Highlights
-
•
Population screening has confirmed circulation of West Nile virus in the southern region of Kazakhstan (Almaty region).
-
•
We report two cases of meningoencephalitis of unknown etiology occurred in the rural area near Tekeli city, Kazakhstan, in August 2019.
-
•
Retrospective analysis showed high titers of anti-WNV IgG in both patients’ serum specimens obtained on day 9 after the onset of symptoms.
-
•
These are the first reports of West Nile virus infection in Kazakhstan.
Abbreviations: CSF, Cerebrospinalfluid; ELISA, Enzyme-linkedimmunosorbent assay; ESR, Erythrocytesedimentation rate; WNV, WestNile virus
Keywords: Neuroinvasive West Nile virus infection, West Nile virus, Meningoencephalitic syndrome of unknown origin, Almaty region, Kazakhstan
Abstract
Background
West Nile virus (WNV) is a member of the genus Flavivirus, which transmitted to humans mainly by mosquitoes. Recent pilot serosurveillance data from the Almaty region, Kazakhstan, suggest widespread WNV circulation in this area. This report includes two cases of neuroinvasive WNV infection in the same family living in a rural area near Tekeli city, Eskeldinsky district, Almaty region, Kazakhstan. Occurring concurrently and manifesting as WNV infection with febrile illness and symptoms of meningoencephalitis.
Methods
The study performed retrospective analysis of clinical histories and achieved serum samples obtained from patients with febrile and meningoencephalitic syndromes of unknown origin in the Almaty region spanning from April 1 to October 31, 2019. All sera samples obtained from patients with clinically suspected cases of acute WNV infection were retrospectively tested for WNV and tick-borne encephalitis virus by commercial immunoassays. Two cases were selected.
Cases presentation
We report two cases that occurred in August 2019 in a rural area near Tekeli city. Previously healthy 28- and 19-year-old husband and wife with febrile illness and neurological manifestations were hospitalized with the diagnosis of meningoencephalitis of unknown etiology and treated empirically. Retrospective serological analysis showed the presence of high titers of IgG against WNV on day 9 after onset of symptoms in cases.
Conclusions
This is the first report of aseptic meningitis with WNV infection in the background in Kazakhstan. The obtained data suggest circulation of WNV in the Almaty region and emphasize importance of laboratory testing for WNV in suspicious cases occurring in the region.
Introduction
West Nile virus (WNV) is a mosquito-borne flavivirus belonging to the Japanese encephalitis serocomplex of the family Flaviviridae [1]. Culex spp. are the most common vectors of WNV transmitting infection to humans and birds serve as reservoirs [1,2]. Person-to-person transmission is not common but can result from blood transfusion, organ transplantation, intrauterine or breastfeeding exposure [2].
In 80 % of the cases, infection is asymptomatic and in the rest clinical manifestations include acute febrile illness with symptoms of a respiratory infection, often with the development of meningitis and meningoencephalitis [3,4]. Neuroinvasive WNV disease occurs in less than 1% of cases, following viral penetration of the blood–brain barrier and direct invasion into neurons, particularly those in the brainstem, deep nuclei, and anterior horn of the spinal cord. Neuroinvasive WNV disease could result in significant morbidity and mortality. Thus, high clinical suspicion is necessary to diagnose this disease.
WNV-related neuroinvasive manifestations are various and include aseptic meningitis, meningoencephalitis and acute flaccid paralysis syndrome (a poliomyelitis-like illness). The proportion of neuroinvasive disease manifesting as meningitis, as opposed to encephalitis or myelitis, has varied greatly within a given epidemic season and locale. A number of nonspecific symptoms may provide a clue to the diagnosis of central nervous system infection with WNV, as in other forms of encephalitis, such as fever (in 70–100 % of patients), headache (50–100 % of patients) and altered mental status (50–100 % of patients), vomiting (30–75 % of patients), diarrhea (15–35 % of patients) and rash (5–50 % of patients). Flaccid paralysis is observed in a significant proportion of patients (30–50 %). Other distinctive findings include cranial neuropathies and most commonly unilateral or bilateral peripheral facial palsy. Also, dyskinesias is common in patients with WNV meningoencephalitis, and can include postural or kinetic tremor (in up to 90 % of patients), parkinsonism (including cogwheel rigidity, bradykinesia and postural instability [70 %]), and myoclonus [5].
WNV is spread globally [1]. Several outbreaks of WNV infection cases have been reported in a number of countries [[6], [7], [8]]. WNV has also been widely registered in the neighboring with Kazakhstan countries: Russia, Turkmenistan, Uzbekistan and China [9]. In Kazakhstan, WNV isolation from mosquitoes and WNV seropositivity in humans have been reported in the West-Kazakhstan region [10,11]. Also, recently, circulation of WNV in the southern region of Kazakhstan (Turkestan region) has been confirmed by the population screening [12].
Due to the facts that WNV occurs in the neighboring with Kazakhstan countries and that the number of cases of meningoencephalitis and fevers of unknown origin has steadily been increasing in the Almaty region in the past decade, we performed a pilot study aimed at the evaluation of WNV seroprevalence in the healthy population and patients with fevers of unknown origin. Commercial immunoassays VectorNile-IgM and VectorNile-IgG, VectorBest (Russia) were used for detection of sera IgM and IgG antibodies against WNV. WNV IgG were detected in 19.8 % (37/187) of the tested asymptomatic residents of the Almaty region. WNV IgM were detected in 2.4 % (5/209) of sera samples obtained from febrile patients with fevers of unknown origin during the 2018–2019 epidemiological season. Thus, preliminary results confirmed active circulation of WNV in the Almaty region of Kazakhstan and allowed us to identify WNV clinical cases among the patients with appropriate clinical manifestation.
Here we describe two cases from one family with clinical symptoms of neuroinvasive WNV infection and positive WNV serology which occurred in the Almaty region of Kazakhstan.
Methods
Study design
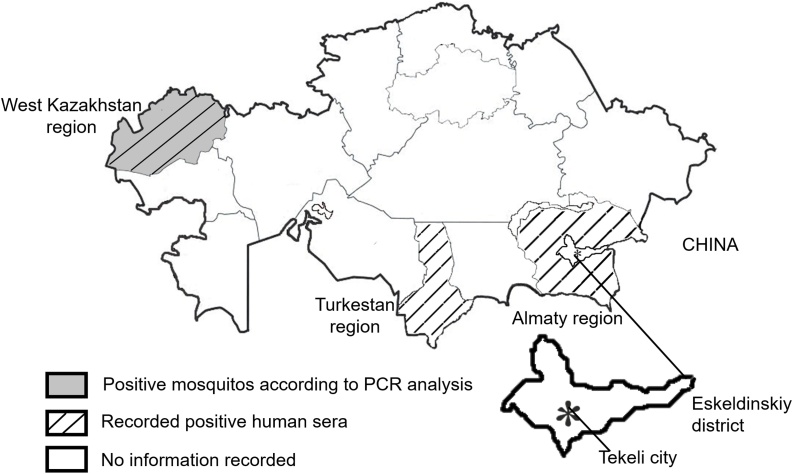
This is a retrospective study of cases of fever of unknown origin with neurological manifestations reported during the 2019 epidemiological season aimed to confirm active circulation of WNV in the Almaty region of Kazakhstan. Archived serum samples were retrospectively tested for WNV and the clinical data of the patients, which serum samples were positive for WNV, were selected and analyzed. The clinical data were gathered from medical records without any personally identifiable information. The local ethics committee of the National Center for Biotechnology has approved the study (Fig. 1).
Fig. 1.
Geographic distribution of WNV by regions in Kazakhstan. Geographic distribution of reported WNV positive human sera and mosquitoes in the regions of Kazakhstan is depicted. ‘*’ indicates the location of described human WNV clinical cases.
Serological analysis
Archived serum samples were retrieved from storage at −20 °C at a local hospital. These serum samples had been obtained from patients on day 9 after onset of symptoms and previously tested for tick-borne encephalitis. For serological analysis, serum samples were thawed and inactivated at 56 °C for 20 min. Commercial assays (VectorNile-IgM and VectorNile-IgG, VectorBest, Russia) were used as directed by the manufacturer. The results were calculated by dividing the optical density (OD) values of the test sera by the OD values of the Cutoff Calibrator. Indicators above 0.478 for IgM and 0.355 for IgG were considered positive.
Cases
Case 1
A 28-year-old male from the rural Syrymbet village (44.8601 °N, 78.7629 °E), Eskeldinsky district, Almaty region, Kazakhstan, was admitted to the emergency department of a hospital in Tekeli city (Almaty region, Kazakhstan) on August 2, 2019, with a 3-day febrile illness. His symptoms started on July 31 (day 1) with fever and headache. On day 2 his fever raised up to 39.0 °C (axillary temperature), headache intensified and the patient began vomiting. Oral analgesics and nonsteroidal anti-inflammatory drugs did not improve patient’s condition.
Upon admission, physical assessment revealed fever (39.0 °C axillary temperature), nausea and vomiting, blood pressure 140/90 mmHg, heart rate 72–88 bpm, muffled heart sounds, respiratory rate 18/min, weakness, malaise, severe headache, and photophobia. Patient’s throat was hyperemic, tongue had white coating, liver was palpated at the edge of the rib arch. Neck muscle rigidity (40 mm), Kernig's sign on the both sides, and lower motor neuron type facial palsy were observed. The patient denied tick bites. Cerebrospinal fluid (CSF) opening pressure was 180 mmH2O. CSF pleocytosis (26 cells/mL) with decreased lymphocyte counts (12.0 cells/mL; 46.1 %), neutrophil predominance (14.4 cells/mL; 53.9 %), and mild protein concentration (33.0 mg/dl) were detected.
On day 4 after symptoms onset, laboratory tests showed that the erythrocyte sedimentation rate (ESR) (26 mm/hr), blood white blood cell counts and urine tests were within the normal range. Bacterial culture of the CSF and the nasopharyngeal swab were negative for Meningococcus and other species of Neisseria. Also, serological analysis for herpes viruses, enterovirus infection and tick-borne encephalitis showed negative results.
Based on the clinical findings, the patient was diagnosed with aseptic meningitis and treated with symptomatic therapy.
The Kernig's sign, vomiting and pharyngeal hyperemia disappeared on day 4 and 5 since onset of illness, respectively. The patient continued to be febrile until day 9. Neck stiffness and increased blood pressure were observed until day 10. Weakness, malaise, and headache lasted for 11 days of illness. He was discharged in 14 days after symptoms onset with near-complete recovery.
The patient’s initial serum sample taken on day 9 after symptom onset was retrospectively assessed for WNV by ELISA. Serum specimen obtained from the patient was negative for WNV IgM and positive for WNV IgG (the OD value of the sample exceeded the OD value of the Cutoff Calibrator more than 15 times (OD value – 4.482)).
Case 2
The first case patient’s 19-year old wife was hospitalized to the Tekeli city hospital, Almaty region, Kazakhstan on August 5, 2019, with the same initial diagnosis of acute respiratory viral infection, meningism. She fell ill on August 4 (day 1), 5 days later her husband. Upon admission, she had high fever (maximum axillary temperature of 39.8 °C), weakness, malaise, dizziness, photophobia, nausea, and moderate headache for 2 days.
Physical findings were as follows: fever (axillary temperature of 39.7 °C); nausea and vomiting; blood pressure 140/90 mmHg; heart rate 94 bpm; muffled heart sounds; respiratory rate 20/min; hyperemic mucosa of the pharynx and posterior pharyngeal wall; thick white coating on the tongue; liver was palpated at the edge of the rib arch. Neck muscle stiffness (30 mm), Kernig's sign, lower motor neuron type facial palsy, tremor in the hands and leg, and carpopedal spasms were also noted. A lumbar puncture revealed a CSF opening pressure at 180 mmH2O, pleocytosis (20 cells/mL) with low lymphocyte (15.0 cells/mL; 75.0 %) and neutrophil (5 cells/mL; 25.0 %) counts, and a significantly elevated protein concentration (264 mg/dl). Laboratory evaluation showed an elevated white blood cell (WBC) count of 10.3/μl with an increase in neutrophil numbers (81.5 %) accompanied with a decrease in lymphocyte numbers (10.0 %). On the second day of illness, the ESR was 23 mm/hr (normal value < 15 mm/hr). Urine tests were within the normal range. Serological analysis and bacterial culture tests showed negative results for Neisseria species, herpes viruses, enterovirus infection and tick-borne encephalitis.
The patient was diagnosed with aseptic meningitis and prescribed symptomatic therapy with additional empiric antiviral therapy (Rimantadine (1.5 mg/kg in 3 doses for 2 days and then in 2 doses for 4 days)).
The patient improved after the initiation of the treatment. She was completely afebrile on day 3 after symptom onset. The Kernig's sign, vomiting and pharyngeal hyperemia disappeared on day 3. Neck stiffness and increased blood pressure were observed until day 8 and 9, respectively. She was discharged after on day 11 of illness with near-complete improvement and without neurologic sequelae.
Retrospective serological analysis showed the absence of IgM and high titers of IgG (OD value of the sample 11 times exceeded the OD value of the Cutoff Calibrator (OD value – 4.212)) against WNV in the patient’s serum sample obtained on day 9 after symptom onset.
Discussion
Neuroinvasive WNV infection is associated with significant morbidity. The diagnosis of neuroinvasive disease in WNV infection is based on several factors like exposure to the vector, living in an endemic area, and the clinical manifestations. In endemic areas, the diagnosis should be considered, especially in the summer [13].
WNV circulation has been previously demonstrated in the West-Kazakhstan and Turkestan regions of Kazakhstan [[10], [11], [12]]. However, despite evidence of the virus circulation, documented cases of WNV infection have not been reported in Kazakhstan. In the Almaty region of Kazakhstan over the past decade, the number of cases of meningoencephalitis and fever of unknown origin has been increasing [14]. However, patients are not suspected for WNV infection in clinics due to a persistent misconception that the disease does not occur in the southern region of the country.
Our pilot screening study showed the positivity for anti-WNV IgG and anti-WNV IgM in healthy residents and patients with fevers of unknown of the Almaty region. We assume that sporadic cases or small clusters of WNV disease had previously occurred in the southern region of Kazakhstan and remained undiagnosed due to the overlapping of the neurological symptoms attributable to several other viral infections of the central nervous system [[15], [16], [17]] and limitations in the availability of diagnostic resources in most hospitals within the Almaty region, Kazakhstan. As an example, here we give a brief summary of the medical histories of two patients whose serum taken on day 9 of illness showed the presence of high titers of IgG for WNV.
Two family members, a husband and his wife, manifested a febrile illness with a difference of 4 days. The symptoms observed in these patients were consistent with meningitis that started abruptly. Laboratory tests for the presence of other etiological factors of meningitis and encephalitis were negative. In both cases, the disease manifestated in August, which is consistent with the seasonal outbreaks of WNV infection [1].
The territory of Almaty region is characterized by a sharply continental climate with warm springs and summers. Tekeli city is located in the south-east of Kazakhstan, at the foothills of the northern of Tien Shan ridge - Jungarsky Alatau and characterized by large amounts of rainfall and high number of surrounded natural reservoirs, which benefit mosquito breeding. In August, the average temperatures vary from 15 °C to 29 °C and average precipitation is 13 mm. The main vectors for WNV are mosquito species of the Culex genus, but WNV has also been previously detected in several Aedes and Anopheles spp. [18]. The mosquito fauna in the Almaty region is represented by 20 species of genera Culex, Anopheles, and Aedes that are potentially able to transmit WNV to humans [19,20] (Cx. theileri, Cx. modestus, Cx. pipiens pipiens, Cx. p. molestus, An. maculipennis, An. pulcherrimus, An. claviger, An. messeae, Ae. caspius caspius, Ae. c. dorsalis, Ae. straminaeus, Ae. cantans, Ae. flavescens, Ae. cyprius, Ae. pionips, Ae. pullatus, Ae. catarphylla, Ae. leucomelas, Ae. vexans, Ae. cinereus). Aedes spp. mosquitoes become active in late March – early April and reach their peak numbers in June. The species of genera Culex and Anopheles appear in middle April reaching two peaks in numbers in early June and in the end of July [21].
Performed retrospective serological analysis showed the absence of anti-WNV IgM and high titers of anti-WNV IgG in both patients. Although detection of IgG alone does not indicate acute infection, but rather a prior infection, the obtained results raise suspicion for WNV endemicity in this region. Also, this study was limited by the unavailability of neutralizing antibody testing and by the absence of convalescent-phase serum samples. Possible cross-reactivity with tick-borne encephalitis virus reported earlier [22] has been excluded due to negative serological results. Also, both patients live in Eskeldinsky district, which borders northwest China, where WNV is widely distributed [23]. Thus, we can assume the background of WNV infection in both patients.
There is no specific treatment for WNV disease [24] and most of the viruses that cause meningitis [25]. Patients with severe meningeal symptoms often require pain control for headaches and antiemetic therapy and rehydration for associated nausea and vomiting [24]. In the observed cases, patients responded favorably to unspecific supportive therapy and recovered within 8–10 days from neurological sequelae.
The results of the study raise suspicion of WNV circulation in the southern region of Kazakhstan. Therefore, patients with meningoencephalitis of suspected viral etiology in the area must also be evaluated for WNV.
Conclusion
To our knowledge these cases represent the first description of acute meningoencephalitis due to WNV infection in the Almaty region, Kazakhstan. These cases illustrate that a compatible clinical syndrome and seasonal context for WNV should prompt testing for the presence of WNV immunoglobulins in the serum and CSF. More research and surveillance studies are needed to understand the true incidence and prevalence of WNV infection in the southern region of Kazakhstan.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
AD: played a key role in the cases analysis and interpretation, contributed to the conception and design, supervised, and reviewed manuscript. YO: prepared manuscript. AS: performed the diagnosis, treatment, and follow-up of the patients; ANiz: and YO: performed ELISA. AZ and YP: assisted in the preparation of the manuscript. ZhB: performed figure. AS, RE, LY, and FI: performed data collection. ANeu: provided sera samples collection and storage. All authors have reviewed and approved the manuscript.
Author statements
The authors declare that informed consent was obtained from the patients upon admission for the treatment, laboratory testing, and data analysis. The privacy rights of human subjects were observed. The local ethics committee of the National Center for Biotechnology has approved the study.
Sources of funding
This study was supported by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan, Kazakhstan, under Grant #AP05135904 "Study of the spread of various genotypes of the virus and the risks of infection of people with West Nile Fever in Kazakhstan".
Consent
Written informed consent was obtained from the patients for the treatment, laboratory testing, and data analysis. The privacy rights of human subjects were observed. The local ethics committee of National Center for Biotechnology has approved the study.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors wish to acknowledge all the patients participating in this study and Gabit Iniyatov, Tekeli city Hospital, Kazakhstan, for his valuable assistance in providing West Nile Fever cases and clinical data described in this report.
References
- 1.Centers for Disease Control and Prevention . 2019. West Nile virus.http://www.cdc.gov/westnile/ [Google Scholar]
- 2.Hayes E.B., Komar N., Nasci R.S., Montgomery S.P., O’Leary D.R., Campbell G.L. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sejvar J.J. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6:606–623. doi: 10.3390/v6020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chancey C., Grinev A., Volkova E., Rios M. The global ecology and epidemiology of West Nile virus. Biomed Res Int. 2015;2015 doi: 10.1155/2015/376230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyler K.L. West Nile virus infection in the United States. Arch Neurol. 2004;61:1190–1195. doi: 10.1001/archneur.61.8.1190. [DOI] [PubMed] [Google Scholar]
- 6.Mavridis K., Fotakis E.A., Kioulos I., Mpellou S., Konstantas S., Varela E. Detection of West Nile Virus—Lineage 2 in Culex pipiens mosquitoes, associated with disease outbreak in Greece, 2017. Acta Trop. 2018;182:64–68. doi: 10.1016/j.actatropica.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Martinez D., Murray K.O., Reyna M., Arafat R.R., Gorena R., Shah U.A. West Nile Virus outbreak in Houston and Harris County, Texas, USA, 2014. Emerg Infect Dis. 2017;23:1372–1376. doi: 10.3201/eid2308.170384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fros J.J., Geertsema C., Vogels C.B., Roosjen P.P., Failloux A.B., Vlak J.M. West Nile Virus: high transmission rate in North-Western European mosquitoes indicates its epidemic potential and warrants increased surveillance. PLoSNegl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger S. Gideon Informatics Inc; Los Angeles: 2019. West Nile fever: global status; pp. 86–89. [Google Scholar]
- 10.Maikanov N.S., Ayazbaev T.Z. Vol. 2. 2016. Epidemic value and specific structure of mosquitoes (Culicidae) of the western Kazakhstan (in Russian) pp. 45–48. (Epidemiology, ecology, clinic, diagnostics and prevention of natural focal infections). [Google Scholar]
- 11.Grazhdanov A.K., Ayazbaev T.Z., Toporkov A.V., Bidashko F.G., Zakharov A.V., Belonozhkina L.B. Concerning the allocation of emerging natural foci of the currently important infectious diseases in the West of Kazakhstan (In russian) Probl Osobo Opas Infekc. 2014;3:20–24. [Google Scholar]
- 12.Kulemin M.V., Sailaubekuly R., Abishova G.K., Abildaev K.B., Shaimerdenova B.E. The researching of some arboviruses in southern Kazakhstan in 2017 (in Russian). Paper Presented at the West Kazakhstan Regional Scientific Practical Conference «Epidemiological Surveillance of Natural Focal Infections. the Ecology of Carriers and Vectors. Biosafety»; March, Uralsk, Kazakhstan; 2018. pp. 13–14. [Google Scholar]
- 13.Rossi S.L., Ross T.M., Evan M.D. West Nile virus. Clin Lab Med. 2010;30:47–65. doi: 10.1016/j.cll.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zh Bekshin, Esmagambetova A., Ahmetov V. Scientific and Practical Center for Sanitary and Epidemiological Expertise and Monitoring" of the Ministry of Healthcare of the Republic of Kazakhstan; Almaty: 2019. Sanitary and epidemiological situation in the Republic of Kazakhstan in 2018 (in Russian) pp. 34–36. [Google Scholar]
- 15.de Figueiredo M.L., Figueiredo L.T. Review on infections of the central nervous system by St. Louis encephalitis, Rocio and West Nile flaviviruses in Brazil, 2004–2014. Adv Microbiol. 2014;4:955–961. [Google Scholar]
- 16.Sejvar J.J., Marfin A.A. Manifestations of West Nile neuroinvasive disease. Rev Med Virol. 2006;6:209–224. doi: 10.1002/rmv.501. [DOI] [PubMed] [Google Scholar]
- 17.Soares C.N., Castro M.J.C., Peralta J.M., Freitas M.R.G., Pucciono-Sohler M. Is West Nile virus a potential cause of central nervous system infection in Brazil? Arq Neuropsiquiatr. 2010;68:761–763. doi: 10.1590/s0004-282x2010000500016. [DOI] [PubMed] [Google Scholar]
- 18.Martinet J.P., Ferté H., Failloux A.B., Schaffner F., Depaquit J. Mosquitoes of North-Western Europe as potential vectors of arboviruses: a review. Viruses. 2019;11:1059. doi: 10.3390/v11111059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilibic-Cavlek T., Savic V., Petrovic T., Toplak I., Barbic L., Petric D. Emerging trends in the epidemiology of West Nile and Usutu virus infections in Southern Europe. Front Vet Sci. 2019;6:437. doi: 10.3389/fvets.2019.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Öncü C., Brinkmann A., Günay F., Kar S., Öter K., Sarikaya Y. West Nile virus, Anopheles flavivirus, a novel flavivirus as well as Merida-like rhabdovirus Turkey in field-collected mosquitoes from Thrace and Anatolia. Infect Genet Evol. 2018;57:36–45. doi: 10.1016/j.meegid.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Koyshybaeva G.S. Blood sucking mosquitoes (Culicidae) in the piedmont steppes of the Northern Tien Shan (in Russian) Eurasian Journal of Ecology. 2016;33:266–267. [Google Scholar]
- 22.Lu Z., Fu S.H., Cao L., Tang C.J., Zhang S., Li Z.X. Human infection with West Nile Virus, Xinjiang, China, 2011. Emerg Infect Dis. 2014;20:1421–1423. doi: 10.3201/eid2008.131433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen L.R., Marfin A.A., Gubler D.J. WestNile virus. JAMA. 2003;290:524–528. doi: 10.1001/jama.290.4.524. [DOI] [PubMed] [Google Scholar]
- 24.Petersen L.R., Brault A.C., Nasci R.S. West Nile virus: review of the literature. JAMA. 2013;310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarrin I., Sellier P., Lopes A., Morgand M., Makovec T., Delcey V. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Bull Sch Med Univ Md. 2016;95:e2372. doi: 10.1097/MD.0000000000002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.