Abstract
Although traditional anaerobic digestion (AD) process to produce methane-rich biogas from wet waste is deep-rooted, high carbon footprint and its low value as compared with other renewable sources demand advanced strategies to avoid its production. An emerging conversion pathway to arrest methanogenesis for producing value-added fuels and chemicals instead of biogas is sought as a sustainable alternative. This research provides a comprehensive analysis on current technology development, process challenges, applications, and economics for producing high-value short-chain carboxylic acids from AD of wet wastes. We show that (1) the theoretical energy yields of acids equal or exceed biogas, and (2) the cost of these acids is competitive with those produced from chemical markets, making this economically viable for mass production. With global abundance of wet waste feedstocks, this process of short-chain acid production provides a promising alternative to conventional biogas production technology, while achieving waste management and carbon mitigation goals.
Subject Areas: Environmental Chemistry, Energy Resources, Energy Sustainability
Graphical Abstract
Environmental Chemistry; Energy Resources; Energy Sustainability.
Introduction
The rise in global population and urbanization has increased total waste generation, causing the need for already limited waste disposal sites, potentially leading to unsanitary conditions affecting human health and exacerbating pollution across the globe through increased use of fossil fuel to meet growing energy demand (Kataki et al., 2017, Mazur, 1994, Stern, 2011, Zabel, 2009). With a finite supply of conventional fossil raw materials and environmental implications caused by their use, research efforts are paving the way to more environmentally friendly, economically viable, and technically feasible alternative energy sources (Koberg and Gedanken, 2012). One option gaining increased attention is the recovery of valuable products from organic-rich waste streams, using waste-to-energy (WTE) pathway. Waste is a pressing environmental challenge worldwide, but with its high energy potential, WTE can provide a sustainable path toward reducing fossil consumption and waste valorization. Conversion of wet waste feedstocks into transportation fuels and chemicals represents a significant opportunity for transforming these underutilized resources (Psomopoulos et al., 2009, Brunner and Rechberger, 2015, Kothari et al., 2010, Beyene et al., 2018).
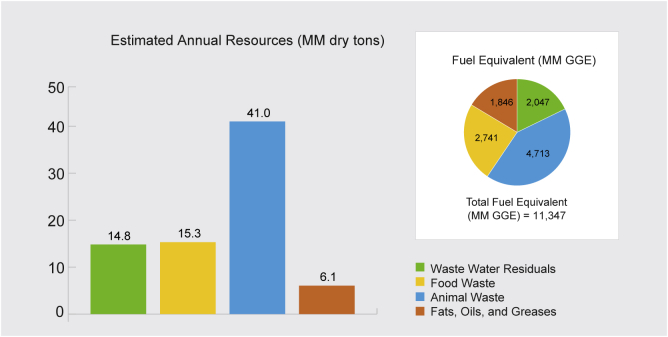
Wet wastes often include wastewater sludge; food waste; animal waste; and fats, oils, and greases (FOG) (see Supplemental Information, section Supplemental Wet Waste Feedstocks: The Untapped Potential) (BETO, 2017). The U.S. Department of Energy estimates approximately 50 million dry tons of combined wet organic wastes in the United States are available annually for conversion to biofuels, bioproducts, or biopower, representing an untapped energy potential of nearly 0.7 quadrillion British thermal units (Btu) (BETO, 2017). Moreover, some of these wastes can be available at negligible or negative prices (avoided tipping fees). Badgett et al. (2019) estimates about 61% of sewage sludge, 27% of animal manure, and 7% of food waste may be potentially available in the United States at negative prices in areas with dense population and high landfill tipping fees. Figure 1 shows the distribution of waste in the United States along with individual energy potential in gallons gasoline equivalent (GGE), resulting in an annual energy resource of 11.3 billion GGE (Skaggs et al., 2018).
Figure 1.
Estimated Wet Waste Quantity and its Fuel Equivalent in the United States (Skaggs et al., 2018)
The bar chart represents the estimated million (MM) dry tons of wet waste, and the embedded pie chart shows the fuel equivalent values of all the wastes in MM gallons gasoline equivalent (GGE).
The most common practices of waste management include (1) direct disposal, landfilling, and incineration (Liu et al., 2015, Rushton, 2003), (2) composting and production of animal feed (Capson-Tojo et al., 2016), and (3) anaerobic digestion (AD) to produce biogas (methane, or CH4, and carbon dioxide, or CO2). Disposing and landfilling are unsustainable, as their use leads to environmental issues such as greenhouse gas emissions (GHG) and odor production (Capson-Tojo et al., 2016), whereas combustion and incineration of waste produces toxic pollutants that are suspected human carcinogens (Rushton, 2003) and are usually present in the form of particulates, gases, and metals (Harrad and Harrison, 1996). Composting and animal feed production by recycling organic matter are potentially better options but produce low-value products and promulgate risk for disease dispersal.
In contrast, all these wet wastes can be converted into renewable fuels (including diesel and aviation fuels), biochemicals, biopower, and many other products. With abundance of wet waste availability, researchers have been focusing on arresting the methanogenesis step in the AD process to produce short-chain carboxylic acids such as volatile fatty acids (VFAs) and lactic acids, which can further be used to produce hydrocarbons or chemicals (Agler et al., 2011, Chang et al., 2010, Kleerebezem and Van Loosdrecht, 2007, Lee et al., 2014, Yuan et al., 2006). Thus, by utilizing the untapped potential of wet wastes, we could create an environmentally sustainable pathway to meet the challenges associated with growing energy demand and help construct the bioeconomy of the future (BETO, 2017).
This study provides a comprehensive and multidisciplinary review on advanced pathways for production of high-value products such as lactates and VFAs (referred to as “short-chain carboxylic acids”) from wet wastes including sludge from wastewater treatment (WWT) plants, food waste, swine manure, and FOG as an alternative to conventional biogas. The focus provides quantitative analysis on both technical and economic perspectives of wet waste-to-short-chain carboxylic acid conversion strategies, considering the fundamental limitation of wet waste conversion theoretical boundaries to value-added chemicals. The insights on the current technology status, process challenges, and economic and market potentials are summarized in the study to provide recommendations for wet waste-to-short-chain carboxylic acid conversion strategies on technology applications, commercialization, and technology features for future research and development guidelines as an alternative to biogas production.
Biogas or Carboxylic Acids: What is the Valorization Pathway?
Conventional AD
AD is a natural process where microorganisms break down organic materials in absence of oxygen to produce biogas, a useful renewable source. Biogas is mainly composed of 40%–75% CH4 and 15%–60% CO2 by volume with small amounts of hydrogen (H2), nitrogen, hydrogen sulfide, oxygen, and water (Ryckebosch et al., 2011). It is a decomposition process that naturally occurs in swamps, water-logged soils, rice fields, and digestive systems of termites and large animals (Olsson, 2012). It is an established technology that has been commercialized in municipal WWT plants since the early 1900s. Since then, it has been applied to treating different types of organic waste including food waste, yard waste, and process residues, providing a clear path to waste management while reducing dependency on conventional sources.
The traditional AD process involves four biological steps that include hydrolysis, acidogenesis, acetogenesis, and methanogenesis. Hydrolysis breaks down insoluble matter to simple monomers via hydrolytic bacteria, whereas the acidogenesis and acetogenesis convert simple sugars and acids to carboxylic acids, alcohols, CO2, and H2 via acidogens and other bacteria. The final methanogenesis step converts these acetates and H2 to biogas via methanogenic bacteria, which is the final product of the process.
Although the production of biogas using AD technology provides an environmentally sustainable approach when compared with landfills, there are several problems associated with the process and its use. These include the possibility of GHG emissions during biogas handling operations, additional costs for biogas refining to remove impurities for use in applications, and cheap availability of natural gas (see Supplemental Information section Supplemental Issues Pertaining to Biogas Production). Also, the second largest constituent in biogas is CO2, which translates to carbon being lost to unusable gas (although there has been some research on converting this CO2 to methane) (Diender et al., 2018), thereby reducing carbon and subsequently energy efficiency. With these obstacles in mind, approximately 250 plants out of 1,500 digesters located at WWT facilities in the United States utilize biogas for heat and/or power, whereas others simply flare the biogas. Therefore, research efforts have been focused on production of higher-value products as opposed to biogas that would reshape the traditional AD process industry and fundamentally improve total energy yields.
Arrested Methanogenesis
To achieve the goals of both economic development and environmental sustainability, development of advanced technologies by utilizing an integrated approach to resource management for producing high-value end products must be a priority. One alternative and a more advanced strategy to avoid the production of biogas is to arrest methanogenesis to a degree so that intermediate short-chain carboxylic acids are produced instead, as a direct precursor to high-value biofuel and bioproduct precursors (BETO, 2017, Tamis et al., 2015). In addition, studies have shown H2 to be produced biologically by fermentation of organic wastes (also referred as “dark fermentation”) (Łukajtis et al., 2018, Batista, 2014, Pecorini et al., 2019), although thermodynamic limitations due to inoculum instability and issues pertaining to optimization of operational parameters for maintaining microbial consortia for continuous H2 production are bottlenecks restricting the hydrogen yield and productivity (Sivagurunathan et al., 2016, Ren et al., 2011).
Arrested methanogenesis refers to different approaches that inhibit or primarily block methanogenesis while promoting carboxylic acid production in acidogenesis and acetogenesis steps. There are several proven strategies to arrest methanogenesis, including change in operating parameters of the process (e.g., pH, temperature, and retention time) to increase activity of acid-forming bacteria (Jankowska et al., 2018), use of an inhibitor to prohibit methanogen activity (Lukitawesa et al., 2020), and use of enhanced waste pretreatment technologies (Yuan et al., 2019, Yin et al., 2014).
Short-Chain Acids
Short-chain carboxylic acids range from C2 to C4 acids such as acetic acid (C2), propionic acid (C3), lactic acid (C3), butyric acid (C4), and succinic acid (C4)—which are potential renewable carbon sources that can be used to produce fuels or chemicals (Chang et al., 2010, Wang et al., 2014). They are used in a variety of applications, including polymers, food additives, pharmaceutical products, and cosmetics.
Carboxylic acids are produced from microbial carbohydrate digestion in ruminants and other herbivores, as a naturally occurring concept, without any need for external enzymes to break down cellulose and complex carbohydrates (Bergman, 1990, Aluwong et al., 2010). Conventionally, carboxylic acids are commercially derived from fossil-based sources through chemical routes (Huang et al., 2002, Eggeman and Verser, 2005). For example, acetic acid is produced through carboxylation of methanol, butyric acid from chemical synthesis through oxidation of butyraldehyde (obtained from propylene), and propionic acid from direct oxidation of hydrocarbons or carboxylation of ethylene with carbon monoxide and water.
Utilizing organic-rich wastes (Figure 1) to produce carboxylic acids would help eliminate waste generated around the world (Akaraonye et al., 2010). It would also help reduce concerns pertaining to using glucose and sucrose as a main carbon source to produce carboxylic acids (Zigová et al., 1999, Kondo and Kondo, 1996). Also, the short-chain acids from waste sources would help reduce GHG emissions and energy and chemical demand, which are typically higher if produced from petroleum-derived sources (Besselink et al., 2017, Wu et al., 2016). Moreover, with the increasing price of oil and scarcity of non-renewable sources, alternative routes such as wet wastes for producing carboxylic acids would be a cost-effective and environmentally friendly option.
Although proven, there are several challenges with arrested AD technology that include identification of microbial consortia for conversion of complex organic streams, low productivity of carboxylic acids, acid toxicity (presence of a high concentration of minerals and carboxylates), and high cost of recovery and separation. There has been progress toward addressing some of these challenges, but a great amount of effort is still needed to move toward commercialization. These challenges are addressed in the next section.
Technical Perspectives: Toward Short-Chain Acid Production
Carboxylic Acid Titers and Yields Using Arrested Methanogenesis
Several studies have demonstrated the feasibility of such strategies by controlling redox, pH, and other operational conditions, leading to production of C2-C6 acids (Lee et al., 2014, Chen et al., 2008). Table 1 summarizes the acid products, product titers, mass, and energy yields from different literature along with the feedstocks used in the arrested AD process. Compared with sludge and food waste, fewer studies investigated swine manure and FOG digestion.
Table 1.
Summary of Literature Values for Short-Chain Acid Composition, Titer, Mass, and Energy Yield
| Feed | Carboxylic Acids | Carboxylic Acid (g/L) | Carboxylic Acid Yield (g/g VS Fed) | Carboxylic Acid Energy Yield (%) | Reference |
|---|---|---|---|---|---|
| Sludge | nr | 10.7 | 0.3 | 42.6 | Rughoonundun et al., 2012 |
| Sludge | nr | nr | 0.11–0.32a | na | Ahn and Speece, 2006 |
| Sludge | AA, BA, VA | 10.7 | 0.34 | 43 | Rughoonundun et al., 2010 |
| Sludge | AA, PA, VA, BA | nr | 0.077–0.141b | na | Cagnetta et al., 2016 |
| Sludge | AA, PA, VA | 3.5a | 0.302b | na | Wu et al., 2009 |
| WAS | nr | nr | 0.159–0.235—Untreateda 0.14–0.24—Pretreateda |
17.1–25.3—Untreated 15.3–25.9—Pretreated |
Ma et al., 2016 |
| WAS | AA, PA, VA | 0.90–1.77 | 0.17–0.31a | 18.3–36.1 | Li et al., 2014 |
| WAS | AA, PA | nr | 0.185–0.421b | na | Luo et al., 2014 |
| WAS | AA, PA, VA | nr | 0.298–0.368b | na | Zhang et al., 2009a |
| Primary sludge & WAS | nr | 4.9–21.6 | 0.15–0.78a | 25.1–64.9 | Morgan-Sagastume et al., 2011 |
| Primary sludge & WAS | AA | nr | 0.54–0.62 | 54.2–61.9 | Jankowska et al., 2015 |
| Primary, secondary sludge, & WAS | nr | nr | 0.44b | 45 | Khiewwijit et al., 2015 |
| Bagasse & sludge | AA, BA | 15.08–60.8 | 0.36–0.45 | 38.4–46.2 | Rughoonundun and Holtzapple, 2017 |
| Bagasse & sludge | AA, BA | 23.2 | 0.23 | 26.3 | Rughoonundun et al., 2010 |
| MSW & SS | AA, BA | 16.3–26.0 | 0.175–0.276 | 25.4–40 | Aiello-Mazzarri et al., 2006 |
| MSW & SS | AA, PA | nr | 0.17–0.389 | 23.3–53.4 | Ross and Holtzapple, 2001 |
| MSW & SS | AA, BA | 10.7–20.5 | 0.15–0.41 | 16.5–51.9 | Chan and Holtzapple, 2003 |
| MSW & SS | AA, PA, BA | 13.6–22.2 | 0.095–0.197 | 12.7–25.7 | Aiello-Mazzarri et al., 2005 |
| FW | AA, BA | 3.94–39.46 (pH) | 0.032–0.316 | 2.3–27.6 | Jiang et al., 2013 |
| AA, PA, BA | 14.9–47.89 (temp) | 0.137–0.44 | 12.5–34.6 | ||
| AA, PA, BA, VA | 12.98–24.93 (OLR) | 0.261–0.504 | 19.2–42.8 | ||
| FW (tofu and egg white) | AA, PA, BA, VA | 7.28–21.07 | 0.16–0.46 | 12.2–36.1 | Shen et al., 2017 |
| FW | LA | nr | 0.23–0.27 (wet basis) | 51.8–60.9 | Kwan et al., 2016 |
| FW & WAS | nr | nr | 0.186—WASa; 0.435—FWa; 0.692—Combineda | 52.9—Combined | Chen et al., 2013c |
| FW & excess sludge | nr | nr | 0.168—Sludge; 0.315—FW; 0.867—Combined | 66.8—Combined | Wu et al., 2016 |
| FW & sludge | BA, AA | nr | 0.124–0.918c | 5.2–52.3 | Wang et al., 2014 |
| Cattle manure | AA, PA, CA | nr | 0.158–0.24 | 24.2–34.1 | Ross and Holtzapple, 2001 |
| Chicken manure | nr | nr | 0.327d | na | Smith and Holtzapple, 2011 |
| Swine manure | AA, PA, VA | nr | 0.09–0.12a | na | Huang et al., 2016 |
| Rice straw & chicken manure | AA, BA, CA | 25–40.8 | 0.16–0.29 | 32.1–59.9 | Agbogbo and Holtzapple, 2007 |
| Swine manure & corn stover | AA, BA | 15.2–25.1 | 0.19–0.38 | 26.2–47.6 | Chan et al., 2011 |
| Bagasse & chicken manure | AA, BA | 15.5–28 | 0.11–0.18 | 15.1–25.7 | Fu and Holtzapple, 2010 |
| Bagasse & chicken manure | AA, BA, PA | 28.3–40.2 | 0.26–0.47 | 34.7–62.9 | Fu and Holtzapple, 2011 |
| Sugarcane trash & chicken manure | AA, BA | 18.4–29.9 | 0.23–0.36 | 36.7–65.9 | Nachiappan et al., 2011 |
| Paper & chicken manure | nr | nr | 0.129–0.183 | 20.4–28 | Smith et al., 2011 |
| Paper & chicken manure | nr | 7.9–14.5 | 0.159–0.481 | 20.4–62.3 | Golub et al., 2012 |
| Freshwater microalgae | AA, PA, BA, VA | 3.6–14.7 | 0.115–0.462 | na | Zhao et al., 2016 |
| Water hyacinths | AA, PA, BA, VA, CA | 8.0–19.9 | 0.12–0.3 | na | Forrest et al., 2010 |
| Paper & yeast | BA, AA | 13.8–16.6 | 0.08–0.09 | 14.1–16.6 | Forrest et al., 2012 |
| Cheese whey | nr | 9.27–16.65 | 0.80–0.85e | na | Domingos et al., 2017 |
| Corn fiber | BA | 11.1 (6.6—BA) | 0.56 (0.33—BA)a | 58.8 | Agler et al., 2012 |
| Kitchen waste | AA, BA | 36 | 0.262 | 23.2 | Zhang et al., 2005 |
VS = volatile solids, MSW = municipal solid waste, SS = sewage sludge, AA = acetic acid, BA = butyric acid, PA = propionic acid, CA = caproic acid, VA = valeric acid, nr = not reported, na = data not available, FW = food waste, LA = lactic acid, WAS = waste activated sludge, OLR = organic loading rate. Note that data not available is included when the yield (g/g VS fed) is available but the composition of carboxylic acids or the lower heating value of component (feed or specific acid) is not available to estimate the energy yield.
Value expressed in g carboxylic acid/g non-acid volatile solids fed.
Value expressed in chemical oxygen demand (COD) units.
Value expressed in g carboxylic acid-COD/g volatile suspended solids (VSS) fed.
Value expressed in g carboxylic acid-COD/g lactose.
Value expressed in g carboxylic acid/g VSS (removed).
The acid product distribution varies significantly with variation of feedstocks and arrested AD technologies. One typical composition of carboxylic acids from wastewater sludge digestion ranges from 30%–65% for acetic acid, 15%–30% for propionic acid, 15%–35% for butyric acid, and 10%–30% for valeric acid. However, high ranges of acetic (70%–80%) and propionic (10%–15%) acids have also been reported from sludge based on varying operational parameters. For food waste digestion, the composition of acetic acid is reported to be higher, in the range of 50%–80%, with 10%–20% propionic acid and butyric acid at lower pH values (<5). The high concentration of acetic acid results from higher carbohydrate and protein concentrations in food waste as compared with sludge.
The product titers of carboxylic acids depend on the loading rate and digester operation mode. For example, acid titers from sludge and food waste digestions are in the range of 1–22 grams per liter (g/L) and 4 to 48 g/L, respectively. As food waste has high soluble organic content and better digestibility, the concentration of carboxylic acids is typically higher as compared with sludge. Co-processing with various organic wastes often reaches higher product titers; up to 61 g/L have been reported (Rughoonundun and Holtzapple, 2017). Higher product titers would often result in high product yield. When food waste is co-digested with sludge, the highest reported mass yield is 0.9 g/g VS. The high product yield is a result of higher amounts of hydrolytic and acidogenic bacteria (Wang et al., 2014).
The production values, however, vary greatly based on feedstock type, operational parameters, type of fermentations such as batch or countercurrent, ratio of co-fermenting feedstocks, and inoculum used for the AD process (Kleerebezem et al., 2015). The carboxylic acid energy yield can reach in excess of 60% for sludge as shown by Jankowska et al. (2015) and Morgan-Sagastume et al. (2011). Also, the energy yield for lactic acid through a fungal hydrolysis process can reach up to 61% for food wastes as shown by Kwan, Hu, and Lin (Kwan et al., 2016). In addition, co-fermenting sludge with food waste yields higher acid concentration with an energy yield of 53% and 67%, as shown by Chen et al. (2013c) and Wu et al. (2016), respectively. To further understand the importance of arresting methanogenesis by varying multiple process parameters, we estimate carboxylic acid theoretical energy yields as described in the section below.
Theoretical Energy Yields: Acids Versus Biogas
The compositional variability—such as amount of cellulose, lipid, protein, and lignin—and conversion of fermentable components plays a vital role in determining the superiority of carboxylic acid production as opposed to CH4 (in biogas) in terms of energy potential. Table 2 shows a summary of key compositions of four types of wet waste organic feedstocks utilized for carboxylic acid production.
Table 2.
Summary of Key Data for Four Types of Wet Waste Organic Feedstocks
| Parameters | Wastewater Sludge | Food Waste | Swine Manure | FOG |
|---|---|---|---|---|
| Typical scale (wet tons/day, unless noted) | 1–300 MGD | 1–250 | 1–250 | 1–200 |
| Ash | 7.5% | 5.0% | 15.2% | 0% |
| Lipids | 18.0% | 21.0% | 3.8% | 78.0% |
| Proteins | 24.0% | 19.0% | 20.0% | 7.0% |
| Fermentable carbohydrates | 16.0% | 55.0% | 36.5% | 15.0% |
| Lignin | 0% | 0% | 21.0% | 0% |
| Extractives (all non-fermentable components) | 34.5% | 0% | 3.5% | 0% |
| Energy density (MMBtu/DT) | 17.7 | 20.8 | 15.5 | 35.4 |
| Dry tons (MM) | 14.8 | 15.3 | 41.0 | 6.1 |
| Trillion Btu | 237.6 | 318.2 | 547.1 | 214.3 |
| Moisture content (%) | 96% | 75% | 93% | 6%–95% |
| TS (%) | Primary: 2%–6% | 25% | 7% | 5%–94% |
| Secondary: 2%–10% | ||||
| COD (mg/L) | ||||
| Range | 47,200–140,000 | 39,800–350,000 | 20,600–35,000 | 92,000–149,000 |
| Mean | 135,711 | 154,000 | 28,430 | 120,500 |
| Assumed COD reduction | 55.5% | 65.0% | 55.0% | 82.0% |
MGD = million gallons per day, DT = dry ton, MMBtu = million British thermal units, MM = million, TS = total solids, COD = chemical oxygen demand.
As described earlier, the main acids produced through the AD process are in the range of C2 to C4 acids with small quantities of C5 and C6 acids. Thus, we focus the analysis on lactic, acetic, propionic, butyric, and succinic acids in this study. In addition, H2 is a main coproduct along with carboxylic acids from the arrested AD process (Alibardi and Cossu, 2016, Grzelak et al., 2015, Zhang et al., 2015, Zhao et al., 2017). Considering the multiple uses and high energy density of H2—103.8 million Btu per ton (MMBtu/ton) (Toolbox, 2003, Tools, nd)—our estimates for theoretical energy yield of carboxylic acids also reflect H2 potential.
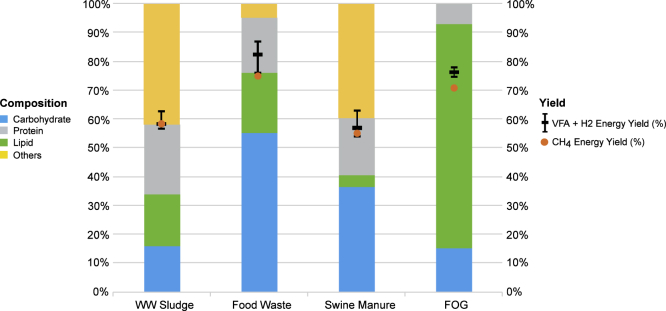
The energy yield is an important parameter to estimate the biogas production values for a given amount of waste. So, would energy yield for carboxylic acids be comparable with that for biogas? Based on the molar conversion ratio of fermentable components in the feed, we divide the weighted average heating values of short-chain carboxylic acids by the lower heating values (LHVs) of the feed to determine the theoretical energy yield of the acids. As illustrated in Figure 2, the theoretical energy yield for CH4 varies with the type of wastes, in comparison with energy yields for short-chain carboxylic acids. The compositions of fermentable components in each waste are also shown in Figure 2. The energy yield of CH4 is estimated at 58% for sludge, 74% for food waste, 54% for manure, and 70% for FOG.
Figure 2.
Theoretical Energy Yields for Carboxylic Acids as Compared with Methane
Left y-axis is percentage composition of the wastes and right y-axis is percentage energy yield of product to feedstocks. The black error bars indicate the variation of C2-C4 acid product distribution for each type of wet waste.
For acid production, high energy yields require not only high molar conversion of fermentable components in waste feedstocks, but also a product with high intrinsic LHV. For instance, butyric acid has the highest LHV among all C2–C4 acids studied here, which would result in a high energy yield assuming it is the sole product. The molar yields to short-chain carboxylic acids (C2–C4) are listed in Table 3. The molar yield from carbohydrate to acetic acid is 3 to 1, leading to high energy yields in food waste because of 55% carbohydrate content in its feed composition. Similarly, high lipid composition in FOG leads to high energy yields of either CH4 or carboxylic acids.
Table 3.
Molar Yields of Carbohydrate, Lipid, and Protein to Carboxylic Acids
| Molar Yield to Carboxylates | Carbohydrate | Lipid | Protein |
|---|---|---|---|
| Acetic acid, H2 | 3, 0 | 18, 73 | 0.22, 0.55 |
| Propionic acid, H2 | 1.5, 1.5 | 12, 61 | 0.15, 0.38 |
| Lactic acid, H2 | 2, 3 | 12, 73 | 0.15, 0.53 |
| Butyric acid, H2 | 1, 2 | 9, 55 | 0.11, 0.33 |
| Succinic acid, H2 | 1, 5 | 9, 82 | 0.11, 0.66 |
| CH4 | 3 | 36 | 0.44 |
The error bars in Figure 2 indicate the variation of acid product distributions. Thus, the energy yield of acids is estimated to exceed CH4 with values of 58%–63% for sludge, 76%–87% for food waste, 54%–63% for swine manure, and 75%–80% for FOG. This demonstrates the importance of exploring alternative approaches such as arrested AD, as energy in wet wastes can be sustained efficiently in carboxylic acids as compared with CH4.
Factors Impacting Acid Production
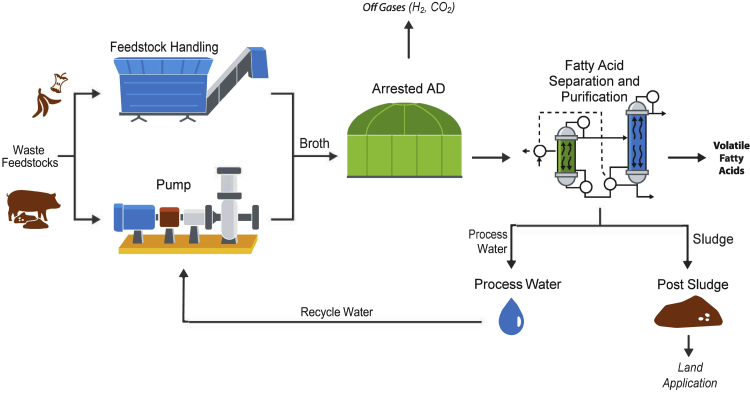
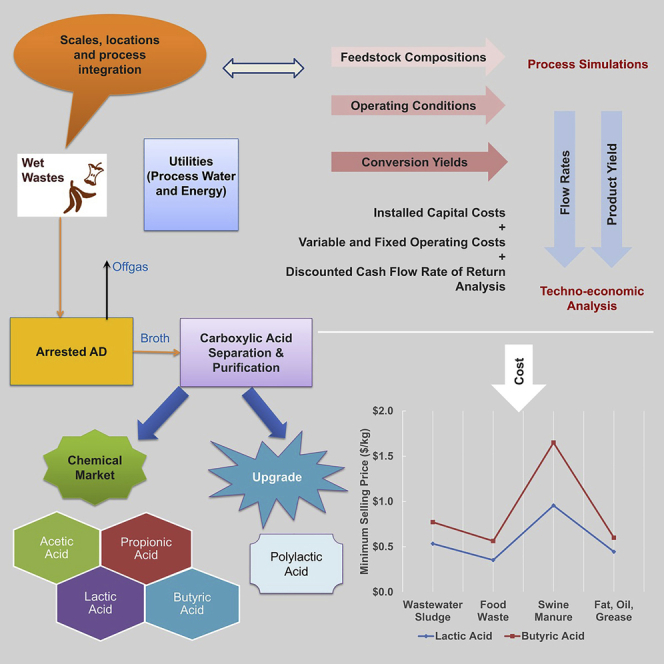
Figure 3 illustrates major unit operations for production of short-chain carboxylic acids through an arrested AD concept and its extraction from the fermentation broth.
Figure 3.
Major Unit Operations for Carboxylic Acid Production
After biomass enters the arrested AD unit, microorganisms can utilize it as an energy source to produce products by converting fermentable components to acids. In acidogenesis, the monomers formed in the hydrolytic phase are taken up by acidogenic bacteria to be degraded into short-chain carboxylic acids, alcohol, H2, and CO2. In this process, a group of microorganisms are inhibited before the methanogenesis stage so that no CH4 is formed, and the main products in the broth are a mixture of carboxylic acids. After acid production from the arrested AD, the broth is sent to the separation and extraction steps to selectively obtain acids from the mixture. The remaining unconverted sludge can be used for land application. Process water is routed back for reuse.
There are several approaches to increase carboxylic acid and simultaneously reduce biogas production during the biological conversion step. This includes inducing acidification by improving the rate of hydrolysis, increasing the amount of acidogens by promoting acidogenesis, and reducing methanogens by inhibiting methanogenesis (Yuan et al., 2006). Recent progress has been made in the new electrofermentation process, where electrodes are used to regulate the redox potential to inhibit methanogenesis (Jiang et al., 2019). The rate-limiting step for carboxylic acid production is hydrolysis (Wang et al., 2014, Li and Noike, 1992), and vital factors impacting its production include pH (Chen et al., 2007, Chen et al., 2013a), temperature (Li et al., 2014), redox potential (Jiang et al., 2018), and hydraulic retention time (HRT) (Jankowska et al., 2015). Other parameters impacting carboxylic acid production include organic loading rate (Yun and Cho, 2016), substrate pretreatment (Morgan-Sagastume et al., 2011), co-fermentation with other wastes (Wu et al., 2016), and feeding pattern (Zheng et al., 2010). Studies have also shown to produce H2 and CO2 along with carboxylic acids (Kleerebezem et al., 2015).
pH is one of the most important parameters impacting product yields. pH levels in the reactor highly impact the rate of hydrolysis and acidogenesis, which is one of the key approaches toward short-chain acid production (Begum et al., 2018, Zhao et al., 2018). Most of the acidogens cannot withstand extreme acidic (pH = 3) or alkaline (pH = 12) environments (Liu et al., 2012), thus it is necessary to maintain an optimal pH for inducing acidification depending on the type of wet wastes and acids of interest. Wu et al. (2009) found that acid productions from primary sludge are higher at alkaline conditions with the highest concentration of 3.5 grams of chemical oxygen demand per liter (g COD/L) at pH = 10, with acetic, propionic, and iso-valeric acids having the highest yields. Zhang, Chen, and Zhou (Zhang et al., 2009a) also studied the production of short-chain acids from waste-activated sludge at different pH conditions and found that acids do not withstand acidic (pH < 7) or strong alkaline conditions (pH > 9). Similarly for food waste, high yields were reported by Wang et al. (2014) from food waste at pH = 6, with acetate and butyrate as the main acids. Jiang et al. (2013) and Zhang et al. (2005) also showed high acid yields from kitchen waste at pH levels close to neutral (pH between 6 and 7) due to high hydrolytic enzyme activity and acidification and low methanogen activity (Strazzera et al., 2018). Huang et al. (2016) showed high acid yields from swine manure at slightly alkaline pH values (8–10) for a short-term AD process. Thus, pH is an influencing factor to induce acidification and inhibit methanogens for selectively producing acids (Garcia-Aguirre et al., 2017).
The AD process corresponds to a cascade of oxidation and reduction reactions carried out by consortia of microorganisms. As a closed system without external inputs of an energy source or electron acceptors, AD tends to reach a thermodynamic equilibrium, with CH4 as the main product because it has the lowest Gibbs energy change per electron than organic compounds. Similar to pH as a measure of proton activity, the redox potential corresponds to the NAD+/NADH ratio within cells, which represents their intracellular oxidation states and controls gene expression and enzyme synthesis for the overall cell metabolic activities (Sporer et al., 2017, Chen et al., 2008). Therefore, recent studies found redox potential could influence fermentation pathways and regulate the product spectrum. Hirano et al. (2013) reported that when a high-redox potential was maintained (+0.2 volts and −0.2 volts versus silver/silver chloride), methanogenesis by Methanothermobacter thermautotrophicus was effectively suppressed; when a low potential −0.8 volts was applied, CH4 production increased dramatically (Hirano et al., 2013). Jiang et al. (2018) investigated the effects of redox potential of the mixed culture anaerobic fermentation reactor and found that by increasing the potential from −1.0 volts to −0.2 volts (versus silver/silver chloride), methanogenesis was reduced by 68% and acetic acid generation was reduced by 33%. This redox-potential-based control presents a new approach to arrest methanogenesis.
Temperature is another important factor impacting acid production. The population of microbes and rate of hydrolysis are highly impacted by change in operational temperature (Zhou et al., 2018). Hao and Wang (Hao and Wang, 2015) and Zhang, Chen, and Zhou (Zhang et al., 2009a) found increased hydrolysis rates and carboxylic acid productions at thermophilic temperatures as compared with mesophilic conditions due to high proportions of carbohydrate and protein in sludge. Jiang et al. (2013) and Komemoto et al. (2009) found maximum acid yields from food waste at lower temperatures (35°C–45°C) demonstrating low solubilization and high acidogenesis with a sharp decrease at high-temperature conditions (>55°C). For both sludge and food waste, mesophilic temperatures favor acetic and propionic acid production, whereas thermophilic temperatures favor butyrate production (Zhang et al., 2009a, Jiang et al., 2013). In contrast, high yields were obtained by Huang et al. (2016) from AD utilizing swine manure with C2–C5 acids as the dominant products. Therefore, temperature conditions impact the microbial population, leading to selective acid formation depending on the type of waste.
HRT is an important operational parameter needed to determine the volume of the reactor used in the AD process. Depending on the type of waste and hydrolysis rate, studies have shown high carboxylic acid yields at long HRTs (Ben et al., 2011, Bengtsson et al., 2008, Sans et al., 1995). When using sludge as a feedstock, an HRT long enough to endorse hydrolysis and short enough to mitigate methanogen production is ideal for acid production (Ferrer et al., 2010). In contrast, Xiong et al. (2012) and Miron et al. (2000) have shown high yields at short HRT from sludge, with iso-valeric, acetic, and butyric as the dominant acids. For vegetable waste, a long HRT helps to increase VS reduction, producing a high concentration of acids (Bolaji and Dionisi, 2017). Similarly, for food waste, Lim et al. (2008) showed an increase in acid production when HRT was increased from 4 to 8 days; however, no significant difference was observed when HRT was further increased to 12 days due to acids being consumed by methanogens. Acetate and propionate were mainly produced for all HRTs, whereas butyrate production reached a maximum at an HRT of 8 days. Therefore, depending on the type of waste, the HRT should be fine-tuned to promote acidification and restrict methanogen activity for distributed acid production.
Apart from pH, redox potential, temperature, and HRT, other factors, including organic loading rate (OLR), substrate pretreatment, co-fermentation, and feeding pattern, also affect methanogen activity. The OLR—or amount of waste fed daily to the digester—shows similar behavior to acid yields as HRT. Studies have shown acid yields decrease with increasing OLR; however, its concentration increases linearly with OLR until a certain optimum rate with drastic reductions with any further increase in OLR (Lim et al., 2008, Oktem et al., 2006, Yu, 2001). In addition, the pretreatment of substrates (e.g., hydrothermal treatment or heat shock) have been shown to increase acid yields from sludge and food waste (Shanableh and Jomaa, 2001, Shen et al., 2016, Yin et al., 2014). Other types of pretreatment, such as acid, alkaline, biological, ozone, and ultrasound, have also been shown to increase the soluble content of COD by more than 25%, which helps to improve hydrolysis by improving enzymatic accessibility of the substrate (Devlin et al., 2011, Doǧan and Sanin, 2009, Kim et al., 2003, Kim et al., 2005, Cesaro and Belgiorno, 2013, Bougrier et al., 2006, Shahriari et al., 2012, Eskicioglu et al., 2008, Yang et al., 2010, Elbeshbishy et al., 2011, Cesaro et al., 2012, Sanders et al., 2000). Wu et al. (2016) have shown an increase in acid production when co-fermenting food waste with excess sludge. The study shows that co-fermentation effectively enhances hydrolysis and acidogenesis yield by increasing hydrolytic and acidogenic bacteria while inhibiting methanogens with self-maintaining pH at around 5.2 to 6.4. Nebot et al. (1995) compared different feeding patterns (continuous versus semi-continuous) and found that production of acids sharply decreases with increase in feed frequency (per day at constant intervals), suggesting a semi-continuous feeding pattern.
In addition to the individual effects of operational parameters, many studies have shown the combined effects to optimize carboxylic acids productions. For example, Jankowska et al. (2015) investigated the impact of pH and HRT on acid production during mixed culture fermentation and showed that acid concentrations were highest at long HRTs at alkaline conditions, whereas short HRTs were favorable at an acidic environment. Acetate was the main acid except at an acidic environment and short HRTs where propionate dominated.
Short-Chain Acid Recovery Methods
The separation or recovery of carboxylic acids drives the economics to sustainable and cost-effective applications. Industrial separation of carboxylic acids such as lactate, acetate, and succinate from the fermentation broth is typically expensive due to significant chemical and energy uses such as in neutralization and physical separation. For instance, in the industrial production of citric acid, the downstream recovery process accounts for 30%–40% of the production cost (Straathof, 2011, López-Garzón et al., 2017). Acid extraction from the fermentation broth will need to be followed by selective acid recovery from the mixture of short-chain carboxylic acids, increasing recovery costs. The solvent that may be used in the pertractive membrane for the extraction of acids could be lost downstream due to ineffective recovery of acids, which increases the separation cost. Also, an additional filtration step to remove ash from the recycled solvent stream increases the overall cost. Moreover, membranes are proven to recover long-chain acids (Outram and Zhang, 2018), so the short-chain carboxylic acids may have losses that increase the cost of separation.
Some of the current and most effective recovery methods include liquid-liquid extraction, membrane separation through electrodialysis, precipitation, distillation, adsorption through ion exchange, and absorption. In addition, the separation of acids in batch mode through resin adsorption and solvent elution are also well known. Other technologies based on extractant or sorbent auxiliary phase include an ion-exchange method that uses ionic liquids to extract acids with greater extraction efficiency (López-Garzón et al., 2017, Reyhanitash et al., 2016, Zhao et al., 2005) and an in situ product recovery method that can be coupled with other separation techniques such as liquid-liquid extraction to recover short- (C2–C4) and medium-chain (C5–C6) carboxylic acids using a combined in situ product recovery/membrane technology (Saboe et al., 2018). Moreover, several novel methods being researched also include membrane separation through nanofiltration, reverse osmosis, membrane contractors, and pervaporation.
Liquid-liquid extraction is a well-established method that uses relative solubility to separate immiscible liquids (Atasoy et al., 2018). Anionic solvents such as alcohols, ketones, or ethers are used to extract carboxylic acids from the fermented broth (Strazzera et al., 2018, Singhania et al., 2013). High extraction efficiencies in the range of 61%–98% have been observed by Alkaya et al. (2009) while using sugar-beet processing wastes, although the pH of the solution might increase during extraction, making it difficult for recovery if it is not controlled (Reyhanitash et al., 2016). A feasible and popular alternative is membrane separation through electrodialysis where carboxylic acids are transferred from one solution to another through a voltage difference between two electrodes with recovery efficiencies of up to 99% (Jones et al., 2015, Huang et al., 2007). However, high costs of membranes, energy requirements, and fouling may limit its application (Huang et al., 2007, Singhania et al., 2013).
Another widely accepted method is precipitation of calcium lactate with calcium hydroxide to recover lactic acid from the fermentation broth. Although common, this method requires large quantities of sulfuric acid and lime and accounts for 50% of the production costs (Wasewar et al., 2003). Ammonia or alcohols have also been utilized to recover carboxylic acids using a distillation process where the mixture is separated based on a difference in volatility. Kumar et al. (2006) have shown the extraction of lactic acid through esterification and hydrolysis in a reactive distillation-based process. However, research is still needed to lower energy demand and capital costs for economic separation and recovery using distillation (Andersen et al., 2015). Another prevelant method is adsorption where acids are recovered from the broth through physical interaction with absorbents such as ion exchange resins (Rebecchi et al., 2016), with yields ranging from 76% to 85% (Atasoy et al., 2018). Although reliable, the regeneration of ion exchange resins and waste stream disposal still needs consideration to make this method cost effective (Wasewar et al., 2004). Air or gas stripping absorption, generally used to remove contaminants from wastewater, has also been applied for acid recovery by Li et al. (2015) where a combination of nitrogen gas stripping with calcium carbonate slurry was used to recover C2 and C4 acids.
Other novel methods for carboxylic acid recovery include reverse osmosis and nanofiltration that basically use semipermeable membranes to separate solute from the solvent based on size and pressure (Zhou et al., 2013); however, the recovered acids are not concentrated (Zacharof and Lovitt, 2013). Studies have shown retention rates up to 90% using nanofiltration membranes depending on the pH, temperature, pressure, and solute concentration (Zacharof et al., 2016, Xiong et al., 2015). Aydin, Yesil, and Tugtas (Aydin et al., 2018) recently investigated the recovery of carboxylic acids from landfill leachate and fermentation broth of anaerobically digested organic waste using a permeation membrane contractor and found recovery efficiency of greater than 86% and 95%, respectively. Another study showed the extraction of acids using a bioelectrochemcial system, such as microbial electrolysis cells that uses microorganisms attached to electrodes to catalyze oxidation or reduction reactions (Cerrillo et al., 2016). Recently, pervaporation separation was investigated for selective reovery of acetic acid from the fermentation broth, but high operating costs limit their application (Woo and Kim, 2019, Chen et al., 2013b).
Economic Perspectives
Economic Potentials of the Arrested AD Technology
Economic perspectives of acid productions provide insights and guidance for emerging R&D needs. The economics of short-chain carboxylic acids are studied based on the range of estimated theoretical energy yields (Figure 2), mass yields, and future market demand. The supporting technical assumptions pertaining to product titer, yield, carboxylic acid separation efficiency, and COD reduction are described in Table 4. Undoubtfully, improving the acid production from arrested AD unit operation is a key process metric to improve overall process economics. Moreover, taking advantage of existing AD units could also bring economic benefits. We consider these technical and economic assumptions in the Aspen Plus (AspenPlus, 2007) software-based techno-economic analysis (TEA) model and consider the change is operating parameters to improve the performance of the microorganism consortium through synthetic biology and genetic engineering to overcome technical challenges stated in the technical perspective section for targeting the selective acid production. With regard to acid separation, an in situ product recovery unit during biological conversion is capable of not only pulling reaction equivalents but also removing acids to improve product final titers to minimize reactor sizes. For the current design, an in situ pertractive unit continuously extracts the acids across a membrane using a solvent such as tri-octyl-phosphine oxide (TOPO) (Saboe et al., 2018). Although membrane separation technology has been widely accepted in the industrial applications, there are still concerns related to fouling of the membrane, type of membrane (e.g., zeolite vs. polymer) utilized for selective acid separation, and loss of solvent during acid recovery process (Singhania et al., 2013, Huang et al., 2007). This may have significant impacts on the process efficiency. There are methods utilized for improving the anti-fouling properties of membranes, which includes pretreatment technologies to remove any undesirable products or chemicals leading to fouling, scheduled cleaning, and changing membrane properties depending on the acid properties (hydrophobic versus hydrophilic) (Abdelrasoul et al., 2013). For the issue related to solvent loss, the cost of additional vacuum distillation unit operation is considered to prevent dimerization and degradation of the TOPO carrier molecule as well as reduce the boiling point to improve the energy efficiency of the integrated plant. See Supplemental Information section Supplemental Carboxylic Acid Recovery Methods using TOPO for detailed description of the process.
Table 4.
Technical Assumptions for Lactic and Butyric Acid Production from Wet Wastes
| Technical Parameters | Sludge | Food Waste | Swine Manure | FOG |
|---|---|---|---|---|
| COD reduction | 55.50% | 65% | 55% | 82% |
| Acid separation efficiency | >99% | >99% | >99% | >99% |
| Lactic acid titer (g/L) | 29.3 | 200.4 | 42.6 | 110.5 |
| Butyric acid titer (g/L) | 18.8 | 121.0 | 23.8 | 76.6 |
| Lactic acid yield (kg/dry ton) | 326.7 | 598.7 | 428.6 | 864.7 |
| Butyric acid yield (kg/dry ton) | 209.1 | 340.6 | 238 | 602.8 |
| Product titers (kg VS/m3/day) | 4.2 | 4.2 | 4.2 | 4.2 |
As illustrative examples, lactic acid and butyric acid are selected for this economic study. It should be noted that although arrested methanogenesis would usually yield a mixture of carboxylic acids in the fermentation broth without specifically targeting high value acids, we select lactic and butyric acids as the sole acid product based on the market demand and cost of acid on a mass and energy basis. The LHV of lactic acid is estimated to be 11.72 MMBtu/ton (AspenPlus, 2007), whereas its current market selling price is $1.30–$2.30/kg in 2011$ (Higson, 2011, Biddy et al., 2016), equivalent to $1.40–$2.40/kg in 2016$ ($109.00–$186.00/MMBtu) using the Inorganic Chemical Index (SRI Consulting, 2008). On the contrary, the LHV of butyric acid is estimated to be higher than lactic acid at 19.8 MMBtu/ton (AspenPlus, 2007), whereas its current market selling price has a similar range from $1.80 (2013$) to $2.40 (2015$)/kg (Thongchul, 2015, Calt, 2015), equivalent to $1.80–$2.40/kg in 2016$ ($83.00–$110.00/MMBtu).
Table 5 summarizes the capital and operating costs associated with the production of carboxylic acids (lactic and butyric acids) along with key parameters used to perform TEA for this process pathway. As shown in the table, the two major unit operations contributing the most to the total installed capital cost estimates are anaerobic digester system (50%–70%) and carboxylic acid separation technology (15%–30%), depending on the type of waste.
Table 5.
Techno-economic Parameters Associated with Lactic and Butyric Acid Production from Wet Wastes
| Parameter | Wastewater Sludge | Food Waste | Swine Manure | Fat, Oil, and Grease | ||||
|---|---|---|---|---|---|---|---|---|
| Product | Lactic Acid | Butyric Acid | Lactic Acid | Butyric Acid | Lactic Acid | Butyric Acid | Lactic Acid | Butyric Acid |
| Plant scale (wet tons/day, unless noted) | 300 million gallons/day | 250 | 220 | 200 | ||||
| Discount rate | 10% | |||||||
| Cost year | 2016 | |||||||
| Plant economic years | 30 | |||||||
| Installed Capital Costs (million$) | ||||||||
| Feedstock handling | 0.3 | 0.3 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Anaerobic digester | 35.7 | 32.9 | 11.1 | 10.6 | 3.97 | 3.79 | 5.27 | 5.0 |
| Acid separation | 15.3 | 14.7 | 1.96 | 1.6 | 1.23 | 1.19 | 1.39 | 1.3 |
| Storage | 0.2 | 0.1 | 0.02 | 0.01 | 0.01 | 0.01 | 0.1 | 0.01 |
| Utilities | 0.4 | 0.3 | 0.2 | 0.15 | 0.04 | 0.04 | 0.1 | 0.1 |
| Total capital costs | 51.9 | 50.0 | 13.3 | 12.5 | 5.3 | 5.1 | 6.8 | 6.5 |
| Direct and indirect costs | 16.4 | 13.3 | 4.2 | 3.9 | 1.6 | 1.6 | 2.2 | 2.0 |
| Total capital investment | 68.3 | 63.3 | 17.5 | 16.4 | 6.9 | 6.7 | 9.0 | 8.5 |
| Annualized Operating Costs (million$/year) | ||||||||
| Variable operating costs | 1.64 | 1.18 | 0.51 | 0.3 | 0.1 | 0.06 | 0.27 | 0.2 |
| Fixed operating costs | 6.92 | 6.71 | 1.63 | 1.58 | 1.09 | 1.08 | 1.12 | 1.1 |
| Total operating costs | 8.57 | 7.89 | 2.14 | 1.88 | 1.19 | 1.14 | 1.39 | 1.3 |
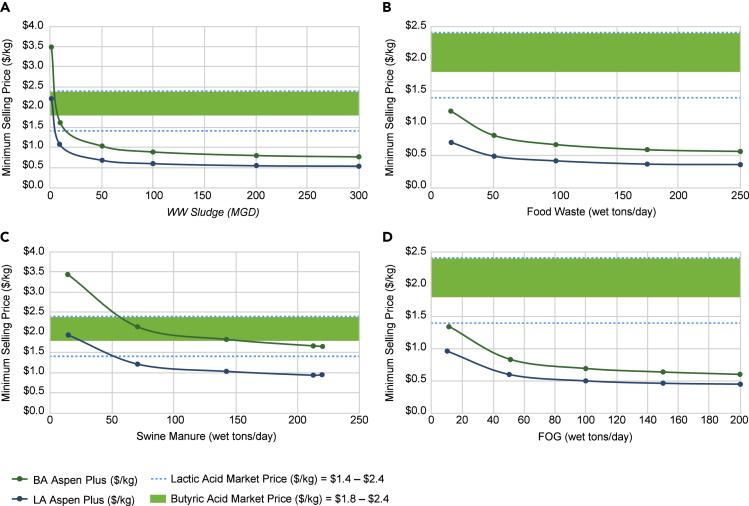
Combining arrested AD with an in situ pertractive separation unit, Figure 4 presents the minimum selling price of the two acids from four waste feedstocks. At a scale higher than 10 million gallons per day (MGD) for wastewater sludge or other wastes of 10–50 wet tons/day, the waste to lactic acid production is cost competitive with production from fossil-based processes. Although the cost estimates for butyric acid are higher than lactic acid, it is still competitive to market price at a scale of 15 MGD for wastewater sludge and 10–140 wet tons/day for other wet wastes. The higher costs of butyrate in $/kg as compared with lactate is because the butyrate pathway produces more CO2 than lactate, which reduces the overall biomass conversion yield to the targeted acids. Also, butyrate has low oxygen to carbon ratio, which also impacts its mass yield.
Figure 4.
Lactic and Butyric Acid Production Costs in Comparison with Current Market Selling Price
(A–D) show the carboxylic acid production costs in $/kg for AD processing wastewater sludge, food waste, swine manure, and FOG, respectively, for corresponding plant scales.
In addition, some of the wet waste feedstocks are currently available for negligible or even negative prices (depending on the type of waste and location), as facilities such as animal feeding operations or WWT plants need to pay some amount in the form of tipping fees to dispose the waste. The average tipping fees in the United States for 2018 were $55.22/ton as compared with $51.82/ton in 2017, a 7% increase over one year (Waste360, 2018). Once the value of these wastes becomes apparent, the demand and its corresponding cost would upsurge with increasing technology readiness as more WTE operations would compete for them. Moreover, biogas has a possibility to leak if mishandled, diminishing the overall positive impacts.
Wet wastes are geographically distributed with a wide range of compositional and temporal variability. Although distributed in areas with high population density for maximum market opportunities (except for manure), there are often challenges that restrict technologies to one family of feedstocks when considering stringent handling regulations. The distributed nature of wet wastes as opposed to fossil-based feedstocks requires a decentralized approach and tailored production strategies. With carboxylic acid production from wet waste via arrested AD still in its nascent stage, mass production could help reduce costs and increase production volumes by more than three times (18% versus 6%) compared with centralized facilities by applying technical expertise (Daugaard et al., 2015). Moreover, our analysis shows lactic and butyric acid production costs to be competitive at lower plant scales, making distributed conversion more appealing than traditional methods. However, choosing a centralized system could help economize costs with high-quality products. Thus, there are factors for favorable strategies that determine the economics and should be carefully considered before making a final decision.
In all, the costs of acids from swine manure are lower as compared with other wastes, whereas food waste and FOG show the highest economic potential for lactic and butyric acid production. The production of these acids from wastewater sludge and swine manure needs to attain high yields on a mass basis before it can compete with market prices at lower plant scales. Our analysis does not consider any feedstock costs in the TEA for production of carboxylic acids. However, it is important to note that the production cost of acids could be further reduced by incorporating negative feedstock costs (where applicable, depending on the type of waste and location), usually incurred by the facilities (e.g., WWT plants) in terms of tipping fees. Therefore, this study shows that waste to acids could provide an economical alternative, with options of retrofitting existing AD facilities. We conclude that converting waste to short-chain carboxylic acids could be cost competitive with acids from chemical markets.
Potential Uses of Acids Produced from Wastes
Carboxylic acids can be used in a variety of applications including biological nutrient removal, polyhydroxyalkanoate (PHA), renewable plastics (such as polylactic acid (PLA)), chemical or chemical precursors, and bioenergy production (Atasoy et al., 2018, Lee et al., 2014).
Carboxylic acids can be used in the production of PHAs, which are biodegradable polyesters synthesized by different types of microorganisms and stored in microbial cells as carbon and energy storage materials (Li et al., 2016, Raza et al., 2018, Khanna and Srivastava, 2005). The main acids responsible for PHA production include acetate, butyrate, and lactate (Colombo et al., 2016, Agler et al., 2011). They can also be utilized as a feed for biofuel production or used in production of a variety of chemicals such as acrylate and propene (Chen, 2009, Gao et al., 2011, Spekreijse et al., 2012, Zhang et al., 2009b). PHAs can be accumulated in the range of 40%–77% from different types of feed including sludge (Jiang et al., 2009) and food waste (Reddy et al., 2012). Bengtsson et al. (2017) conducted a pilot-scale experiment to remove carbon and nitrogen from wastewater and observed a 49% accumulation of PHA from volatile suspended solids with acetate and other organic residues as a substrate. Korkakaki, van Loosdrecht, and Kleerebezem (Korkakaki et al., 2016) increased the PHA storage capacity from 48 to 70 wt% by introducing a sedimentation step to selectively force carbon in the substrate for PHA accumulation in contrast to microbial growth. See Supplemental Information section Supplemental Uses of Carboxylic Acids.
Lactic acid is typically produced by fermentation or by chemical synthesis. A fermentation process converts carbohydrates—such as glucose and sucrose—to lactate using Lactobacillus microorganisms. Chemical synthesis uses acetaldehyde and hydrogen cyanide and the hydrolysis of lactonitrile with sulfuric acid to lactic acid and ammonium sulfate. About 77% of lactate is consumed to make PLA, 19% is applied for food and beverages, whereas the remaining is used for industrial applications, pharmaceuticals, and personal care products. The global production of lactic acid was estimated to be over 0.3 million tons in 2013, and its demand is expected to reach around 2 million tons by 2020 (Biddy et al., 2016, Innorex, nd). Although the global PLA capacity is expected to exceed 800,000 tons by 2020, its demand is expected to rise at a much higher rate to 1.2 million tons in 2020. This robust growth in the PLA market would enable the use of unconventional feedstocks and exploration of alternative pathways to meet the market demand.
Butyric acid is typically produced by oxidation of butyraldehyde derived from oxosynthesis of propylene obtained from crude oil (Baroi et al., 2017, Thongchul, 2015). The primary application of butyric acid is mainly in plastic production (Cao et al., 2011), with its derivatives being used in food, chemical, and pharmaceutical production (Baroi et al., 2017, Thongchul, 2015). It has also been utilized in animal feed to replace antibiotics. The global production of butyric acid was 88,000 tons annually in 2013 with the United States alone producing 39,000 tons. The demand is expected to rise to 105,000 tons in 2020 at a compound annual growth rate of 15.1% (Atasoy et al., 2018).
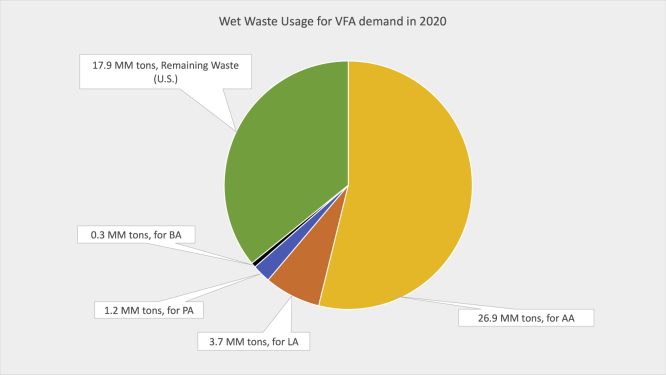
The wet waste in the United States has the capacity to meet and surpass the global demand of acids, provided all the available waste is diverted to produce these promising acids. Figure 5 shows the amount of wet waste required for meeting the global demand of acetic, propionic, lactic, and butyric acids in 2020 (Atasoy et al., 2018).
Figure 5.
Summary of Wet Waste Usage to Meet the Global Short-chain Acids Demand (2020)
The mass yield of carboxylic acids from all animal waste is assumed to be similar to swine manure.
All the wet wastes have enough inherent energy to meet the global demand, with an additional 17.9 million tons available for use in acid derivative markets or fuel production. The demand for acetic acid is highest followed by lactic acid, propionic acid, and butyric acid. The demand for lactic acid is 18-fold as compared with butyric acid, whose main market is fast-growing PLA. As most of the lactic acid is used for PLA production, the excess production potential could positively disrupt the global PLA demand beyond the predicted values. This allows its market to expand and potentially drive down prices, considering its high potential to replace polyethylene terephthalate, which has a large demand worldwide. Alternatively, the demand for other acids will continue to rise, potentially requiring alternative ways to produce the supply without disrupting the market economy.
Conclusions and Outlook
Increasing waste around the world and dependency on fossil fuels have created the need for developing alternative approaches such as WTE that lead to more environmentally friendly and economically attractive alternative energy sources. Conventional methods to produce biogas from wastes are well-known commercialized processes implemented all over the world. However, with issues related to GHG emissions from fossil-based feedstocks, research efforts are driving toward the production of high-value products such as carboxylic acids through an arrested AD process. This study presents the potentials of acid production through an arrested AD process on both technical and economic aspects. One key finding from this study is that arrested AD, as alternative WTE strategy, can sustain energy efficiently from waste feedstocks as compared with CH4. This study provides insights on current technology development, process challenges, applications, and economics for producing high-value chemicals such as short-chain carboxylic acids from wet wastes. Although the optimum set of operating parameters for acid production and its separation from the fermentation broth continues to be a challenge, the high energy potential and competitive cost makes it a strong contender for AD technology. Our economic analysis shows that at larger plant scales (>15 MGD or >140 wet tons per day), an arrested AD process could produce acids that could beat the current market selling price, providing a potentially viable pathway to meet the future demand of acids by switching to eco-friendly wastes. Although the costs of lactate and butyrate are competitive with market prices, there are still challenges to be addressed technically on improving arrested AD technology and cost-effective, low-capital, and energy-intensive separation technologies to increase economic attractiveness. Last, the wet waste in the United States has the capacity to meet and surpass the global demand of acids, provided all the available waste is diverted to produce these promising acids. The acid existing market capacity can be saturated by converting 65% of wastes, with an additional 17.9 million tons available for use in acid derivative markets or fuel production. This research aims to help the WTE community broaden their perspective beyond conventional AD with the potential to direct future research and development. For the next steps, we plan to integrate the carboxylic acid upgrading technologies using arrested methanogenesis as the core conversion step linking with downstream separation and purification strategies. This will be achieved through detailed research and development in the area of conversion of carboxylic acids to hydrocarbon fuels and chemicals that will help us understand the process technology and techno-economic advances over chemical markets or reach the projected cost targets to compete with gasoline and diesel.
Limitations of the Study
Although this study demonstrates that the cost of producing lactic and butyric acid is cost competitive to chemical markets, these results are based on technical and economic assumptions in TEA and would need to be revisited based on new experimental data and refined cost estimates. Moreover, an arrested AD technology would usually produce a mixed slate of carboxylic acid products unlike pure acids assumed in this study, which may have additional cost implications based on acid separation efficiency and technology utilized. Moreover, the fundamental limitation associated with sensitivity of industries to use carboxylic acids produced from wet waste, especially in the pharmaceutical sector, may hinder faster adoption of this technology. However, this may be overcome through pilot- and demonstration-scale facilities showing high productivities and purity levels required by industries.
Resource Availability
Lead Contact
Further information and requests should be directed to and will be fulfilled by the Lead Contact, Ling Tao (Ling.Tao@nrel.gov).
Materials Availability
This study did not generate any new materials.
Data and Code Availability
Any data utilized in this study can be found the main manuscript and Supplemental Information.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The work was supported by the U.S. Department of Energy's Bioenergy Technologies Office under Contract No. DE-AC36-08GO28308 with the National Renewable Energy Laboratory. The views and opinions of the authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. We appreciate all the editing help from our technical editor. We thank Beau Hoffman and Mark Philbrick of BETO, Meltem Urgun Demirtas of Argonne National Laboratory and Violeta Sanchez I Nogue of NREL for their generous in-depth review and suggestions. We are grateful to Justin Rickard, Dorothy Breazeale, and Julia Laser of NREL for edits to the paper and figures.
Author Contributions
Conceptualization, L.T.; Literature, A.B.; Chemical Process Modeling, A.B. and L.T.; Writing—Original Draft, A.B. and L.T.; Writing—Support, Z.R.; Review & Editing, A.B., L.T., and Z.R.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101221.
Supplemental Information
References
- Abdelrasoul A., Doan H., Lohi A. Fouling in membrane filtration and remediation methods. In: Nakajima H., editor. Mass transfer-advances in sustainable energy and environment oriented numerical modeling. InTech; Rijeka, Croatia: 2013. pp. 195–218. [Google Scholar]
- Agbogbo F.K., Holtzapple M.T. Fixed-bed fermentation of rice straw and chicken manure using a mixed culture of marine mesophilic microorganisms. Bioresour. Technol. 2007;98:1586–1595. doi: 10.1016/j.biortech.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Agler M.T., Wrenn B.A., Zinder S.H., Angenent L.T. Waste to bioproduct conversion with undefined mixed cultures: the carboxylate platform. Trends Biotechnol. 2011;29:70–78. doi: 10.1016/j.tibtech.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Agler M.T., Werner J.J., Iten L.B., Dekker A., Cotta M.A., Dien B.S., Angenent L.T. Shaping reactor microbiomes to produce the fuel precursor n-butyrate from pretreated cellulosic hydrolysates. Environ. Sci. Technol. 2012;46:10229–10238. doi: 10.1021/es302352c. [DOI] [PubMed] [Google Scholar]
- Ahn Y.H., Speece R.E. Elutriated acid fermentation of municipal primary sludge. Water Res. 2006;40:2210–2220. doi: 10.1016/j.watres.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Aiello-Mazzarri C., Coward-Kelly G., Agbogbo F.K., Holtzapple M.T. Conversion of municipal solid waste into carboxylic acids by anaerobic countercurrent fermentation: effect of using intermediate lime treatment. Appl. Biochem. Biotechnol. 2005;127:79–94. doi: 10.1385/abab:127:2:079. [DOI] [PubMed] [Google Scholar]
- Aiello-Mazzarri C., Agbogbo F.K., Holtzapple M.T. Conversion of municipal solid waste to carboxylic acids using a mixed culture of mesophilic microorganisms. Bioresour. Technol. 2006;97:47–56. doi: 10.1016/j.biortech.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Akaraonye E., Keshavarz T., Roy I. Production of polyhydroxyalkanoates: the future green materials of choice. J. Chem. Technol. Biotechnol. 2010;85:732–743. [Google Scholar]
- Alibardi L., Cossu R. Effects of carbohydrate, protein and lipid content of organic waste on hydrogen production and fermentation products. Waste Manage. 2016;47:69–77. doi: 10.1016/j.wasman.2015.07.049. [DOI] [PubMed] [Google Scholar]
- Alkaya E., Kaptan S., Ozkan L., Uludag-Demirer S., Demirer G.N. Recovery of acids from anaerobic acidification broth by liquid–liquid extraction. Chemosphere. 2009;77:1137–1142. doi: 10.1016/j.chemosphere.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Aluwong T.K., Ishaku P., Abdullahi A. Volatile fatty acids production in ruminants and the role of monocarboxylate transporters: a review. Afr. J. Biotechnol. 2010;9:6229–6232. [Google Scholar]
- Andersen S.J., Candry P., Basadre T., Khor W.C., Roume H., Hernandez-Sanabria E., Coma M., Rabaey K. Electrolytic extraction drives volatile fatty acid chain elongation through lactic acid and replaces chemical pH control in thin stillage fermentation. Biotechnol. Biofuels. 2015;8:221. doi: 10.1186/s13068-015-0396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AspenPlus . Aspen Technology Inc; 2007. Release 7.2. [Google Scholar]
- Atasoy M., Owusu-Agyeman I., Plaza E., Cetecioglu Z. Bio-based volatile fatty acid production and recovery from waste streams: current status and future challenges. Bioresour. Technol. 2018;268:773–786. doi: 10.1016/j.biortech.2018.07.042. [DOI] [PubMed] [Google Scholar]
- Aydin S., Yesil H., Tugtas A.E. Recovery of mixed volatile fatty acids from anaerobically fermented organic wastes by vapor permeation membrane contactors. Bioresour. Technol. 2018;250:548–555. doi: 10.1016/j.biortech.2017.11.061. [DOI] [PubMed] [Google Scholar]
- Badgett A., Newes E., Milbrandt A. Economic analysis of wet waste-to-energy resources in the United States. Energy. 2019;176:224–234. [Google Scholar]
- Baroi G.N., Gavala H.N., Westermann P., Skiadas I.V. Fermentative production of butyric acid from wheat straw: economic evaluation. Ind. Crops Prod. 2017;104:68–80. [Google Scholar]
- Batista F. Hydrogen production by dark fermentation. Chem. Eng. Trans. 2014;38:481. [Google Scholar]
- Begum S., Anupoju G.R., Sridhar S., Bhargava S.K., Jegatheesan V., Eshtiaghi N. Evaluation of single and two stage anaerobic digestion of landfill leachate: effect of pH and initial organic loading rate on volatile fatty acid (VFA) and biogas production. Bioresour. Technol. 2018;251:364–373. doi: 10.1016/j.biortech.2017.12.069. [DOI] [PubMed] [Google Scholar]
- Ben M., Mato T., Lopez A., Vila M., Kennes C., Veiga M.C. Bioplastic production using wood mill effluents as feedstock. Water Sci. Technol. 2011;63:1196–1202. doi: 10.2166/wst.2011.358. [DOI] [PubMed] [Google Scholar]
- Bengtsson S., Hallquist J., Werker A., Welander T. Acidogenic fermentation of industrial wastewaters: effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008;40:492–499. [Google Scholar]
- Bengtsson S., Karlsson A., Alexandersson T., Quadri L., Hjort M., Johansson P., Morgan-Sagastume F., Anterrieu S., Arcos-Hernandez M., Karabegovic L. A process for polyhydroxyalkanoate (PHA) production from municipal wastewater treatment with biological carbon and nitrogen removal demonstrated at pilot-scale. New Biotechnol. 2017;35:42–53. doi: 10.1016/j.nbt.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Bergman E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- Besselink H., Brouwer B., Van Der Burg B. Validation and regulatory acceptance of bio-based approaches to assure feedstock, water and product quality in a bio-based economy. Ind. Crops Prod. 2017;106:138–145. [Google Scholar]
- BETO (2017). Biofuels and Bioproducts from Wet and Gaseous Waste Streams: Challenges and Opportunities.
- Beyene H.D., Werkneh A.A., Ambaye T.G. Current updates on waste to energy (WtE) technologies: a review. Renew. Energy Focus. 2018;24:1–11. [Google Scholar]
- Biddy M.J., Scarlata C., Kinchin C. National Renewable Energy Laboratory; 2016. Chemicals from Biomass: A Market Assessment of Bioproducts with Near-Term Potential. [Google Scholar]
- Bolaji I.O., Dionisi D. Acidogenic fermentation of vegetable and salad waste for chemicals production: effect of pH buffer and retention time. J. Environ. Chem. Eng. 2017;5:5933–5943. [Google Scholar]
- Bougrier C., Albasi C., Delgenès J.P., Carrère H. Vol. 45. 2006. Effect of ultrasonic, thermal and ozone pre-treatments on waste activated sludge solubilisation and anaerobic biodegradability; pp. 711–718. (Chem. Eng. Process.). [Google Scholar]
- Brunner P.H., Rechberger H. Waste to energy – key element for sustainable waste management. Waste Manag. 2015;37:3–12. doi: 10.1016/j.wasman.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Cagnetta C., Coma M., Vlaeminck S.E., Rabaey K. Production of carboxylates from high rate activated sludge through fermentation. Bioresour. Technol. 2016;217:165–172. doi: 10.1016/j.biortech.2016.03.053. [DOI] [PubMed] [Google Scholar]
- Calt E.A. Products Produced from Organic Waste Using Managed Ecosystem Fermentation. J. Sustain. Dev. 2015;8:43. [Google Scholar]
- Cao Y., Li H., Zhang J. Homogeneous synthesis and characterization of cellulose acetate butyrate (CAB) in 1-Allyl-3-methylimidazolium chloride (AmimCl) ionic liquid. Ind. Eng. Chem. Res. 2011;50:7808–7814. [Google Scholar]
- Capson-Tojo G., Rouez M., Crest M., Steyer J.-P., Delgenès J.-P., Escudié R. Food waste valorization via anaerobic processes: a review. Rev. Environ. Sci. Biotechnol. 2016;15:499–547. [Google Scholar]
- Cerrillo M., Viñas M., Bonmatí A. Removal of volatile fatty acids and ammonia recovery from unstable anaerobic digesters with a microbial electrolysis cell. Bioresour. Technol. 2016;219:348–356. doi: 10.1016/j.biortech.2016.07.103. [DOI] [PubMed] [Google Scholar]
- Cesaro A., Belgiorno V. Sonolysis and ozonation as pretreatment for anaerobic digestion of solid organic waste. Ultrason. Sonochem. 2013;20:931–936. doi: 10.1016/j.ultsonch.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Cesaro A., Naddeo V., Amodio V., Belgiorno V. Enhanced biogas production from anaerobic codigestion of solid waste by sonolysis. Ultrason. Sonochem. 2012;19:596–600. doi: 10.1016/j.ultsonch.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Chan W.N., Holtzapple M.T. Conversion of municipal solid wastes to carboxylic acids by thermophilic fermentation. Appl. Biochem. Biotechnol. 2003;111:93–112. doi: 10.1385/abab:111:2:93. [DOI] [PubMed] [Google Scholar]
- Chan W.N., Fu Z., Holtzapple M.T. Co-digestion of swine manure and corn stover for bioenergy production in MixAlco™ consolidated bioprocessing. Biomass Bioenerg. 2011;35:4134–4144. [Google Scholar]
- Chang H., Kim N., Kang J., Moon Jeong C. Biomass-derived Volatile Fatty Acid Platform for Fuels and Chemicals. Biotechnol. Bioproc. E. 2010;15:1–10. [Google Scholar]
- Chen G.Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009;38:2434–2446. doi: 10.1039/b812677c. [DOI] [PubMed] [Google Scholar]
- Chen Y., Jiang S., Yuan H., Zhou Q., Gu G. Hydrolysis and acidification of waste activated sludge at different pHs. Water Res. 2007;41:683–689. doi: 10.1016/j.watres.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Chen Y., Cheng J.J., Creamer K.S. Inhibition of anaerobic digestion process: a review. Bioresour. Technol. 2008;99:4044–4064. doi: 10.1016/j.biortech.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Chen H., Meng H., Nie Z., Zhang M. Polyhydroxyalkanoate production from fermented volatile fatty acids: effect of pH and feeding regimes. Bioresour. Technol. 2013;128:533–538. doi: 10.1016/j.biortech.2012.10.121. [DOI] [PubMed] [Google Scholar]
- Chen J.H., Zheng J.Z., Liu Q.L., Guo H.X., Weng W., Li S.X. Pervaporation dehydration of acetic acid using polyelectrolytes complex (PEC)/11-phosphotungstic acid hydrate (PW11) hybrid membrane (PEC/PW11) J. Membr. Sci. 2013;429:206–213. [Google Scholar]
- Chen Y., Luo J., Yan Y., Feng L. Enhanced production of short-chain fatty acid by co-fermentation of waste activated sludge and kitchen waste under alkaline conditions and its application to microbial fuel cells. Appl. Energy. 2013;102:1197–1204. [Google Scholar]
- Colombo B., Sciarria T.P., Reis M., Scaglia B., Adani F. Polyhydroxyalkanoates (PHAs) production from fermented cheese whey by using a mixed microbial culture. Bioresour. Technol. 2016;218:692–699. doi: 10.1016/j.biortech.2016.07.024. [DOI] [PubMed] [Google Scholar]
- Daugaard T., Mutti L.A., Wright M.M., Brown R.C., Componation P. Learning rates and their impacts on the optimal capacities and production costs of biorefineries. Biofuel Bioprod. Bior. 2015;9:82–94. [Google Scholar]
- Devlin D.C., Esteves S.R.R., Dinsdale R.M., Guwy A.J. The effect of acid pretreatment on the anaerobic digestion and dewatering of waste activated sludge. Bioresour. Technol. 2011;102:4076–4082. doi: 10.1016/j.biortech.2010.12.043. [DOI] [PubMed] [Google Scholar]
- Diender M., Uhl P.S., Bitter J.H., Stams A.J.M., Sousa D.Z. High rate biomethanation of carbon monoxide-rich gases via a thermophilic synthetic coculture. ACS Sustain. Chem. Eng. 2018;6:2169–2176. doi: 10.1021/acssuschemeng.7b03601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doǧan I., Sanin F.D. Alkaline solubilization and microwave irradiation as a combined sludge disintegration and minimization method. Water Res. 2009;43:2139–2148. doi: 10.1016/j.watres.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Domingos J.M.B., Martinez G.A., Scoma A., Fraraccio S., Kerckhof F.-M., Boon N., Reis M.A.M., Fava F., Bertin L. Effect of operational parameters in the continuous anaerobic fermentation of cheese whey on titers, yields, productivities, and microbial community structures. ACS Sustain. Chem. Eng. 2017;5:1400–1407. [Google Scholar]
- Eggeman T., Verser D. 21-124. 2005. Recovery of organic acids from fermentation broths; pp. 605–618. (Appl. Biochem. Biotechnol.). [DOI] [PubMed] [Google Scholar]
- Elbeshbishy E., Hafez H., Dhar B.R., Nakhla G. Single and combined effect of various pretreatment methods for biohydrogen production from food waste. Int. J. Hydrogen Energy. 2011;36:11379–11387. [Google Scholar]
- Eskicioglu C., Prorot A., Marin J., Droste R.L., Kennedy K.J. Synergetic pretreatment of sewage sludge by microwave irradiation in presence of H2O2 for enhanced anaerobic digestion. Water Res. 2008;42:4674–4682. doi: 10.1016/j.watres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Ferrer I., Vázquez F., Font X. Long term operation of a thermophilic anaerobic reactor: process stability and efficiency at decreasing sludge retention time. Bioresour. Technol. 2010;101:2972–2980. doi: 10.1016/j.biortech.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Forrest A.K., Hernandez J., Holtzapple M.T. Effects of temperature and pretreatment conditions on mixed-acid fermentation of water hyacinths using a mixed culture of thermophilic microorganisms. Bioresour. Technol. 2010;101:7510–7515. doi: 10.1016/j.biortech.2010.04.049. [DOI] [PubMed] [Google Scholar]
- Forrest A.K., Hollister E.B., Gentry T.J., Wilkinson H.H., Holtzapple M.T. Comparison of mixed-acid fermentations inoculated with six different mixed cultures. Bioresour. Technol. 2012;118:343–349. doi: 10.1016/j.biortech.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Fu Z., Holtzapple M.T. Fermentation of sugarcane bagasse and chicken manure to calcium carboxylates under thermophilic conditions. Appl. Biochem. Biotechnol. 2010;162:561–578. doi: 10.1007/s12010-009-8748-z. [DOI] [PubMed] [Google Scholar]
- Fu Z., Holtzapple M.T. Anaerobic thermophilic fermentation for carboxylic acid production from in-storage air-lime-treated sugarcane bagasse. Appl. Microbiol. Biotechnol. 2011;90:1669–1679. doi: 10.1007/s00253-011-3178-6. [DOI] [PubMed] [Google Scholar]
- Gao X., Chen J.C., Wu Q., Chen G.Q. Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr. Opin. Biotechnol. 2011;22:768–774. doi: 10.1016/j.copbio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Garcia-Aguirre J., Aymerich E., González-Mtnez De Goñi J., Esteban-Gutiérrez M. Selective VFA production potential from organic waste streams: assessing temperature and pH influence. Bioresour. Technol. 2017;244:1081–1088. doi: 10.1016/j.biortech.2017.07.187. [DOI] [PubMed] [Google Scholar]
- Golub K.W., Golub S.R., Meysing D.M., Holtzapple M.T. Propagated fixed-bed mixed-acid fermentation: effect of volatile solid loading rate and agitation at near-neutral pH. Bioresour. Technol. 2012;124:146–156. doi: 10.1016/j.biortech.2012.08.094. [DOI] [PubMed] [Google Scholar]
- Grzelak J., Ślęzak R., Krzystek L. Kinetics of volatile fatty acids and hydrogen production during anaerobic digestion of organic waste material. Challenges of Modern Technology. 2015;6:48–52. [Google Scholar]
- Hao J., Wang H. Volatile fatty acids productions by mesophilic and thermophilic sludge fermentation: biological responses to fermentation temperature. Bioresour. Technol. 2015;175:367–373. doi: 10.1016/j.biortech.2014.10.106. [DOI] [PubMed] [Google Scholar]
- Harrad S.H., Harrison R.M. Institute of Public and Environmental Health, University of Birmingham; 1996. The Health Effects of the Products of Waste Combustion. [Google Scholar]
- Higson A. The National Non-Food Crops Center; 2011. Lactic Acid Factsheet. [Google Scholar]
- Hirano S., Matsumoto N., Morita M., Sasaki K., Ohmura N. Electrochemical control of redox potential affects methanogenesis of the hydrogenotrophic methanogen Methanothermobacter thermautotrophicus. Lett. Appl. Microbiol. 2013;56:315–321. doi: 10.1111/lam.12059. [DOI] [PubMed] [Google Scholar]
- Huang Y.L., Wu Z., Zhang L., Ming Cheung C., Yang S.-T. Production of carboxylic acids from hydrolyzed corn meal by immobilized cell fermentation in a fibrous-bed bioreactor. Bioresour. Technol. 2002;82:51–59. doi: 10.1016/s0960-8524(01)00151-1. [DOI] [PubMed] [Google Scholar]
- Huang C., Xu T., Zhang Y., Xue Y., Chen G. Application of electrodialysis to the production of organic acids: state-of-the-art and recent developments. J. Membr. Sci. 2007;288:1–12. [Google Scholar]
- Huang W., Huang W., Yuan T., Zhao Z., Cai W., Zhang Z., Lei Z., Feng C. Volatile fatty acids (VFAs) production from swine manure through short-term dry anaerobic digestion and its separation from nitrogen and phosphorus resources in the digestate. Water Res. 2016;90:344–353. doi: 10.1016/j.watres.2015.12.044. [DOI] [PubMed] [Google Scholar]
- Innorex Global PolyLactic Acid and Lactic Acid market forecast to see robust growth to 2020. http://www.innorex.eu/detalle_noticia.php?no_id=3194
- Jankowska E., Chwiałkowska J., Stodolny M., Oleskowicz-Popiel P. Effect of pH and retention time on volatile fatty acids production during mixed culture fermentation. Bioresour. Technol. 2015;190:274–280. doi: 10.1016/j.biortech.2015.04.096. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Duber A., Chwialkowska J., Stodolny M., Oleskowicz-Popiel P. Conversion of organic waste into volatile fatty acids – the influence of process operating parameters. Chem. Eng. J. 2018;345:395–403. [Google Scholar]
- Jiang Y., Chen Y., Zheng X. Efficient polyhydroxyalkanoates production from a waste-activated sludge alkaline fermentation liquid by activated sludge submitted to the aerobic feeding and discharge process. Environ. Sci. Technol. 2009;43:7734–7741. doi: 10.1021/es9014458. [DOI] [PubMed] [Google Scholar]
- Jiang J., Zhang Y., Li K., Wang Q., Gong C., Li M. Volatile fatty acids production from food waste: effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013;143:525–530. doi: 10.1016/j.biortech.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Lu L., Wang H., Shen R., Ge Z., Hou D., Chen X., Liang P., Huang X., Ren Z.J. Electrochemical control of redox potential arrests methanogenesis and regulates products in mixed culture electro-fermentation. ACS Sustain. Chem. Eng. 2018;6:8650–8658. [Google Scholar]
- Jiang Y., May H.D., Lu L., Liang P., Huang X., Ren Z.J. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation. Water Res. 2019;149:42–55. doi: 10.1016/j.watres.2018.10.092. [DOI] [PubMed] [Google Scholar]
- Jones R.J., Massanet-Nicolau J., Guwy A., Premier G.C., Dinsdale R.M., Reilly M. Removal and recovery of inhibitory volatile fatty acids from mixed acid fermentations by conventional electrodialysis. Bioresour. Technol. 2015;189:279–284. doi: 10.1016/j.biortech.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Kataki R., Chutia R.S., Bordoloi N.J., Saikia R., Sut D., Narzari R., Gogoi L., Nikhil G.N., Sarkar O., Venkata Mohan S. Biohydrogen production scenario for Asian countries. In: Singh A., Rathore D., editors. Biohydrogen Production: Sustainability of Current Technology and Future Perspective. Springer India; 2017. pp. 207–235. [Google Scholar]
- Khanna S., Srivastava A.K. Recent advances in microbial polyhydroxyalkanoates. Process Biochem. 2005;40:607–619. [Google Scholar]
- Khiewwijit R., Temmink H., Labanda A., Rijnaarts H., Keesman K.J. Production of volatile fatty acids from sewage organic matter by combined bioflocculation and alkaline fermentation. Bioresour. Technol. 2015;197:295–301. doi: 10.1016/j.biortech.2015.08.112. [DOI] [PubMed] [Google Scholar]
- Kim J., Park C., Kim T.H., Lee M., Kim S., Kim S.W., Lee J. Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge. J. Biosci. Bioeng. 2003;95:271–275. doi: 10.1016/s1389-1723(03)80028-2. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Choi Y.G., Kim D.Y., Kim D.H., Chung T.H. Water Science and Technology; 2005. Effect of Pretreatment on Acid Fermentation of Organic Solid Waste. [PubMed] [Google Scholar]
- Kleerebezem R., Van Loosdrecht M.C.M. Mixed culture biotechnology for bioenergy production. Curr. Opin. Biotechnol. 2007;18:207–212. doi: 10.1016/j.copbio.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Kleerebezem R., Joosse B., Rozendal R., Van Loosdrecht M.C.M. Anaerobic digestion without biogas? Rev. Environ. Sci. Biotechnol. 2015;14:787–801. [Google Scholar]
- Koberg M., Gedanken A. Optimization of bio-diesel production from oils, cooking oils, microalgae, and castor and jatropha seeds: probing various heating sources and catalysts. Energy Environ. Sci. 2012;5:7460–7469. [Google Scholar]
- Komemoto K., Lim Y.G., Nagao N., Onoue Y., Niwa C., Toda T. Effect of temperature on VFA’s and biogas production in anaerobic solubilization of food waste. Waste Manag. 2009;29:2950–2955. doi: 10.1016/j.wasman.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Kondo T., Kondo M. Efficient production of acetic acid from glucose in a mixed culture of Zymomonas mobilis and Acetobacter sp. J. Ferment. Bioeng. 1996;81:42–46. [Google Scholar]
- Korkakaki E., Van Loosdrecht M.C.M., Kleerebezem R. Survival of the fastest: selective removal of the side population for enhanced PHA production in a mixed substrate enrichment. Bioresour. Technol. 2016;216:1022–1029. doi: 10.1016/j.biortech.2016.05.125. [DOI] [PubMed] [Google Scholar]
- Kothari R., Tyagi V.V., Pathak A. Waste-to-energy: a way from renewable energy sources to sustainable development. Renew. Sustain. Energy Rev. 2010;14:3164–3170. [Google Scholar]
- Kumar R., Nanavati H., Noronha S.B., Mahajani S.M. A continuous process for the recovery of lactic acid by reactive distillation. J. Chem. Technol. Biotechnol. 2006;81:1767–1777. [Google Scholar]
- Kwan T.H., Hu Y., Lin C.S. Valorisation of food waste via fungal hydrolysis and lactic acid fermentation with Lactobacillus casei Shirota. Bioresour. Technol. 2016;217:129–136. doi: 10.1016/j.biortech.2016.01.134. [DOI] [PubMed] [Google Scholar]
- Lee W.S., Chua A.S.M., Yeoh H.K., Ngoh G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014;235:83–99. [Google Scholar]
- Li, Y.Y. and Noike, T. (1992). Upgrading of anaerobic digestion of waste activated sludge by thermal pretreatment. Proceedings of the 16th Biennial Conference of the International Association on Water Pollution Research and Control - Water Quality International '92 26, 857–866.
- Li X., Peng Y., Ren N., Li B., Chai T., Zhang L. Effect of temperature on short chain fatty acids (SCFAs) accumulation and microbiological transformation in sludge alkaline fermentation with Ca(OH)2 adjustment. Water Res. 2014;61:34–45. doi: 10.1016/j.watres.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Li X., Swan J.E., Nair G.R., Langdon A.G. Preparation of volatile fatty acid (VFA) calcium salts by anaerobic digestion of glucose. Biotechnol. Appl. Biochem. 2015;62:476–482. doi: 10.1002/bab.1301. [DOI] [PubMed] [Google Scholar]
- Li Z., Yang J., Loh X.J. Polyhydroxyalkanoates: opening doors for a sustainable future. NPG Asia Mater. 2016;8:e265. [Google Scholar]
- Lim S.J., Kim B.J., Jeong C.M., Choi J.D.R., Ahn Y.H., Chang H.N. Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresour. Technol. 2008;99:7866–7874. doi: 10.1016/j.biortech.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Liu H., Wang J., Liu X., Fu B., Chen J., Yu H.Q. Acidogenic fermentation of proteinaceous sewage sludge: effect of pH. Water Res. 2012;46:799–807. doi: 10.1016/j.watres.2011.11.047. [DOI] [PubMed] [Google Scholar]
- Liu A., Ren F., Lin W.Y., Wang J.-Y. A review of municipal solid waste environmental standards with a focus on incinerator residues. Int. J. Sustain. Built Environ. 2015;4:165–188. [Google Scholar]
- López-Garzón C.S., Van Der Wielen L.a.M., Straathof A.J.J. Strong anion exchange recovery of aqueous dicarboxylates: extraction and sorption equilibrium comparison. Sep. Purif. Technol. 2017;175:9–18. [Google Scholar]
- Lukitawesa, Patinvoh R.J., Millati R., Sárvári-Horváth I., Taherzadeh M.J. Factors influencing volatile fatty acids production from food wastes via anaerobic digestion. Bioengineered. 2020;11:39–52. doi: 10.1080/21655979.2019.1703544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Feng L., Zhang W., Li X., Chen H., Wang D., Chen Y. Improved production of short-chain fatty acids from waste activated sludge driven by carbohydrate addition in continuous-flow reactors: influence of SRT and temperature. Appl. Energy. 2014;113:51–58. [Google Scholar]
- Ma H., Chen X., Liu H., Liu H., Fu B. Improved volatile fatty acids anaerobic production from waste activated sludge by pH regulation: alkaline or neutral pH? Waste Manag. 2016;48:397–403. doi: 10.1016/j.wasman.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Mazur A. How does population growth contribute to rising energy consumption in America? Popul. Environ. 1994;15:371–378. [Google Scholar]
- Miron Y., Zeeman G., Van Lier J.B., Lettinga G. The role of sludge retention time in the hydrolysis and acidification of lipids, carbohydrates and proteins during digestion of primary sludge in CSTR systems. Water Res. 2000;34:1705–1713. [Google Scholar]
- Morgan-Sagastume F., Pratt S., Karlsson A., Cirne D., Lant P., Werker A. Production of volatile fatty acids by fermentation of waste activated sludge pre-treated in full-scale thermal hydrolysis plants. Bioresour. Technol. 2011;102:3089–3097. doi: 10.1016/j.biortech.2010.10.054. [DOI] [PubMed] [Google Scholar]
- Nachiappan B., Fu Z., Holtzapple M.T. Ammonium carboxylate production from sugarcane trash using long-term air-lime pretreatment followed by mixed-culture fermentation. Bioresour. Technol. 2011;102:4210–4217. doi: 10.1016/j.biortech.2010.12.059. [DOI] [PubMed] [Google Scholar]
- Nebot E., Romero L.I., Quiroga J.M., Sales D. Effect of the feed frequency on the performance of anaerobic filters. Anaerobe. 1995;1:113–120. doi: 10.1006/anae.1995.1006. [DOI] [PubMed] [Google Scholar]
- Oktem Y.A., Ince O., Donnelly T., Sallis P., Ince B.K. Determination of optimum operating conditions of an acidification reactor treating a chemical synthesis-based pharmaceutical wastewater. Process Biochem. 2006;41:2258–2263. [Google Scholar]
- Olsson G. IWA Publishing; 2012. Water and Energy: Threats and Opportunities. [Google Scholar]
- Outram V., Zhang Y. Solvent-free membrane extraction of volatile fatty acids from acidogenic fermentation. Bioresour. Technol. 2018;270:400–408. doi: 10.1016/j.biortech.2018.09.057. [DOI] [PubMed] [Google Scholar]
- Pecorini I., Francesco B., Iannelli R. Biochemical hydrogen potential tests using different inocula. Sustainability. 2019;11:622. [Google Scholar]
- Psomopoulos C.S., Bourka A., Themelis N.J. Waste-to-energy: a review of the status and benefits in USA. Waste Manag. 2009;29:1718–1724. doi: 10.1016/j.wasman.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Raza Z.A., Abid S., Banat I.M. Polyhydroxyalkanoates: characteristics, production, recent developments and applications. Int. Biodeterior. Biodegradation. 2018;126:45–56. [Google Scholar]
- Rebecchi S., Pinelli D., Bertin L., Zama F., Fava F., Frascari D. Volatile fatty acids recovery from the effluent of an acidogenic digestion process fed with grape pomace by adsorption on ion exchange resins. Chem. Eng. J. 2016;306:629–639. [Google Scholar]
- Reddy M.M., Deshpande A., Sattler M. Targeting JAK2 in the therapy of myeloproliferative neoplasms. Expert Opin. Ther. Targets. 2012;16:313–324. doi: 10.1517/14728222.2012.662956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren N., Guo W., Liu B., Cao G., Ding J. Biological hydrogen production by dark fermentation: challenges and prospects towards scaled-up production. Curr. Opin. Biotechnol. 2011;22:365–370. doi: 10.1016/j.copbio.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Reyhanitash E., Zaalberg B., Kersten S.R.A., Schuur B. Extraction of volatile fatty acids from fermented wastewater. Sep. Purif. Technol. 2016;161:61–68. [Google Scholar]
- Ross M.K., Holtzapple M.T. Laboratory method for high-solids countercurrent fermentations. Appl. Biochem. Biotechnol. 2001;94:111–126. doi: 10.1385/abab:94:2:111. [DOI] [PubMed] [Google Scholar]
- Rughoonundun H., Holtzapple M.T. Converting wastewater sludge and lime-treated sugarcane bagasse to mixed carboxylic acids – a potential pathway to ethanol biofuel production. Biomass Bioenerg. 2017;105:73–82. [Google Scholar]
- Rughoonundun H., Granda C., Mohee R., Holtzapple M.T. Effect of thermochemical pretreatment on sewage sludge and its impact on carboxylic acids production. Waste Manag. 2010;30:1614–1621. doi: 10.1016/j.wasman.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Rughoonundun H., Mohee R., Holtzapple M.T. Influence of carbon-to-nitrogen ratio on the mixed-acid fermentation of wastewater sludge and pretreated bagasse. Bioresour. Technol. 2012;112:91–97. doi: 10.1016/j.biortech.2012.02.081. [DOI] [PubMed] [Google Scholar]
- Rushton L. Health hazards and waste management. Br. Med. Bull. 2003;68:183–197. doi: 10.1093/bmb/ldg034. [DOI] [PubMed] [Google Scholar]
- Ryckebosch E., Drouillon M., Vervaeren H. Techniques for transformation of biogas to biomethane. Biomass Bioenerg. 2011;35:1633–1645. [Google Scholar]
- Saboe P.O., Manker L.P., Michener W.E., Peterson D.J., Brandner D.G., Deutch S.P., Kumar M., Cywar R.M., Beckham G.T., Karp E.M. In situ recovery of bio-based carboxylic acids. Green. Chem. 2018;20:1791–1804. [Google Scholar]
- Sanders W.T., Geerink M., Zeeman G., Lettinga G. Anaerobic hydrolysis kinetics of particulate substrates. Water Sci. Technol. 2000;41:17–24. [PubMed] [Google Scholar]
- Sans C., Mata-Alvarez J., Cecchi F., Pavan P., Bassetti A. Acidogenic fermentation of organic urban wastes in a plug-flow reactor under thermophilic conditions. Bioresour. Technol. 1995;54:105–110. [Google Scholar]
- Shahriari H., Warith M., Hamoda M., Kennedy K.J. Anaerobic digestion of organic fraction of municipal solid waste combining two pretreatment modalities, high temperature microwave and hydrogen peroxide. Waste Manage. 2012;32:41–52. doi: 10.1016/j.wasman.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Shanableh A., Jomaa S. Production and transformation of volatile fatty acids from sludge subjected to hydrothermal treatment. Water Sci. Technol. 2001;44:129–135. [PubMed] [Google Scholar]
- Shen D., Wang K., Yin J., Chen T., Yu X. Effect of phosphoric acid as a catalyst on the hydrothermal pretreatment and acidogenic fermentation of food waste. Waste Manag. 2016;51:65–71. doi: 10.1016/j.wasman.2016.02.027. [DOI] [PubMed] [Google Scholar]
- Shen D., Yin J., Yu X., Wang M., Long Y., Shentu J., Chen T. Acidogenic fermentation characteristics of different types of protein-rich substrates in food waste to produce volatile fatty acids. Bioresour. Technol. 2017;227:125–132. doi: 10.1016/j.biortech.2016.12.048. [DOI] [PubMed] [Google Scholar]
- Singhania R.R., Patel A.K., Christophe G., Fontanille P., Larroche C. Biological upgrading of volatile fatty acids, key intermediates for the valorization of biowaste through dark anaerobic fermentation. Bioresour. Technol. 2013;145:166–174. doi: 10.1016/j.biortech.2012.12.137. [DOI] [PubMed] [Google Scholar]
- Sivagurunathan P., Kumar G., Bakonyi P., Kim S.-H., Kobayashi T., Xu K.Q., Lakner G., Tóth G., Nemestóthy N., Bélafi-Bakó K. A critical review on issues and overcoming strategies for the enhancement of dark fermentative hydrogen production in continuous systems. Int. J. Hydrogen Energy. 2016;41:3820–3836. [Google Scholar]
- Skaggs R.L., Coleman A.M., Seiple T.E., Milbrandt A.R. Waste-to-Energy biofuel production potential for selected feedstocks in the conterminous United States. Renew. Sustain. Energy Rev. 2018;82:2640–2651. [Google Scholar]
- Smith A.D., Holtzapple M.T. Investigation of the optimal carbon-nitrogen ratio and carbohydrate-nutrient blend for mixed-acid batch fermentations. Bioresour. Technol. 2011;102:5976–5987. doi: 10.1016/j.biortech.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Smith A.D., Lockman N.A., Holtzapple M.T. Investigation of nutrient feeding strategies in a countercurrent mixed-acid multi-staged fermentation: experimental data. Appl. Biochem. Biotechnol. 2011;164:426–442. doi: 10.1007/s12010-010-9145-3. [DOI] [PubMed] [Google Scholar]
- Spekreijse J., Le Nôtre J., Van Haveren J., Scott E.L., Sanders J.P.M. Simultaneous production of biobased styrene and acrylates using ethenolysis. Green. Chem. 2012;14:2747–2751. [Google Scholar]
- Sporer A.J., Kahl L.J., Price-Whelan A., Dietrich L.E.P. Redox-based regulation of bacterial development and behavior. Annu. Rev. Biochem. 2017;86:777–797. doi: 10.1146/annurev-biochem-061516-044453. [DOI] [PubMed] [Google Scholar]
- SRI Consulting . Chemical Economics Handbook. SRI Consulting; 2008. U.S. producer price indexes – chemicals and allied products/industrial inorganic chemicals index. [Google Scholar]
- Stern D. The role of energy in economic growth. Ecol. Econ. Rev. 2011;1219:26–51. doi: 10.1111/j.1749-6632.2010.05921.x. [DOI] [PubMed] [Google Scholar]
- Straathof A. The Proportion of Downstream Costs in Fermentative Production Processes. In: Moo-Young M., editor. Comprehensive biotechnology. 2nd edn. Elsevier; 2011. pp. 811–814. [Google Scholar]
- Strazzera G., Battista F., Garcia N.H., Frison N., Bolzonella D. Volatile fatty acids production from food wastes for biorefinery platforms: a review. J. Environ. Manage. 2018;226:278–288. doi: 10.1016/j.jenvman.2018.08.039. [DOI] [PubMed] [Google Scholar]
- Tamis J., Joosse B.M., Loosdrecht M.C., Kleerebezem R. High-rate volatile fatty acid (VFA) production by a granular sludge process at low pH. Biotechnol. Bioeng. 2015;112:2248–2255. doi: 10.1002/bit.25640. [DOI] [PubMed] [Google Scholar]
- Thongchul N. Production of Lactic Acid and Polylactic Acid for Industrial Applications. In: Yang S.-T., El-Ensashy H., Thongchul N., editors. Bioprocessing technologies in biorefinery for sustainable production of fuels, chemicals, and polymers. Second Edition. Vol. 43. John Wiley and Sons; 2015. pp. 1556–1557. [Google Scholar]
- Toolbox E. Fuels - higher and lower Calorific values. 2003. https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html
- Tools H. Lower and higher heating values of fuels. https://www.h2tools.org/hyarc/calculator-tools/lower-and-higher-heating-values-fuels
- Wang K., Yin J., Shen D., Li N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: effect of pH. Bioresour. Technol. 2014;161:395–401. doi: 10.1016/j.biortech.2014.03.088. [DOI] [PubMed] [Google Scholar]
- Wasewar K.L., Pangarkar V.G., Heesink A.B.M., Versteeg G.F. Intensification of enzymatic conversion of glucose to lactic acid by reactive extraction. Chem. Eng. Sci. 2003;58:3385–3393. [Google Scholar]
- Wasewar K.L., Yawalkar A.A., Moulijn J.A., Pangarkar V.G. Fermentation of glucose to lactic acid coupled with reactive extraction: a review. Ind. Eng. Chem. Res. 2004;43:5969–5982. [Google Scholar]
- Waste360 EREF study Reveals increase in average MSW landfilling tipping Fee. 2018. https://www.waste360.com/landfill-operations/eref-study-shows-continued-increase-average-msw-landfill-tip-fees
- Woo H.C., Kim Y.H. Eco-efficient recovery of bio-based volatile C2-6 fatty acids. Biotechnol. Biofuels. 2019;12:92. doi: 10.1186/s13068-019-1433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Yang D., Zhou Q., Song Z. The effect of pH on anaerobic fermentation of primary sludge at room temperature. J. Hazard. Mater. 2009;172:196–201. doi: 10.1016/j.jhazmat.2009.06.146. [DOI] [PubMed] [Google Scholar]
- Wu Q.-L., Guo W.-Q., Zheng H.-S., Luo H.-C., Feng X.-C., Yin R.-L., Ren N.-Q. Enhancement of volatile fatty acid production by co-fermentation of food waste and excess sludge without pH control: the mechanism and microbial community analyses. Bioresour. Technol. 2016;216:653–660. doi: 10.1016/j.biortech.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Xiong H., Chen J., Wang H., Shi H. Influences of volatile solid concentration, temperature and solid retention time for the hydrolysis of waste activated sludge to recover volatile fatty acids. Bioresour. Technol. 2012;119:285–292. doi: 10.1016/j.biortech.2012.05.126. [DOI] [PubMed] [Google Scholar]
- Xiong B., Richard T.L., Kumar M. Integrated acidogenic digestion and carboxylic acid separation by nanofiltration membranes for the lignocellulosic carboxylate platform. J. Membr. Sci. 2015;489:275–283. [Google Scholar]
- Yang Q., Luo K., Li X.M., Wang D.B., Zheng W., Zeng G.M., Liu J.J. Enhanced efficiency of biological excess sludge hydrolysis under anaerobic digestion by additional enzymes. Bioresour. Technol. 2010;101:2924–2930. doi: 10.1016/j.biortech.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Yin J., Wang K., Yang Y., Shen D., Wang M., Mo H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresour. Technol. 2014;171:323–329. doi: 10.1016/j.biortech.2014.08.062. [DOI] [PubMed] [Google Scholar]
- Yu J. Production of PHA from starchy wastewater via organic acids. J. Biotechnol. 2001;86:105–112. doi: 10.1016/s0168-1656(00)00405-3. [DOI] [PubMed] [Google Scholar]
- Yuan H., Chen Y., Zhang H., Jiang S., Zhou Q., Gu G. Improved bioproduction of short-chain fatty acids (SCFAs) from excess sludge under alkaline conditions. Environ. Sci. Technol. 2006;40:2025–2029. doi: 10.1021/es052252b. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Hu X., Chen H., Zhou Y., Zhou Y., Wang D. Advances in enhanced volatile fatty acid production from anaerobic fermentation of waste activated sludge. Sci. Total Environ. 2019;694:133741. doi: 10.1016/j.scitotenv.2019.133741. [DOI] [PubMed] [Google Scholar]
- Yun J., Cho K.S. Effects of organic loading rate on hydrogen and volatile fatty acid production and microbial community during acidogenic hydrogenesis in a continuous stirred tank reactor using molasses wastewater. J. Appl. Microbiol. 2016;121:1627–1636. doi: 10.1111/jam.13316. [DOI] [PubMed] [Google Scholar]
- Zabel G. Peak people: the interrelationship between population growth and energy resources. Energy Bull. 2009;20 [Google Scholar]
- Zacharof M.P., Lovitt R.W. Complex effluent streams as a potential source of volatile fatty acids. Waste Biomass Valorization. 2013;4:557–581. [Google Scholar]
- Zacharof M.P., Mandale S.J., Williams P.M., Lovitt R.W. Nanofiltration of treated digested agricultural wastewater for recovery of carboxylic acids. J. Clean. Prod. 2016;112:4749–4761. [Google Scholar]
- Zhang B., Zhang L.L., Zhang S.C., Shi H.Z., Cai W.M. The influence of pH on hydrolysis and acidogenesis of kitchen wastes in two-phase Anaerobic digestion. Environ. Technol. 2005;26:329–340. doi: 10.1080/09593332608618563. [DOI] [PubMed] [Google Scholar]
- Zhang P., Chen Y., Zhou Q. Waste activated sludge hydrolysis and short-chain fatty acids accumulation under mesophilic and thermophilic conditions: effect of pH. Water Res. 2009;43:3735–3742. doi: 10.1016/j.watres.2009.05.036. [DOI] [PubMed] [Google Scholar]
- Zhang X., Luo R., Wang Z., Deng Y., Chen G.Q. Application of (R)-3-hydroxyalkanoate methyl esters derived from microbial polyhydroxyalkanoates as novel biofuels. Biomacromolecules. 2009;10:707–711. doi: 10.1021/bm801424e. [DOI] [PubMed] [Google Scholar]
- Zhang F., Chen Y., Dai K., Shen N., Zeng R.J. The glucose metabolic distribution in thermophilic (55°C) mixed culture fermentation: a chemostat study. Int. J. Hydrogen Energy. 2015;40:919–926. [Google Scholar]
- Zhao H., Xia S., Ma P. Use of ionic liquids as 'green' solvents for extractions. J. Chem. Technol. Biotechnol. 2005;80:1089–1096. [Google Scholar]
- Zhao B., Liu J., Frear C., Holtzapple M., Chen S. Consolidated bioprocessing of microalgal biomass to carboxylates by a mixed culture of cow rumen bacteria using anaerobic sequencing batch reactor (ASBR) Bioresour. Technol. 2016;222:517–522. doi: 10.1016/j.biortech.2016.09.120. [DOI] [PubMed] [Google Scholar]
- Zhao X.-X., Fan X.-L., Xue Z.-X., Yang Z.-M., Yuan X.-Z., Qiu Y.-L., Guo R.-B. Simultaneous production of hydrogen and volatile fatty acids from anaerobic digestion of Macrocystis pyrifera biomass residues. J. Cent. South Univ. 2017;24:1281–1287. [Google Scholar]
- Zhao J., Wang D., Liu Y., Ngo H.H., Guo W., Yang Q., Li X. Novel stepwise pH control strategy to improve short chain fatty acid production from sludge anaerobic fermentation. Bioresour. Technol. 2018;249:431–438. doi: 10.1016/j.biortech.2017.10.050. [DOI] [PubMed] [Google Scholar]
- Zheng X., Chen Y., Liu C. Waste activated sludge alkaline fermentation liquid as carbon source for biological nutrients removal in anaerobic followed by alternating aerobic-anoxic sequencing batch reactors. Chin. J. Chem. Eng. 2010;18:478–485. [Google Scholar]
- Zhou F., Wang C., Wei J. Separation of acetic acid from monosaccharides by NF and RO membranes: performance comparison. J. Membr. Sci. 2013;429:243–251. [Google Scholar]
- Zhou M., Yan B., Wong J.W.C., Zhang Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: a mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018;248:68–78. doi: 10.1016/j.biortech.2017.06.121. [DOI] [PubMed] [Google Scholar]
- Zigová J., Šturdík E., Vandák D., Schlosser S. Butyric acid production by Clostridium butyricum with integrated extraction and pertraction. Process Biochem. 1999;34:835–843. [Google Scholar]
- Łukajtis R., Hołowacz I., Kucharska K., Glinka M., Rybarczyk P., Przyjazny A., Kamiński M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018;91:665–694. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any data utilized in this study can be found the main manuscript and Supplemental Information.