Abstract
Inflammatory myofibroblastic tumor (IMT) is a rare pathologic entity that has been mostly described in the lung. It has been reported in nearly every organ, with occurrence in the kidney being extremely rare. IMT has nonspecific clinical and radiological findings and often mimics a malignant process. This is a case discussing the radiologic and pathologic findings of a renal IMT in a 35-year-old female who underwent radical nephrectomy for a right renal pelvis mass that was mistaken for transitional cell carcinoma.
Keywords: Inflammatory myofibroblastic tumor, Pseudotumor, CT, MRI, Kidney
Introduction
Inflammatory myofibroblastic tumor (IMT), inflammatory pseudotumor, plasma cell granuloma, and xanthogranuloma are synonymous terms of the same pathologic entity [1]. IMT has been described in almost every organ, the lung being the most common location [2]. The urinary bladder is the most common location of IMT in the genitourinary tract [2]. It rarely originates in the kidney. The first case of renal IMT was described in 1972 by Davides et al., referred to as plasma cell granuloma [3]. Renal IMT has been described in different age groups, and tends to occur more in males [2]. The etiology of IMT remains unclear. IMT has unpredictable biological potential ranging from inflammatory to metastatic behavior. It was previously described as a benign lesion, but the current WHO classification of this lesion is intermediate grade tumor since it has potential for recurrence and rare metastasis [4].
IMT represents a challenging diagnostic entity due to its nonspecific clinical presentation, laboratory findings, and most importantly radiologic findings. IMT in the kidney is often misdiagnosed as renal cell carcinoma, transitional cell carcinoma of the pelvis or lymphoma based on radiological features [2]. Patients are often treated with nephrectomy to determine the final diagnosis. Here we aimed to present the radiologic and pathologic findings of a case of IMT with a recent literature review.
Case report
A 35-year-old female patient presented with a 4-year history of right flank pain that was thought to be due to urolithiasis. The patient has a medical history of primary amenorrhea of unknown cause, for which she is receiving oral contraceptive pills. The patient had no history of prior malignancy, renal trauma or pyelonephritis. No abnormality detected in the physical examination and urine analysis.
An initial ultrasound (US) performed 4 years prior to surgery showed mildly dilated right renal pelvis and calyceal system of the upper and mid pole and was not further investigated at that time (Fig. 1). Four years later, a second US was showed the same findings. Plain computed tomography (CT) showed mild renal hydronephrosis with thinning of the parenchyma at the upper pole and perinephric fat stranding. Intravenous contrast-enhanced CT showed ill-defined right renal central infiltrative, slightly hyperdense lesion that fills the renal pelvis (Fig. 2a). The lesion is obstructing calyxes especially at the upper moiety and causing caliectasis of upper and middle parts of the collecting system. The upper third parenchyma of the right kidney was thinned and showed delayed enhancement (Fig. 2b, c). The right renal artery is encased by the mass in the renal pelvis, but the right renal vein was difficult to assess (Fig. 2b). The lesion shows homogenous delayed progressive contrast enhancement (Fig. 2d). There was no associated lymphadenopathy or distant metastatic lesions. Peri-renal collateral venous vascular structures draining to the inferior vena cava were seen, but no renal vein occlusion detected (Fig. 3). Additional magnetic resonance imaging (MRI) study done confirmed the presence of renal pelvis mass showing hypointense signal on T1 (Fig. 4a), hypointense signal on T2 (Fig. 4b), no signal drop on opposed phase images. Post gadolinium images show homogenous delayed contrast enhancement, less intense compared to renal parenchyma (Fig. 4c, d). No diffusion restriction is noted. The main differential diagnosis was transitional cell carcinoma but renal cell carcinoma was not a top deferential as the mass is not cortical in origin. Chest CT was free of suspicious nodules. The patient underwent uneventful right nephrectomy due to suspicion of malignancy.
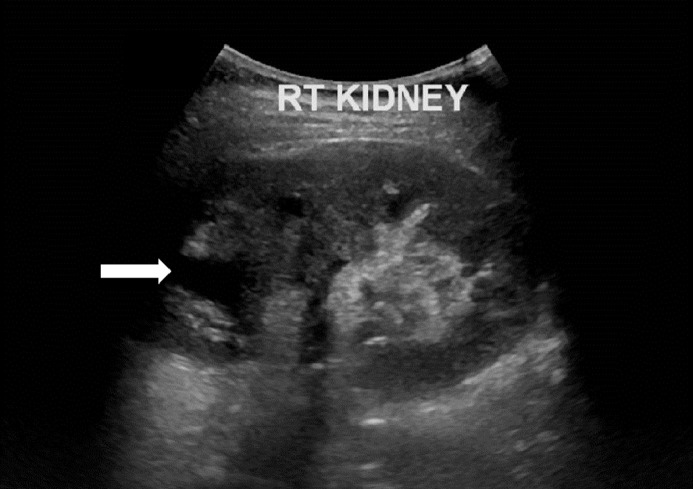
Fig. 1.
Ultrasound image of the right kidney 4 years prior to surgical resection. There is upper calyceal dilatation. Retrospectively a hyperechoic lesion can be questioned in the lower pole, but was not reported at that time.
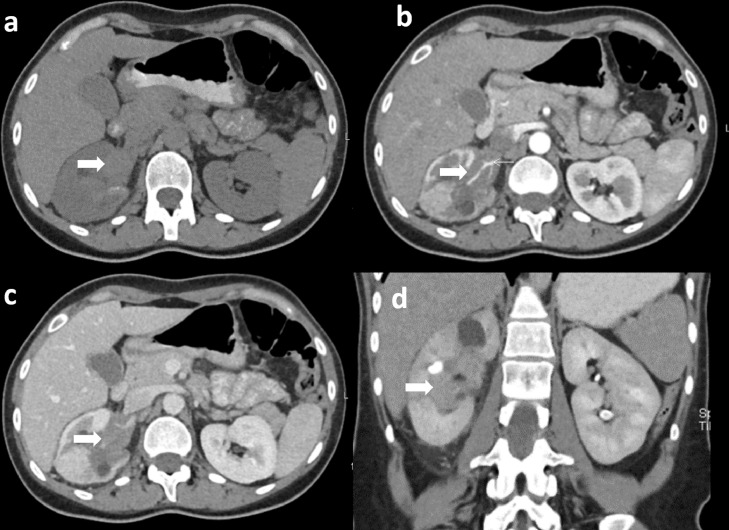
Fig. 2.
Plain and postcontrast CT abdomen. Axial plain CT abdomen shows a mild hyperdense lesion (thick arrow) filling the right renal pelvis (a). Axial postcontrast CT in corticomedullary and nephrographic phase show a lesion filling the right renal pelvis with upper and mid calyx dilatation and thinning of upper pole parenchyma (b, c). Encasement the right renal artery (arrow head) is noted. Coronal pyelographic phase shows progressive postcontrast enhancement (d).
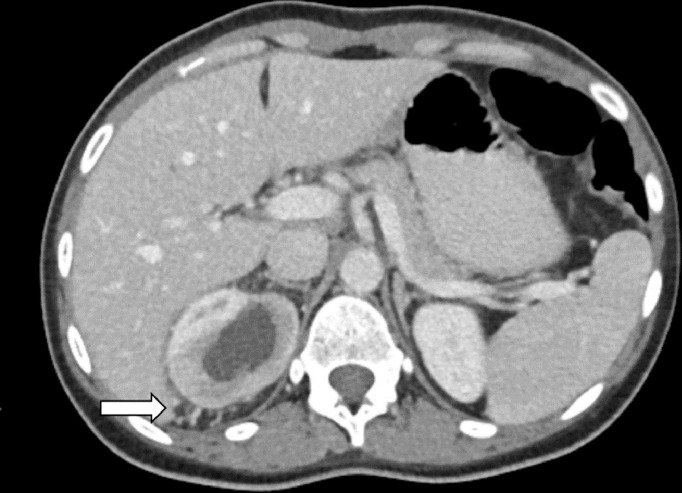
Fig. 3.
Axial postcontrast CT shows peri-renal venous collaterals that can be traced to the inferior vena cava.
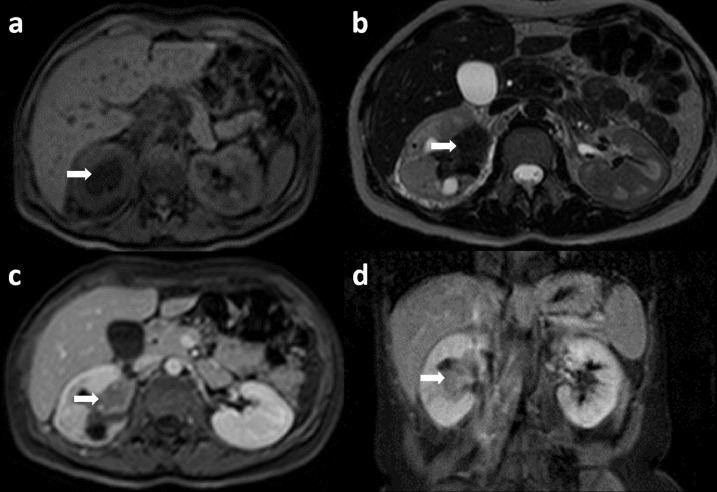
Fig. 4.
MRI with intravenous contrast. Axial T1 (a) and T2 (b) weighted images show a hypointense renal pelvis mass (arrow). Axial (c) and coronal (d) post-contrast T1 weighted image shows mild post contrast enhancement.
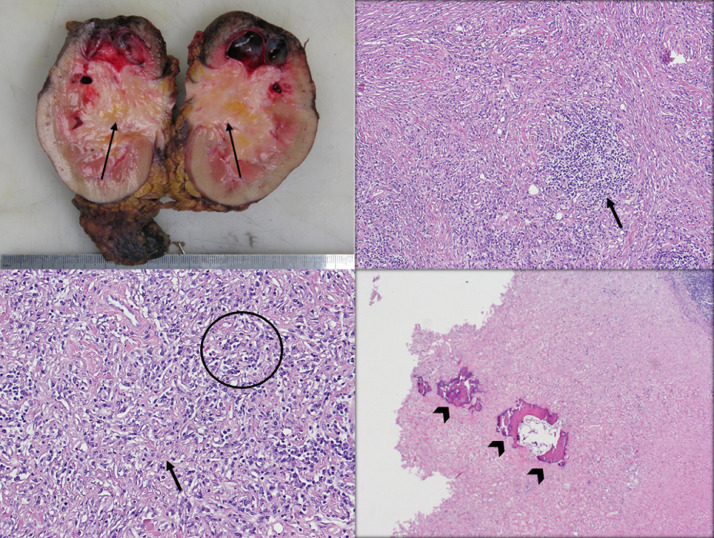
Gross examination of the specimen revealed a 50 × 44 × 40 mm ill-defined, unencapsulated, grayish white, homogenous tumor in the middle zone (Fig. 5a). On microscopic examination, a moderately vascular tumor with increased cellularity was noted. It is cellular at areas and formed by a bland plump spindle cell proliferation with dense chronic mononuclear inflammatory cell infiltrate, mainly plasmacytes, including cellular aggregates and follicles (Fig. 5b, c). In other areas, hypocellular keloid-like areas with prominent hyalinization are also found. Occasional islands of dystrophic calcifications with secondary bone metaplasia are found (Fig. 5d). No significant cellular atypia or necrosis is seen. There is no papillary architecture and no clear cell, hence excluding the possibility of renal cell carcinoma. The absence of atypical transitional epithelium has excluded the diagnosis of transitional cell carcinoma, while the presence of normal lymphocytic infiltrate excluded the diagnosis of lymphoma. On immunohistochemistry, the tumor cells are positive for SMM, Vimentin, and SMA, while the reactive lymphoid cells are positive for CD3, BCL-2, and BCL-6. The latter findings support the diagnosis of IMT. The tumor cells are negative for activin receptor-like kinase (ALK), S-100, CD34, P53, Desmin, Pan Cytokeratin, ER and PR. The ureter and renal vasculature are free of tumor. The surgical resection margins were free.
Fig. 5.
Gross section of the kidney showing the tumor, which is ill-defined, grayish white and a homogenously firm appearance (arrows) (a). On medium power, the cellular part of the tumor showing the spindle cell proliferation with the chronic mononuclear inflammatory cell infiltrate (arrow) seen near the center (H&E) (b). On high power, the cellular part of the tumor shows bland plump spindle cells (circle) with proliferation, with keloid-like areas (arrow) and hyalinization in between the cells (H&E) (c). On medium power, areas of dystrophic calcification (arrowheads) can be seen within the tumor. (H&E) (d).
Twelve months postoperative CT chest, abdomen and pelvis and PET-CT were free of recurrence and metastasis.
Discussion
The complex nature of the histopathology of IMT is reflected by the presence of many descriptive terms such as inflammatory pseudotumor, plasma cell tumor, inflammatory myofibroblastic hyperplasia, histiocytoma or xanthogranuloma and others [1]. Histologically, an IMT contains T and B lymphocytes, myofibroblastic spindle cells, and collagen [1]. IMT of the urinary system can be clinically silent, presents with flank pain, or microscopic and macroscopic hematuria [2]. Etiology has been linked to surgery, chronic inflammation, infection, trauma, and autoimmune reaction; however, it remains unclear [2]. The occasionally seen aggressive features and distant metastasis cannot be well explained by the inflammatory etiology alone. However, the reported successful response to steroid therapy suggests the inflammatory nature [5].
The current WHO classification of this lesion is intermediate grade malignant, with the potential for recurrence and rare metastasis [4]. Many studies tried to link the aggressive behavior with certain molecular markers such as translocations of ALK-1 gene, and P-53, yet inconsistent results among these studies make a prediction of prognosis difficult [6]. Our case was negative for ALK gene. A previously reported case of tracheal IMT in pregnant woman showed strong estrogen receptor expression [7]. Our patient has history of long-term use of oral contraceptive pills. Therefore, the specimen was tested for estrogen and progesterone receptors and turned out negative for both.
Ultrasound features of IMT cannot be concluded in our case as only the consequent upper and mid calyx dilatation was described. Most cases were reported to be hypo or hyperechoic with internal vascularity on color Doppler [2].
The reported CT manifestations of renal IMT are highly variable due to the complex histology of variable amount of inflammatory cells and fibrosis. The lesion could arise from the cortex or pelvis with well or ill-defined margins. The density on plain images ranges from hypodense to slightly hyperdense, homogenous, or heterogeneous. Almost all cases showed postcontrast enhancement but with variable degrees. Also, the pattern of enhancement could be persistent in some cases but also flow like enhancement and washout pattern have been described. Delayed enhancement can be explained by the presence of fibrous tissue [8]. Moreover, the presence of cystic degeneration of renal IMT and calcifications was reported [9,10]. Aggressive behavior was seen in some cases in the form of renal artery penetration, invasion of the perineal fat but rarely metastasis [11]. Thinning of the parenchyma as a result of long-standing hydronephrosis was described also as in our case [12]. Differentiation based on CT findings is difficult and misdiagnosis is almost inevitable unless tissue biopsy was acquired before nephrectomy. Co-occurrence of IMT in patients with bladder or renal cell carcinoma was also reported, but was initially described metastatic, synchronous or recurrent malignant lesion based on radiological appearance alone [13].
Few articles described the MRI findings of IMT. Masses usually are of low signal, homogenous, or heterogeneous with postcontrast enhancement [8]. Typical MRI findings of renal cell carcinoma, apart from papillary RCC, and transitional cell carcinoma are low to isointense signal on T1, hyperintense on T2-weighted images, with enhancement less than renal parenchyma. While these tumors typically show diffusion restriction, the lesion in our case was not. Hence, diffusion restriction could be a good differentiating factor although further studies are needed to confirm the consistency of this finding.
There is no consensus regarding the management of renal IMT due to rarity of the disease. Total nephrectomy is still advocated due to subset of rare cases of metastasis and recurrence [11]. The stable appearance of the lesion in our case, inferred from the presence of hydronephrosis 4 years prior to surgery on sonography favors the nonaggressive nature of the lesion. A tissue biopsy if done would spare the patient from total nephrectomy. A trial of steroid therapy or at least partial nephrectomy followed by close follow-up could be an option. However, encasement of the renal artery and location of the lesion would pose a risk of hemorrhage during a biopsy or preclude the option of partial nephrectomy in our case.
Conclusion
IMT of the kidney is a rare but important entity to recognize and include in the differential diagnosis of renal masses. Diagnosis based on radiological findings may be difficult. Further studies are required to establish radiological based diagnostic criteria.
References
- 1.Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003;23(3):719–729. doi: 10.1148/rg.233025073. [DOI] [PubMed] [Google Scholar]
- 2.Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, Menias CO. Inflammatory pseudotumor: the great mimicker. AJR Am J Roentgenol. 2012;198(3):W217–W227. doi: 10.2214/AJR.11.7288. [DOI] [PubMed] [Google Scholar]
- 3.Davides KC. Plasma cell granuloma of the renal pelvis. J Urol. 1972;107:938–939. doi: 10.1016/s0022-5347(17)61175-3. [DOI] [PubMed] [Google Scholar]
- 4.Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46(2):95–104. doi: 10.1097/PAT.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Zieske AW, Fenves AZ. Bilateral infiltrating renal inflammatory pseudotumor responsive to corticosteroid therapy. Am J Kidney Dis. 2008;51(1):116–120. doi: 10.1053/j.ajkd.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 6.Telugu RB, Prabhu AJ, Kalappurayil NB, Mathai J, Gnanamuthu BR, Manipadam MT. Clinicopathological study of 18 cases of inflammatory myofibroblastic tumors with reference to ALK-1 expression: 5-year experience in a tertiary care center. J Pathol Transl Med. 2017;51(3):255–263. doi: 10.4132/jptm.2017.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Li J, Rao X, Ao Q, Cao X, Huang Y. A case report of tracheal inflammatory myofibroblastic tumor in a 34-week pregnant woman misdiagnosed with asthma. Medicine (Baltimore) 2017;96(33):e7872. doi: 10.1097/MD.0000000000007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang W, Zhou X, Xu S, Lin S. CT manifestations of inflammatory myofibroblastic tumors (inflammatory pseudotumors) of the urinary system. AJR Am J Roentgenol. 2016;206(6):1149–1155. doi: 10.2214/AJR.15.14494. [DOI] [PubMed] [Google Scholar]
- 9.Liang W. A renal inflammatory myofibroblastic tumor similar to cystic renal cell carcinoma: one case report. Medicine (Baltimore) 2015;94(28):e1181. doi: 10.1097/.0000000000001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navale P, Menon S, Bakshi G, Pruthy R, Desai S. Inflammatory myofibroblastic tumor of kidney with heterotopic bone formation: An unusual case mimicking a renal malignancy. Indian J Med Paediatr Oncol. 2013;34(4):320–322. doi: 10.4103/0971-5851.125257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura Y, Urashima M, Nishihara R, Matsuura A, Bekku K, Iguchi H. Inflammatory pseudotumor of the kidney with renal artery penetration. Radiat Med. 2007;25(10):541–547. doi: 10.1007/s11604-007-0174-y. [DOI] [PubMed] [Google Scholar]
- 12.Selvan DR, Philip J, Manikandan R, Helliwell TR, Lamb GH, Desmond AD. Inflammatory pseudotumor of the Kidney. World J Surg Oncol. 2007;24(5):106. doi: 10.1186/1477-7819-5-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SH, Cho JY, Kim SH. Inflammatory myofibroblastic pseudotumor of the urinary bladder in a patient with bilateral renal cell carcinoma. J Ultrasound Med. 2008;27(3):483–486. doi: 10.7863/jum.2008.27.3.483. [DOI] [PubMed] [Google Scholar]