglandin E2 (PGE2), which promote the degeneration of Type II Collagen and aggrecan.39,40 On the other hand, abnormal osteoclast differentiation accelerates bone loss, which promotes the development of OA.36 Researches have demonstrated that activation of GPR40 using GW9508 has an inhibitory effect in osteoclast differentiation.41 We speculate that the administration of GW9508 might have a beneficial impact on subchondral bone tissues. Although evidence has demonstrated the effect of GPR40 on osteoblasts, more researches are required to clarify further the function of GW9508 in subchondral bone.42
In summary, our findings indicate that GPR40 has the potential to be a novel treatment target for OA. However, more research is required to determine the effects of GPR40 in OA.
Ethics Statement
Human SW1353 chondrosarcoma cell line was purchased from ATCC, USA. Our experimental protocols were approved by the ethics committee of Yangzhou University.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 2.Heinegård D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7(1):50–56. doi: 10.1038/nrrheum.2010.198 [DOI] [PubMed] [Google Scholar]
- 3.Chen CT, Burton-Wurster N, Borden C, Hueffer K, Bloom SE, Lust G. Chondrocyte necrosis and apoptosis in impact damaged articular cartilage. J Orthop Res. 2001;19(4):703–711. doi: 10.1016/S0736-0266(00)00066-8 [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri J, Bains Y, Guha S, et al. The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab. 2018;28(3):337–352. doi: 10.1016/j.cmet.2018.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YJ, Sheu ML, Tsai KS, Yang RS, Liu SH, Zissel G. Advanced glycation end products induce peroxisome proliferator-activated receptor γ down-regulation-related inflammatory signals in human chondrocytes via Toll-like receptor-4 and receptor for advanced glycation end products. PLoS One. 2013;8(6):e66611. doi: 10.1371/journal.pone.0066611 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Dong N, Chang L, Wang B, Chu L. Retinal neuronal MCP-1 induced by AGEs stimulates TNF-α expression in rat microglia via p38, ERK, and NF-κB pathways. Mol Vis. 2014;20:616–628. [PMC free article] [PubMed] [Google Scholar]
- 7.Nonaka K, Kajiura Y, Bando M, et al. Advanced glycation end-products increase IL-6 and ICAM-1 expression via RAGE, MAPK and NF-κB pathways in human gingival fibroblasts. J Periodontal Res. 2018;53(3):334–344. doi: 10.1111/jre.12518 [DOI] [PubMed] [Google Scholar]
- 8.Nah SS, Choi IY, Yoo B, Kim YG, Moon HB, Lee CK. Advanced glycation end products increases matrix metalloproteinase-1, −3, and −13, and TNF-alpha in human osteoarthritic chondrocytes. FEBS Lett. 2007;581(9):1928–1932. doi: 10.1016/j.febslet.2007.03.090 [DOI] [PubMed] [Google Scholar]
- 9.Verma P, Dalal K. ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. J Cell Biochem. 2011;112:3507–3514. doi: 10.1002/jcb.23298 [DOI] [PubMed] [Google Scholar]
- 10.Rayasam GV, Tulasi VK, Davis JA, Bansal VS. Fatty acid receptors as new therapeutic targets for diabetes. Expert Opin Ther Targets. 2007;11(5):661–671. doi: 10.1517/14728222.11.5.661 [DOI] [PubMed] [Google Scholar]
- 11.Briscoe CP, Tadayyon M, Andrews JL, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278(13):11303–11311. doi: 10.1074/jbc.M211495200 [DOI] [PubMed] [Google Scholar]
- 12.Hara T, Hirasawa A, Ichimura A, Kimura I, Tsujimoto G. Free fatty acid receptors FFAR1 and GPR120 as novel therapeutic targets for metabolic disorders. J Pharm Sci. 2011;100(9):3594–3601. doi: 10.1002/jps.22639 [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara K, Maekawa F, Yada T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am J Physiol Endocrinol Metab. 2005;289(4):E670–E677. doi: 10.1152/ajpendo.00035.2005 [DOI] [PubMed] [Google Scholar]
- 14.Usui R, Yabe D, Fauzi M, et al. GPR40 activation initiates store-operated Ca2+ entry and potentiates insulin secretion via the IP3R1/STIM1/Orai1 pathway in pancreatic β-cells. Sci Rep. 2019;9(1):15562. doi: 10.1038/s41598-019-52048-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016;7(17):354–395. doi: 10.4239/wjd.v7.i17.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Defossa E, Wagner M. Recent developments in the discovery of FFA1 receptor agonists as novel oral treatment for type 2 diabetes mellitus. Bioorg Med Chem Lett. 2014;24(14):2991–3000. doi: 10.1016/j.bmcl.2014.05.019 [DOI] [PubMed] [Google Scholar]
- 17.Briscoe CP, Peat AJ, McKeown SC, et al. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol. 2006;148(5):619–628. doi: 10.1038/sj.bjp.0706770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto T, Mogami H, Tsuriya D, et al. G-protein-coupled receptor 40 agonist GW9508 potentiates glucose-stimulated insulin secretion through activation of protein kinase Cα and ε in INS-1 cells. PLoS One. 2019;14(9):e0222179. doi: 10.1371/journal.pone.0222179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Tian X, Xu D, et al. GPR40 modulates epileptic seizure and NMDA receptor function. Sci Adv. 2018;4(10):eaau2357. doi: 10.1126/sciadv.aau2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umar S, Li J, Hannabass K, et al. Free fatty acid receptor G-protein-coupled receptor 40 mediates lipid emulsion-induced cardioprotection. Anesthesiology. 2018;129(1):154–162. doi: 10.1097/ALN.0000000000002195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moriyama R, Yamazaki T, Kato T, Kato Y. Long-chain unsaturated fatty acids reduce the transcriptional activity of the rat follicle-stimulating hormone β-subunit gene. J Reprod Dev. 2016;62(2):195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamoto K, Nishinaka T, Sato N, et al. Hypothalamic GPR40 signaling activated by free long chain fatty acids suppresses CFA-induced inflammatory chronic pain. PLoS One. 2013;8(12):e81563. doi: 10.1371/journal.pone.0081563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson WH, Lepus CM, Wang Q, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(10):580. doi: 10.1038/nrrheum.2016.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19(1):248. doi: 10.1186/s13075-017-1454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu H, Li J, Wu L, Chen W. Trichostatin A increases the TIMP-1/MMP ratio to protect against osteoarthritis in an animal model of the disease. Mol Med Rep. 2016;14(3):2423–2430. doi: 10.3892/mmr.2016.5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita T, Matsuoka T, Honda T, Kabashima K, Hirata T, Narumiya S. A GPR40 agonist GW9508 suppresses CCL5, CCL17, and CXCL10 induction in keratinocytes and attenuates cutaneous immune inflammation. J Invest Dermatol. 2011;131(8):1660–1667. doi: 10.1038/jid.2011.123 [DOI] [PubMed] [Google Scholar]
- 27.Stevens AL 1, Wheeler CA, Tannenbaum SR, Grodzinsky AJ. Nitric oxide enhances aggrecan degradation by aggrecanase in response to TNF-alpha but not IL-1beta treatment at a post-transcriptional level in bovine cartilage explants. Osteoarthritis Cartilage. 2008;16(4):489–497. doi: 10.1016/j.joca.2007.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye S, Wang J, Yang S, et al. Specific inhibitory protein Dkk-1 blocking Wnt/β-catenin signaling pathway improve protectives effect on the extracellular matrix. J Huazhong Univ Sci Technolog Med Sci. 2011;31(5):657. doi: 10.1007/s11596-011-0577-y [DOI] [PubMed] [Google Scholar]
- 29.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–478. doi: 10.1097/BOR.0b013e328349c2b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roshak AK, Callahan JF, Blake SM. Small-molecule inhibitors of NF-κB for the treatment of inflammatory joint disease. Curr Opin Pharmacol. 2002;2:316–321. doi: 10.1016/S1471-4892(02)00165-0 [DOI] [PubMed] [Google Scholar]
- 31.Ito R, Tsujihata Y, Suzuki M, Miyawaki K, Matsuda K, Takeuchi K. Fasiglifam/TAK-875, a selective GPR40 agonist, improves hyperglycemia in rats unresponsive to sulfonylureas and acts additively with sulfonylureas. J Pharmacol Exp Ther. 2016;357:217–227. doi: 10.1124/jpet.115.230730 [DOI] [PubMed] [Google Scholar]
- 32.Kaku K, Enya K, Nakaya R, Ohira T, Matsuno R. Efficacy and safety of fasiglifam (TAK-875), a G protein-coupled receptor 40 agonist, in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise: a randomized, doubleblind, placebo-controlled, Phase III trial. Diabetes Obes Metab. 2015;17:675–681. doi: 10.1111/dom.12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li G, Yin J, Gao J, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15(6):223. doi: 10.1186/ar4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mansell JP, Bailey AJ. Abnormal cancellous bone collagen metabolism in osteoarthritis. J Clin Invest. 1998;101:1596–1603. doi: 10.1172/JCI867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansell JP, Tarlton JF, Bailey AJ. Biochemical evidence for altered subchon- dral bone collagen metabolism in osteoarthritis of the hip. Br J Rheumatol. 1997;36:16–19. doi: 10.1093/rheumatology/36.1.16 [DOI] [PubMed] [Google Scholar]
- 36.Westacott CI, Webb GR, Warnock MG, et al. Alteration of cartilage metabolism by cells from osteoarthritic bone. Arthritis Rheum. 1997;40(7):1282–1291. doi: [DOI] [PubMed] [Google Scholar]
- 37.Dequeker J, Mohan R, Finkelman RD, et al. Generalized osteoarthritis asso- ciated with increased insulin-like growth factor types I and II and transforming growth factor beta in cortical bone from the iliac crest. Possible mechanism of increased bone density and protection against osteoporosis. Arthritis Rheum. 1993;36(12):1702–1708. doi: 10.1002/art.1780361209 [DOI] [PubMed] [Google Scholar]
- 38.Donell S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 2019;4(6):221–229. doi: 10.1302/2058-5241.4.180102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thielen NGM, van der Kraan PM, van Caam APM. TGFβ/BMP signaling pathway in cartilage homeostasis. Cells. 2019;8(9):E969. doi: 10.3390/cells8090969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SA, Park BR, Moon SM, Hong JH, Kim DK, Kim CS. Chondroprotective effect of cynaroside in IL-1β-induced primary rat chondrocytes and organ explants via NF-κB and MAPK signaling inhibition. Oxid Med Cell Longev. 2020;2020:9358080. doi: 10.1155/2020/9358080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wauquier F, Philippe C, Léotoing L, et al. The free fatty acid receptor G protein-coupled receptor 40 (GPR40) protects from bone loss through inhibition of osteoclast differentiation. J Biol Chem. 2013;288(9):6542–6551. doi: 10.1074/jbc.M112.429084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Philippe C, Wauquier F, Lyan B, Coxam V, Wittrant Y. GPR40, a free fatty acid receptor, differentially impacts osteoblast behavior depending on differentiation stage and environment. Mol Cell Biochem. 2016;412(1–2):197–208. doi: 10.1007/s11010-015-2626-5 [DOI] [PubMed] [Google Scholar]