Abstract
Chemically-induced diabetic animal models have been employed in many areas of diabetes mellitus (DM) research, but managing post-induction animal survival rates remains one of the main downsides.
The aim of the present study was to propose a reliable approach to animal management and monitoring after DM induction in a rabbit model in order to reduce animal mortality rates.
DM was induced by injecting alloxan in 12 New Zealand White rabbits. A preventive subcutaneous glucose administration to counteract a potentially lethal hypoglycemic phase following alloxan injection was performed on individual bases. Blood glucose level (BGL) was checked hourly for the first 36 h, then every 2 h until the hyperglycemic state was confirmed.
All 12 rabbits survived a 48-hour post-induction phase. The critical hypoglycemic phase's start points and duration differed significantly among the rabbits, lasting from 6.7 to 37 h (19.75 ± 8.44). The rabbits entered the final hyperglycemic phase 18 h at the earliest and 42 h at the latest after induction (26.63 ± 7.07). The average daily BGLs throughout the study period ranged from 268 to 512 mg/dL (413.73 ± 76.69). Eleven rabbits survived until the end of the experiment.
The variability of rabbits' responses to alloxan injection emphasizes the importance of monitoring rabbit behavior and thoroughly checking BGLs, followed by a preventive glucose administration based on rabbits' individual needs for up to 36 h after alloxan injection. The proposed approach seems to reduce animal mortality.
Keywords: Veterinary medicine, Health sciences, Endocrinology, Metabolic disorder, Diabetes mellitus, Diabetes induction, Chemically-induced diabetes, Alloxan-induced diabetes, Mortality rates, Diabetic rabbit
Veterinary medicine; Health sciences; Endocrinology; Metabolism; Metabolic disorder; Diabetes mellitus; Diabetes induction; Chemically-induced diabetes; Alloxan-induced diabetes; Mortality rates; Diabetic rabbit
1. Introduction
The main characteristic of type 1 diabetes mellitus (DM) is an autoimmune destruction of pancreatic beta cells, leading to the lack of insulin production. To achieve this in an animal model, several approaches have been investigated, including the breeding of animals that spontaneously develop autoimmune DM, genetically- or virus-induced models, and experimental models in which the metabolic disorder is induced chemically or surgically [1, 2].
Chemically-induced DM in animal models has been employed in many areas of DM research, including cardiovascular research, ophthalmology, nephrology, transplantation therapies, and testing of new formulations of insulin [2, 3, 4]. Chemically-induced DM is comparatively cheaper and easier to develop and maintain than many other diabetic animal models [5]. Streptozotocin and alloxan are by far the most frequently used drugs shown to cause the necrosis of pancreatic islets and subsequent generation of DM. However, inter- and intra-species variations in the toxicity of these chemicals have been described before [6, 7, 8]. Increasing the dose of the diabetogenic agent may lead to renal and hepatic toxicity, and even light overdosing may result in general toxicity and animal loss, suggesting a narrow window of efficacy [9, 10]. Additionally, reported death rates differ substantially depending on the animal species, dose, chemicals used, and protocols employed.
Both streptozotocin and alloxan have been used to produce experimental DM in animals such as mice, rats, rabbits, and dogs [11], and while streptozotocin is the most widely used chemical in rodents, alloxan is considered the drug of choice for DM induction in rabbits [12]. A varying dose of alloxan, ranging from 50 to 200 mg/kg, has been reported as required to induce DM in rabbits. Interestingly, even in instances when the same dose of alloxan is provided to rabbits of identical gender, and similar maturity and weight, and using the same administration route (intravenous injection), there are inconsistent reports on survival rates [13, 14, 15]. Moreover, many studies do not report data on survival/death rates in the studied models of chemically-induced DM, or offer vague reports such as “an unusually low mortality rate” [16] or “no significant mortality” [17]. However, studies report mortality rates specifically in rabbits as high as 91.6% [18]. A few studies have reported no mortality post-alloxan treatment [13, 19]. Our study protocol was based on the one published by Wang et al. [13] Indeed, alloxan administration leads to characteristic blood glucose responses including a severe, transitional hypoglycemia that may be fatal [9]. The conclusions of the above-mentioned reports lead to the assumption that the management and monitoring of animals during a critical post-induction period might be of paramount importance for successful DM induction.
The guidelines underpinning the use of animals in scientific research, represented by three “Rs”—Replacement, Reduction, and Refinement—are set to ensure the welfare of laboratory animals, making sure that animals are used only when essential, used in minimum numbers, and looked after properly, receiving the best quality care [20]. Later, Banks introduced the 4th “R” representing Responsibility in the conduct of animal-based science [21]. Currently, numerous discussions in the research community and on research forums show that not only good ethical and scientific principles, but also legal and economic reasons, motivate researchers to reduce animal death rates in DM-induced models.
The overall objective of our research project was to study biomaterials and bone regeneration in diabetic versus healthy conditions. As diabetic rat model, as objectively easier model for diabetic induction, was not adequate for our research objectives (mainly due to the limited animal size), and rabbit model is one of the standard models used to study calvarial bone defect healing and sinus lift procedures, in addition to healthy rabbits, it was necessary to develop its diabetic counterparts. However, as one of the main limitations of diabetic rabbit model is known to be high animal mortality rates [18], the aim of the present report was to propose a reliable management strategy to improve animal survival rates after inducing DM using alloxan in rabbit models.
2. Materials and methods
All experimental procedures and protocols used in this investigation were reviewed and approved by the Institutional Animal Care and Use Ethics Committee of the University of Liege, Belgium (approval number: 13-1570). The Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines were carefully followed, as well as national and European legislated guidelines.
2.1. Animals
The overall study involved 24 SPF New Zealand White rabbits randomly divided into two groups: 12 DM-induced rabbits and 12 rabbits which served as healthy controls; however, this report focused on the DM-induced rabbits. The rabbits were males 10–12 weeks old with an average body weight of 3.01 ± 0.34 kg. The animals were acclimatized for 7 days before DM induction, single-housed in cages with standard environmental enrichment (pieces of wood and hay), with a 12-h light/dark cycle and controlled temperature and humidity, and were offered food and water ad libitum. The animals did not fast prior to alloxan administration.
2.2. Induction protocol
All rabbits received a single dose of alloxan monohydrate (Sigma-Aldrich BVBA, Overijse, Belgium) at 100 mg/kg, through a marginal ear vein using an intravenous (iv) cannula (24 gauge) as previously described [13]. Alloxan solution was freshly prepared by dissolving it in sterile normal saline to achieve a concentration of 5% (W/V) and used immediately (since it is known to be very unstable). The injection was performed over a period of 1 min. To reduce risk of nephrotoxicity from hyperuricemia, a 7 ml/kg body weight iv injection of 0.9% saline was given immediately after the injection of alloxan at the rate of 1 mL/min. In order to relief pain and distress from alloxan injection, the injecting procedure was done on anesthetized animals. Anesthesia was administered using a medetomidine/ketamine bolus (0.25/25 mg/kg respectively, IM) after fentanyl/dehydrobenzperidol premedication (0.22 ml/kg of a bolus 25 mg/1.25 mg/ml IM).
To avoid mortalities due to hypoglycemic shock after the induction, subcutaneous injections of 5% glucose solution (5 mL) were administrated, based on individual blood glucose levels (BGL) at the time of measurement, whenever recorded values were lower than 100 mg/dL; IV injections of 5% glucose solution were to be given if a BGL <50 mg/dL was measured. BGLs were measured using a blood glucometer (Accu-Chek, Roche Diabetes Care, Inc., Indianapolis, IN, USA) every hour for the first 36 h, then every 2 h until the diabetic (hyperglycemic) state was confirmed and blood glucose curve was obtained for each rabbit. Additionally, an oral solution of 20% glucose in tap water was provided ad libitum for 2 days after alloxan induction. Animals exhibiting a significant elevation in BGL above 250 mg/dL were considered diabetic, and this value was chosen based on previous studies [22, 23, 24].
2.3. Insulin treatment
Once stable hyperglycemia (constant hyperglycemic state without relapse) was confirmed, BGLs in the rabbits were measured twice a day using the same blood glucometer. If the BGL was higher than 350 mg/dL, long-acting insulin (Insulatard®, Novo Nordisk A/S, Denmark) was administered subcutaneously once or twice daily with an insulin pen. The dose was adjusted according to the BGL for the individual rabbit based on the following protocol previously reported by Wang et al. [13]:
-
(1)
BGL = 350–450 mg/dL received 1 U/kg
-
(2)
BGL = 450–550 mg/dL received 2 U/kg
-
(3)
BGL = 550–600 mg/dL received 3 U/kg
-
(4)
BGL >600 mg/dL received 4 U/kg
2.4. Determination of insulin dose
Because of individual variation and sensitivity to insulin, the insulin dose for each rabbit was determined shortly after the induction of diabetes as previously described by Wang et al. [13] Briefly, a blood glucose curve was acquired by first administering a test dose of insulin (based on the previously mentioned dosing rate) after obtaining the BGL in the morning, and then modifying the insulin dose if necessary. The final insulin dose was determined when a glucose curve was obtained where the peak BGL was < 300 mg/dL and the trough BGL was ≥ 80 mg/dL. If rabbits developed symptoms of illness (such as a loss of appetite or severe weight loss) during the experiment, a new BG curve was obtained and a new insulin dose implemented.
2.5. Humane endpoints
Humane endpoints were established before the start of the study. Interventions to alleviate pain and distress were applied whenever possible, and in accordance with the scientific endpoints, throughout the duration of the experiment (e.g. careful animal handling, providing analgesics, providing adequate diet and its modification if necessary, changing insulin dosage, monitoring clinical signs of hypoglycemia during induction, monitoring for changes in clinical, physiological, and behavioural signs, monitoring rabbit weight, monitoring blood glucose levels, ensuring proper environment with food and water ad libitum, etc.). As automortality is not an acceptable outcome, every condition that was not promptly manageable (e.g. severe weight loss) was considered an indicaton for immediate euthanasia, regardless of scientific endpoints; that is, in cases where it was not possible to balance humane endpoints with scientific endpoints, humane endpoints were considered more important and human killing of the affected animal was performed.
2.6. Statistical method
Descriptive statistics were used to describe the data from the study and summary data are presented as means, standard deviations (SD), minimum, and maximum, where applicable. Statistical analyses were performed using SPSS software (version 18.0, SPSS Inc, Chicago, IL, USA).
3. Results
3.1. Monitoring
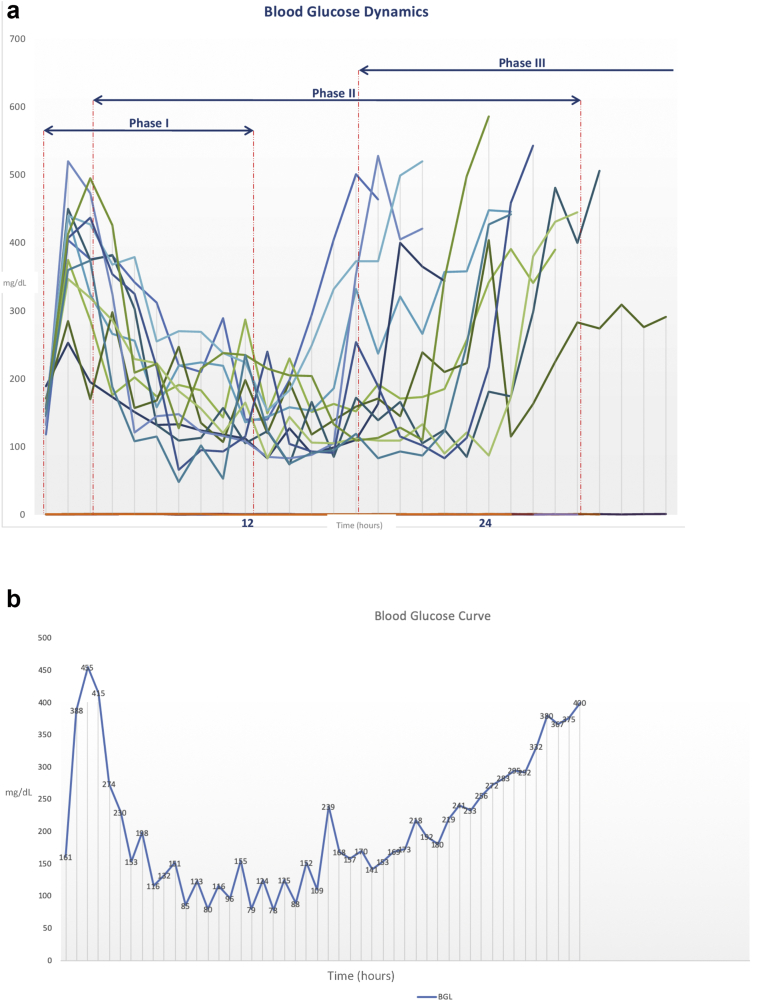
Baseline BGLs were in a normal range (120.17 ± 12.45 mg/dL) (Table 1). A triphasic blood glucose response to the diabetogenic dose of alloxan was observed: reactive hyperglycemic phase (phase 1), hypoglycemic phase (phase 2), and hyperglycemic – diabetic phase (phase 3). The details in terms of BGLs and timeframes are displayed in Figures 1a,b, 2, and 3.
Table 1.
The rabbits' data: weight, baseline, and average blood glucose levels (BGLs) throughout the study period.
| Rabbit | Weight | Baseline BGL | Average BGL |
|---|---|---|---|
| 1 | 3.2 | 138 | 371 |
| 2 | 2.85 | 116 | 268 |
| 3 | 2.5 | 117 | 493 |
| 4 | 3.3 | 145 | 349 |
| 5 | 2.85 | 128 | 334 |
| 6 | 2.6 | 116 | 512 |
| 7 | 3.4 | 124 | 458 |
| 8 | 3.12 | 106 | 390 |
| 9 | 3.45 | 113 | 478 |
| 10 | 2.5 | 102 | 457 |
| 11 | 3.17 | 124 | lost |
| 12 | 3.12 | 113 | 441 |
| Mean | 3.01 | 120.17 | 413.73 |
| SD | 0.34 | 12.45 | 76.69 |
| Range | 2.5–3.45 | 102–145 | 268–512 |
Figure 1.
a: The graphic representation of blood glucose dynamics in 12 rabbits during the first 36 h of monitoring post-induction; a triphasic time course can be observed: the hyperglycemic, hypoglycemic, and hyperglycemic phases. b: The graphic representation of blood glucose curve in a single rabbit during the 48 h of post-induction monitoring; a triphasic time course can be observed: the hyperglycemic, hypoglycemic, and hyperglycemic phases.
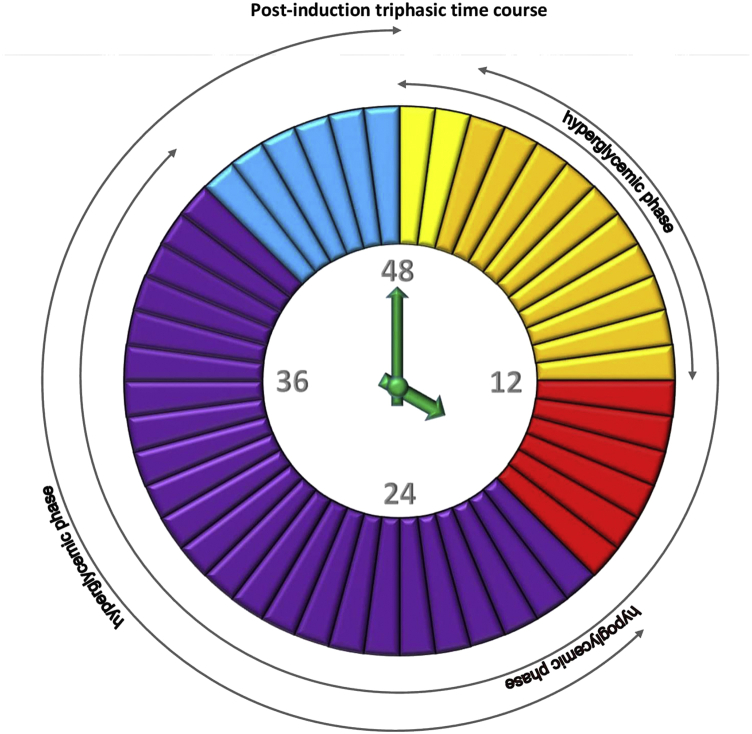
Figure 2.
Each piece of a clock-pie represents 1 h. The clock shows a 48-hour time span in rabbit monitoring, with 3 phases (1st hyperglycemic, 2nd hypoglycemic, 3rd hyperglycemic) that rabbits went through before all of them became diabetic; yellow – hyperglycemic phase for all; orange – hyperglycemic phase for some and hypoglycemic phase for others; red – hypoglycemic phase for all; violet – hypoglycemic phase for some and hyperglycemic phase for others; blue – final hyperglycemic phase for all rabbits.
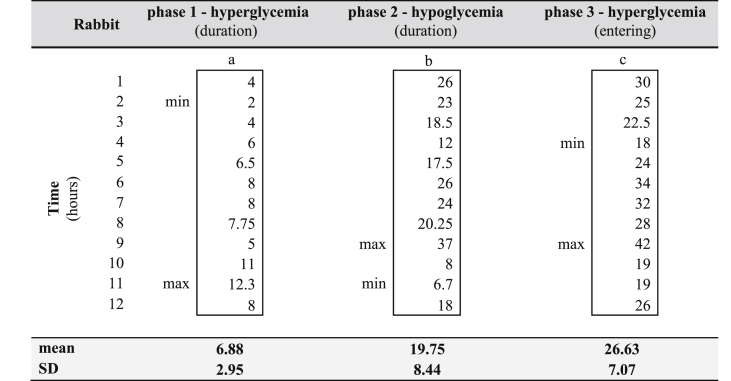
Figure 3.
a) The duration of the first phase in hours; b) the duration of the second phase in hours; c) the hours passed before the rabbits entered the third phase. The data is arranged so as to correspond to the respective rabbit numbers in Table 1 (i.e. from rabbit N°1 to rabbit N°12).
3.2. Hyperglycemic phase (phase 1)
At 1 h after alloxan administration, BGLs increased in all rabbits. However, huge variations between the rabbits were noticeable even at this point. This first phase reached its peak at about 2 h after the alloxan injection for the majority of rabbits and lasted 6.88 ± 2.95 h on average (Figure 3a).
3.3. Hypoglycemic phase (phase 2)
Thereafter, the glucose levels started to decline progressively and the rabbits entered the second phase – the hypoglycemic phase. This phase lasted from 6.7 up to 37 h (19.75 ± 8.44) (Figure 3b). It is worth noting that the majority of rabbits that experienced a shorter phase 1 had longer phase 2. The lowest measured BG value was 48 mg/dL in one rabbit (rabbit N°12) at 9 h post-alloxan treatment. This rabbit was the only one that needed iv injections of glucose. In the same rabbit, a BGL of 61 mg/dL was recorded at 17 h post-induction. Huge oscillations and quick drops in BGLs were observed. Very few signs typical of hypoglycemia, and no signs of convulsions, were noticed. Only five rabbits (N°1, 3, 4, 5, 11) were observed drinking water during the first 24 h post-induction.
3.4. Hyperglycemic – diabetic phase (phase 3)
The third phase—the second hyperglycemic phase—developed 18 h at the earliest and 42 h at the latest after alloxan administration (26.63 ± 7.07) (Figure 3c). Once the rabbits entered this phase, the BGLs continued rising, except for one rabbit (N°2) who relapsed again into a hypoglycemic phase before achieving a stable hyperglycemic state. Two rabbits (N°3 and N°7) did not develop persistent hyperglycemia after this first alloxan treatment, and were given a second dose of alloxan one week later. The same dose and the same protocol were applied this time with success in both rabbits.
Table 2.
The rabbits divided into 3 groups based on their average blood glucose levels (BGLs) throughout the study: mild, moderate, and severe diabetic groups. The rabbit (N°6) that was in ‘severe group’ had average BGL of 512 mg/dL.
| Diabetes | BGL (mg/dL) | No. of rabbits |
|---|---|---|
| mild | 200–300 | 1 |
| moderate | 300–400 | 4 |
| 400–500 | 5 | |
| severe | 500–600 | 1 |
3.5. Insulin administration
Once a stable hyperglycemic state was reached, the average BGLs in rabbits throughout the study period ranged from 268 to 512 mg/dL (413.73 ± 76.69) (Table 1). The rabbits could be divided into 3 groups based on their average BGLs throughout the study: mild, moderate, and severe diabetic groups (Table 2). The majority of rabbits received insulin twice per day, according to the previously provided scheme. The regimen of insulin treatment was the same throughout the study, except for one rabbit (N°11), as explained further below in the text.
3.6. Survival rates
All 12 rabbits survived the 48-hour-long post-induction phase. However, over a follow-up period, one rabbit (N°11) experienced a significant weight loss 1 week after alloxan administration. The BGL in this rabbit ranged from 500-600 mg/dL (data not included in Table 2), and it was injected with insulin twice per day. The insulin therapy was modified by increasing the insulin dose, changing insulin, and adding a rapid-acting insulin. In addition, the type of food was adapted to stimulate the animal to eat more because, unlike the other rabbits, this rabbit did not show the typical signs of polyphagia. However, the rabbit continued to lose weight (weight loss of 23.65% in 12 days post-induction) and in accordance to the established humane endpoints, it was euthanized on the 12th day after DM induction. The remaining 11 rabbits were in good health and survived until the end of the experiment (8 weeks).
4. Discussion
According to the present findings, a triphasic time course was observed after alloxan administration: the first phase marked with an increase in BGL (a reactive hyperglycemic phase), followed by the second phase characterized by a decrease in BGL (a hypoglycemic phase), and ending with the third phase with another rise in BGL (a permanent hyperglycemic phase). These common phasic changes in BGLs were observed in each animal, but a significant heterogeneity in the phases' occurrence and duration among the rabbits was noticed. The initial hypoglycemic phase as described by Lenzen [9], which starts within minutes of alloxan injection and lasts up to 30 min, could not be observed in the present study, as the first BGL measurement was performed only 1 h after the alloxan administration.
The reactive hyperglycemic phase, caused by the inhibition of insulin secretion, on average lasted longer than the previously reported 2–4 h [9]. The following hypoglycemic phase has been described as a result of beta-cytotoxic action of alloxan that leads to secretory granule and cell membrane rupture, and a consequential flooding of the circulatory system with insulin, resulting in hyperinsulinemia [9]. This phase is unavoidable in chemically induced DM models, and it has been held responsible for high animal mortality post-induction. Indeed, Saleem Mir et al have shown that alloxan-induced acute hypoglycemia can result in severe pathological alterations in several vital organs, including the brain, kidneys, and liver, eventually leading to death [12]. A preventive glucose administration combined with a constant monitoring of rabbits' BGLs proved to obviate the occurrence of severe hypoglycemia and consequently prevented animal deaths in the present study.
According to previous reports, the critical hypoglycemic phase typically occurs 4–8 h after alloxan injection and lasts several hours [9]. The present results emphasize heterogeneity in both the onset and duration of this phase; in one rabbit the BGLs started declining just 2 h post-induction and in another only after 12 h; this phase could last for up to 37 h. Individual blood glucose dynamics were observed; some rabbits needed only few preventive injections of glucose to counteract the anticipated hypoglycemia, while others required iv injections at points when the BGL rapidly dropped under 50 mg/dL. This steady monitoring of rabbit behavior, BGL checking, and preventive subcutaneous injections when glucose levels showed a tendency to decrease allowed the operator to have full control and to react promptly as needed. We decided to monitor hourly based on the results of the studies of Reddy and Kinsey [16] and Golay et al. [25] The former showed that a combination of intraperitoneal injections (20 ml of sterile 10% glucose solution) every 4 h for the first 48 h, with iv injections (5 ml of glucose solution) at 24 and 34 h and at any signs of convulsions, resulted in about a 10 % death rate; the latter reported that even though preventive glucose injections were performed intraperitoneally every 2–4 h for the first 24 h, death from hypoglycemia could not be entirely avoided.
Considering that the present results showed that BGL changes were entirely individual and differed greatly among the rabbits, limiting the preventive injections to 4, 8, and 12 h post-induction, as suggested by Wang et al. [13], might not be sufficient care for some rabbits. Moreover, BGL can drop by as much as 100 mg/dL in less than 1 h, and missing one timely BGL check could be lethal for a rabbit in distress. In addition, although some of the rabbits showed a few characteristic behavioral changes indicating a hypoglycemic status, some others appeared normal, and few physical signs would obviously alert us to low BGLs.
We also noted that one of the rabbits (N°2) that seemed to have entered the final hyperglycemic phase at 20 h relapsed into the hypoglycemic state at 25 h after alloxan administration. Therefore, at least 24 h of constant monitoring seems to be required, in order to prevent any adverse late effects of alloxan. This is in disagreement with Wang et al. [13], who indicated that 24-hour continuous observation was not necessary. Moreover, one rabbit had low BGLs up to 37 h post-induction; hence a prolonged surveillance may be needed for some rabbits.
Some researchers reported providing rabbits with only an oral solution of glucose in water, with no additional glucose administration [26]. We have noticed that only some rabbits were drinking water during the first 24 h post-induction, even though we tried to stimulate the rabbits to do so. Based on this, we believe that giving only an oral glucose solution is not sufficient to counterbalance severe hypoglycemia. Another aspect to consider is subjecting animals to fasting prior to induction procedure, which is recommended by some authors as it is believed that fasted animals tend to be more susceptible [2]. Indeed, due to the similarity in structure between glucose and alloxan [27], glucose can compete with alloxan and fasting could therefore be effective in eliminating competition. However, it was previously reported that hypoglycemia is more pronounced in fasted animals [28], which is one of the main reasons the induction procedure in the present study was performed on non-fasted animals.
Another potential cause of animal death may be related to alloxan dosage. Alloxan has a narrow diabetogenic dose, and even light overdosing can cause general toxicity, especially in the kidneys [10]. Even so, literature reports that different doses of as little as 50 mg/kg and up to 200 mg/kg have been employed to induce DM [29, 30, 31, 32], with toxicity generally increasing with the dose. A stable diabetic state seems to be more predictable with a higher dose (100–150 mg/kg), but this usually involves a higher animal mortality. On the other hand, lower doses (50–70 mg/kg) have been found ineffective or less effective in DM induction [15]. However, the data are inconsistent, as some researchers reported mortality rates of up to 75% with an alloxan dose of 80 mg/kg [15], while others reported 100% survival rates with a dose of 150 mg/kg [19]. A dose of 100 mg/kg was chosen for the present study, as it was considered a good balance between animal mortality rates and DM induction success rates according to our literature search, and its efficacy had been proved previously in our pilot study (DM induction in 3 rabbits; 100% survival rate). However, in the present study, the induction had to be repeated in two rabbits which did not develop permanent hyperglycemia after the first alloxan injection; these rabbits could be referred to as “alloxan-recovered” animals. The same observation was made by Wang et al. [13], who used the same dose in their study. Alloxan-resistance has been attributed to individual variations in susceptibility to the beta-cytotoxic effects of alloxan, which may be associated with anti-oxidant status [9, 12], while alloxan-recovery may be the consequence of spontaneous regeneration, which may include multiplication of cells that survived, or new beta-cells that formed from either the duct epithelium or the exocrine pancreas [5, 33, 34]. It has been also proposed that insulin administration might alter islet function or recovery [4]. Gongora et al have shown that with a higher dose of 125 mg/kg, there was no need for a second alloxan dose [35]. However, surprisingly, more than 50% percent of rabbits in their study had a BGL between 180 and 250 mg/dL, which might not be sufficiently “severe” for some DM study models. Battell et al (1999) have previously concluded that the incidence of developed DM was not proportionately related to increasing the dose of alloxan [36].
Furthermore, some researchers suggested alloxan administration at several subsequent time points in order to lower animal mortality. Sun et al [18] have shown that a single alloxan injection at 150 mg/kg led to a mortality rate of 91.67% while the same dose injected as 3 × 50 mg/kg, at three subsequent time points in 1 week intervals, reduced the mortality rate to 8.33%. However, this complicates the whole procedure, is time-consuming, and requires animals to be anesthetized each time.
One more possible factor that could contribute to higher mortality rates is the speed of iv injections of alloxan. Rapid iv injection was associated with a significantly increased death rate; a mortality rate of about 80% was reported with rapid injections, compared to a 30% mortality rate with slow injections [37]. On the other hand, it was also demonstrated that slower injection rates reduced the efficacy of the agent [38]. The injections in our study were performed over a period of 1 min which, based on the results, may present an acceptable balance—balancing risk and the pursuit of effectiveness.
One may argue that the proposed protocol is based on continuous and possibly over-exaggerated BGL checking, which also requires the researchers involved in a study to spend as much as 36 h alongside the induced rabbits. However, the present data suggest that at least two rabbits which experienced severe hyperglycemia in our study probably would have not survived this phase if they were not monitored as presented here, with timely glucose administration. On the other hand, as it turned out, some rabbits did not need to be checked for BGL every hour for 24 h. Nevertheless, we do not consider it safe to stop monitoring during the first 36 h, namely because of the possibility of a relapse into the hypoglycemic state, as observed in one rabbit, and the fact that BGL changes occur quickly and often unpredictably.
As we used a controlled model of DM with daily insulin administration for 8 weeks, it is reasonable to expect higher mortality rates in an uncontrolled model or in one that lasts for a longer period. Moreover, the present findings should be interpreted with caution as they are based on a relatively small number of animals. It is also worth noting that we did not have a control group of alloxan-induced rabbits that received no post-induction monitoring as this was deemed highly unethical. Indeed, our main goal was not to compare different induction protocols and this report should be seen just as practical considerations that may help other researchers in the field to reduce well-known mortality rates in this model.
5. Conclusion
Offsetting a potentially lethal hypoglycemic phase that inevitably follows alloxan administration is of paramount importance. Monitoring rabbit behavior and BGLs with hourly checking for up to 36 h post induction, followed by a preventive glucose administration based on rabbits' individual needs, should be mandatory, as it seems to be an effective way to keep animal mortality rates to a minimum.
Indeed, we consider that the rigorous implementation of methods—from the simplest (as described herein) to the most sophisticated ones, which contribute to improving the well-being of animals recruited for research—goes far beyond the notion of ethics, and substantially establishes a moral obligation.
Declarations
Author contribution statement
M. Bacevic: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
E. Rompen: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
R. Radermecker: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
P. Drion: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
F. Lambert: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to express their gratitude to Professor Sufan Chien for replying to our emails and providing us with very valuable advice regarding the study protocol, and to Luc Duvez for his assistance in animal care.
References
- 1.Chatzigeorgiou A., Halapas A., Kalafatakis K., Kamper E. The use of animal models in the study of diabetes mellitus. In Vivo. 2009;23(2):245–258. [PubMed] [Google Scholar]
- 2.King A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012;166(3):877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheshala R., Peh K.K., Darwis Y. Preparation, characterization, and in vivo evaluation of insulin-loaded PLA-PEG microspheres for controlled parenteral drug delivery. Drug Dev. Ind. Pharm. 2009;35(11):1364–1374. doi: 10.3109/03639040902939213. [DOI] [PubMed] [Google Scholar]
- 4.Deeds M.C., Anderson J.M., Armstrong A.S., Gastineau D.A., Hiddinga H.J., Jahangir A. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab. Anim. 2011;45(3):131–140. doi: 10.1258/la.2010.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan K., Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J. Med. Res. 2007;125(3):451–472. [PubMed] [Google Scholar]
- 6.Tyrberg B., Andersson A., Borg L.A. Species differences in susceptibility of transplanted and cultured pancreatic islets to the beta-cell toxin alloxan. Gen. Comp. Endocrinol. 2001;122(3):238–251. doi: 10.1006/gcen.2001.7638. [DOI] [PubMed] [Google Scholar]
- 7.Dufrane D., van Steenberghe M., Guiot Y., Goebbels R.M., Saliez A., Gianello P. Streptozotocin-induced diabetes in large animals (pigs/primates): role of GLUT2 transporter and beta-cell plasticity. Transplantation. 2006;81(1):36–45. doi: 10.1097/01.tp.0000189712.74495.82. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z.-H., Watschinger B., Brown C., Beyer M.M., Friedman E. Variations of susceptibility to alloxan induced diabetes in the rabbit. Horm. Metab. Res. 1987;19(11):534–537. doi: 10.1055/s-2007-1011876. [DOI] [PubMed] [Google Scholar]
- 9.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 10.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001;50(6):537–546. [PubMed] [Google Scholar]
- 11.Etuk E. 2010. Animals Models for Studying Diabetes Mellitus. [Google Scholar]
- 12.Saleem Mir M., Maqbool Darzi M., Musadiq Khan H., Ahmad Kamil S., Hassan Sofi A., Ahmad Wani S. Pathomorphological effects of Alloxan induced acute hypoglycaemia in rabbits. Alexandria J. Med. 2013;49(4):343–353. [Google Scholar]
- 13.Wang J., Wan R., Mo Y., Zhang Q., Sherwood L.C., Chien S. Creating a long-term diabetic rabbit model. Exp. Diabetes Res. 2010;2010:289614. doi: 10.1155/2010/289614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burski K., Ueland T., Maciejewski R. Serum amylase activity disorders in the course of experimental diabetes in rabbits. Veterinarni Medicina Czech. 2004;49(6):197–200. [Google Scholar]
- 15.Alam S., Khan A.H., Sirhindi G., Khan S. Alloxan induced diabetes in rabbits. Pakistan J. Pharmacol. 2005;22(2):41–45. [Google Scholar]
- 16.Reddy D.V., Kinsey V.E. Transport of amino acids into intraocular fluids and lens in diabetic rabbits. Invest. Ophthalmol. 1963;2:237–242. [PubMed] [Google Scholar]
- 17.Mumtaz F., Lau D., Siddiqui E., Morgan R., Thompson C., Mikhailidis D. Changes in cholinergic and purinergic neurotransmission in the diabetic rabbit bladder. In Vivo. 2006;20(1):1–4. [PubMed] [Google Scholar]
- 18.Sun W.T., Lei C.L., Bi C.C., Chen Z.L., Zhang L. Effect of alloxan time administerDrug on establishing diabetic rabbit model. Int. J. Ophthalmol. 2010;3(3):200–202. doi: 10.3980/j.issn.2222-3959.2010.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Loughlin A., Kulkarni M., Vaughan E.E., Creane M., Liew A., Dockery P. Autologous circulating angiogenic cells treated with osteopontin and delivered via a collagen scaffold enhance wound healing in the alloxan-induced diabetic rabbit ear ulcer model. Stem Cell Res. Ther. 2013;4(6):158. doi: 10.1186/scrt388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell W.M.S., Burch R.L. 1959. The Principles of Humane Experimental Technique. [Google Scholar]
- 21.Banks R. The 4th R of research. Contemp. Top. Lab. Anim. Sci. Am. Assoc. Lab. Anim. Sci. 1995;34(1):50–51. [PubMed] [Google Scholar]
- 22.Akhtar M.S., Khan M.A., Malik M.T. Hypoglycaemic activity of Alpinia galanga rhizome and its extracts in rabbits. Fitoterapia. 2002 Dec;73(7–8):623–628. doi: 10.1016/s0367-326x(02)00235-6. [DOI] [PubMed] [Google Scholar]
- 23.Fuentes O., Arancibia-Avila P., Alarcón J. 2004 Sep. Hypoglycemic Activity of Bauhinia Candicans in Diabetic Induced Rabbits.https://linkinghub.elsevier.com/retrieve/pii/S0367326X04001005 Fitoterapia [Internet][cited 2020 May 12];75(6):527–32. Available from: [DOI] [PubMed] [Google Scholar]
- 24.Díez R., García J.J., Diez M.J., Sierra M., Sahagún A.M., Calle Á.P. Hypoglycemic and hypolipidemic potential of a high fiber diet in healthy versus diabetic rabbits. BioMed Res. Int. 2013:1–8. doi: 10.1155/2013/960568. http://www.hindawi.com/journals/bmri/2013/960568/ [Internet] [cited 2020 May 12] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golay A., Zech L., Shi M.Z., Jeng C.Y., Chiou Y.A., Reaven G.M. Role of insulin in regulation of high density lipoprotein metabolism. J. Lipid Res. 1987;28(1):10–18. [PubMed] [Google Scholar]
- 26.Park J.K., Lee S.O., Cui W.S., Kim S.Z., Koh G.Y., Cho K.W. Activity of angiotensin peptides in clitoral cavernosum of alloxan induced diabetic rabbit. Eur. Urol. 2005;48(6):1042–1050. doi: 10.1016/j.eururo.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Bansal R., Ahmad N., Kidwai J.R. Alloxan-glucose interaction: effect on incorporation of 14C-leucine into pancreatic islets of rat. Acta Diabetol. Lat. 1980;17:135–143. doi: 10.1007/BF02580995. [DOI] [PubMed] [Google Scholar]
- 28.Spiegelman A.R., Tuchman M. Prevention of hypoglycemia during the induction of alloxan diabetes; the use of glucose and anti-hyaluronidase subcutaneously in the rabbit. Diabetes. 1955;4(6):473. doi: 10.2337/diab.4.6.473. [DOI] [PubMed] [Google Scholar]
- 29.Weekers F., Giulietti A.P., Michalaki M., Coopmans W., Van Herck E., Mathieu C. Metabolic, endocrine, and immune effects of stress hyperglycemia in a rabbit model of prolonged critical illness. Endocrinology. 2003;144(12):5329–5338. doi: 10.1210/en.2003-0697. [DOI] [PubMed] [Google Scholar]
- 30.Torres-Jacome J., Gallego M., Rodriguez-Robledo J.M., Sanchez-Chapula J.A., Casis O. Improvement of the metabolic status recovers cardiac potassium channel synthesis in experimental diabetes. Acta Physiologica (Oxford) 2013;207(3):447–459. doi: 10.1111/apha.12043. [DOI] [PubMed] [Google Scholar]
- 31.Dubey G., Dixit S., Alok S. Alloxan-induced diabetes in rabbits and effect of a herbal formulation D-400. Indian J. Pharmacol. 1994;26(3):225. [Google Scholar]
- 32.Puri D., Prabhu K., Murthy P. Mechanism of action of a hypoglycemic principle isolated from fenugreek seeds. Indian J. Physiol. Pharmacol. 2002;46(4):457–462. [PubMed] [Google Scholar]
- 33.Cheta D. Animal models of type I (insulin-dependent) diabetes mellitus. J. Pediatr. Endocrinol. Metab. 1998;11(1):11–19. doi: 10.1515/jpem.1998.11.1.11. [DOI] [PubMed] [Google Scholar]
- 34.Lazarow A. Spontaneous recovery from alloxan diabetes in the rat. Diabetes. 1952;1(5):363–372. doi: 10.2337/diab.1.5.363. [DOI] [PubMed] [Google Scholar]
- 35.Gongora J., Diaz-Roa A., Ramirez-Hernandez A., Cortes-Vecino J.A., Gaona M.A., Patarroyo M.A. Evaluating the effect of Sarconesiopsis magellanica (Diptera: Calliphoridae) larvae-derived haemolymph and fat body extracts on chronic wounds in diabetic rabbits. J. Diabetes Res. 2015;2015:270253. doi: 10.1155/2015/270253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battell M.L., Yuen V.G., Verma S., McNeil J. Vol. 200. CRC Press; USA: 1999. Other Models of Type 1 Diabetes. Experimental Models of Diabetes Florida; pp. 219–229. [Google Scholar]
- 37.Misra M., Aiman U. Alloxan: an unpredictable drug for diabetes induction? Indian J. Pharmacol. 2012;44(4):538. doi: 10.4103/0253-7613.99348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pincus I., Hurwitz J., Scott M. Effect of rate of injection of alloxan on development of diabetes in rabbits. Proc. Soc. Exp. Biol. Med. 1954;86(3):553–554. doi: 10.3181/00379727-86-21162. [DOI] [PubMed] [Google Scholar]