Abstract
Background/purpose
Platelet-rich fibrin (PRF) can be obtained by centrifuging fresh blood in the absence of anticoagulants. Varying the centrifugation speeds may produce tougher and richer concentrated growth factors (CGF). This study examines tensile strength, growth factor content, and the potential of CGF and PRF in promoting periodontal cell proliferation.
Materials and methods
Blood (40 mL/subject) was collected from 44 healthy subjects. PRF and CGF were prepared by centrifuging at 3000 rpm and switching speeds ranging within 3000 rpm, respectively. Fibrin strip was prepared and its tensile strength was measured. Transforming growth factor beta 1 (TGF-β1), platelet-derived growth factor BB (PDGF-BB), and epidermal growth factor (EGF) in the residual serum and fibrin clots were determined by enzyme-linked immunosorbent assay, and their effects on the proliferation of hFOB1.19 osteoblasts and human gingival fibroblasts were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay.
Results
Compared with PRF, tensile strength of CGF was significantly higher. Concentrations and amounts of PDGF-BB and EGF in CGF were significantly higher than those in PRF. Osteoblast number was significantly higher in the cultures with fetal bovine serum (FBS, 10%) and with PRF or CGF fibrin clots (5%, 10%, and 50%) compared to that without FBS. Moreover, osteoblast number in CGF, regardless of the preparation of 10% and 50%, was significantly greater than that in PRF. Similar findings were also observed for gingival fibroblasts among the various subjects.
Conclusion
Varying centrifugation speeds can modify the tensile strength and biological activities of platelet fibrin clots.
Keywords: Concentrated growth factor, Growth factors, Platelet-rich fibrin, Periodontal cell proliferation, Tensile strength
Introduction
Autologous platelet concentrate of platelet-rich plasma (PRP) has been used in various surgeries, including neck surgery, otolaryngology, cardiovascular surgery, and maxillofacial surgery, because PRP contains high concentration of growth factors.1 However, there are some drawbacks of employing PRP such as having to add animal-derived thrombin for clotting, low handling efficiency, and fundamental individual differences.2
Platelet-rich fibrin (PRF) is developed in the absence of anticoagulant.3 The preparation has been simplified to collecting blood and centrifuging immediately to form fibrin clots. In dental practice, PRF has received wide application in dental surgeries, including periodontal regeneration,4,5 root coverage,6 alveolar ridge preservation,7 and sinus lift,8 due to its potential ability to regulate vascular response9 and thus achieve accelerating wound healing via earlier vessel formation and tissue maturation.6
By varying the centrifugation speed, the concentrated growth factor (CGF), originally developed by Sacco, has been characterized as a relatively stiffer fibrin clot.10 CGF can significantly promote the proliferation and osteo-induction of periodontal ligament stem cells in vitro11 and bone tissue engineering in vivo.12 As such, CGF shows great potential in numerous clinical and biotechnological applications. Although different centrifugation speeds may theoretically permit the isolation of a fibrin matrix that is larger, denser, and richer in growth factors,10 the knowledge about the physical and biological activities of the fibrin clots of PRF and CGF is still limited. In the present study, the tensile strength, growth factor contents, and the proliferation activities of osteoblasts and gingival fibroblasts of the PRF and CGF were evaluated and compared in vitro.
Materials and methods
Patient pool and blood preparation
A total of 44 subjects (19 females and 25 males) with the mean age of 42 years (range: 22–84 years) were recruited for this study. The study was approved by the Institutional Review Board of Buddhist Tzu Chi General Hospital (New Taipei City, Taiwan; No. 01-X23-044), and the blood samples were collected from July to December 2015. After each collection, half of the blood was used for PRF preparation and the other half for CGF preparation.
PRF and CGF preparation
PRF and CGF were prepared as stated in previous studies.3,13 In brief, 40 mL of blood was drawn from the forelimb vein and gently pushed into four 10 mL venous blood collection tubes (BD, Franklin Lakes, NJ, USA) without anticoagulant. Two tubes, containing 20 mL blood, were immediately centrifuged at 3000 rpm for 12 min using a HSIANGTA centrifuge (HSIANGTA, New Taipei City, Taiwan). After discarding the red blood cells, the PRF and the remaining serum were collected and immediately stored at −80 °C. Another two tubes containing 18 mL blood were centrifuged using MEDIFUGE centrifuge (MEDIFUGE MF 200 100, Sofia, Italy) at ∼0–2700 rpm for 20 s, 2700 rpm for 2 min, 2400 rpm for 4 min, 2700 rpm for 2 min, 3000 rpm for 3 min, and then stopped from 3000 to 0 rpm in about 36 s to obtain the CGF. Similar to that for PRF, the CGF and the serum were stored at −80 °C until use, except part of PRF and CGF samples were immediately taken for the tensile strength measuring. For those frozen samples, after defreezing and diluting (using phosphate-buffered saline or culture media (Leibovitz L-15, Invitrogen, Grand Island, NY, USA), 1 mL/g weight), the fibrin clots were sonicated (BRANSON, Danbury, CT, USA) with the frequency of 20/min for 3 min. The supernatants were collected after centrifuging (12,000 rpm for 10 min at 4 °C) to determine the growth factor concentrations.
Protein assessment of growth factors by enzyme-linked immunosorbent assay
The levels of transforming growth factor beta 1 (TGF-β1), platelet-derived growth factor BB (PDGF-BB), and epidermal growth factor (EGF) were assayed using commercially available enzyme-linked immunosorbent assay (ELISA kits, R&D system, Minneapolis, MN, USA).
Tensile strength
The freshly prepared PRF and CGF were immediately placed into a premade acrylic box (1 × 1 × 0.5 cm), and the strip was prepared after a gentle compression to squeeze out the serum. The tensile strength was then measured using the digital sensor (DSCUSB, Mantracourt Electronics Ltd., Exeter, UK).
Human gingival fibroblasts (HGFs)
HGFs were obtained from three patients in this study using methods described in our previous study.14 In brief, gingival specimens were collected from gingivectomy samples using crown lengthening or distal wedge procedure of non-inflamed periodontal tissues. The specimens were immediately immersed in a medium containing 2 mg/mL protease and 10% fetal bovine serum (FBS) (Gibco, Waltham, MA, USA) at 4 °C for 2 days. After separation from the outer epithelial layer, the connective tissue was minced and digested in a medium containing 10% FBS and 2 mg/mL collagenase (Sigma–Aldrich, St. Louis, MO, USA) for 24 h. HGFs under five to seven passages were used for experiments. All the procedures were approved by the ethics committee of the Faculty of Medicine, Taipei Tzu Chi Hospital, Taiwan.
hFOB1.19 cells
hFOB1.19 cell line was obtained from the Bioresource Collection and Research Center, Hsinchu, Taiwan. Cells were maintained in Dulbecco's minimal essential medium (DMEM/F12, Invitrogen, Grand Island, NY, USA) without phenol red supplementation and containing 10% heat-inactivated FBS and 0.3 mg/mL G418 at 34 °C in a humidified atmosphere with 5% CO2.
Cell proliferation
HGF cells (1.5 × 104 cells/mL) and hFOB1.19 cells (104 cells/mL) were cultured in 96-well culture plates separately. After confirming the adherence of the cells to the plates, the medium was replaced with 5%, 10%, and 50% PRF- or CGF-containing medium, as well as with the new medium containing 25% and 50% serum, and the cells were cultured for 48 h. Cell proliferation was determined by the 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Sigma–Aldrich Corp, St. Louis, MO, USA).
Statistical analysis
Data are presented as mean and standard deviation. The paired t-test was used to compare the tensile strengths obtained and the concentrations of the growth factors in the serum or fibrin clots prepared by PRF and CGF. One-way ANOVA was used to examine the effects of FBS, PRF/CGF serum, or fibrin clots on cell proliferation, while the post hoc analysis by Duncan's test was further used to check if significance was obtained. A value of p < 0.05 was considered as statistically significant.
Results
The tensile strength obtained in the CGF group (2.2 ± 0.7 N) was significantly greater than that in the PRF group (1.7 ± 0.7 N) (Fig. 1). The PDGF and EGF levels, including the concentrations and amounts, in CGF were significantly greater than those in PRF; however, TGF levels were similar in both PRF and CGF. In the serum, the concentrations of the growth factors were much less than those in the fibrin clots. For each growth factor examined, no statistical difference was noted between the CGF and PRF groups, except for EGF levels (Table 1).
Figure 1.
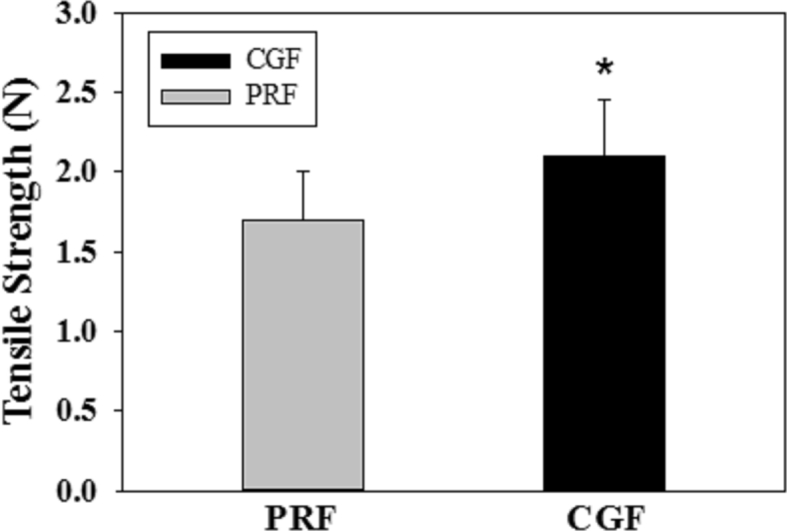
Comparison of the tensile strengths obtained from PRF and CGF fibrin clots (n = 11; N = Newton; ∗: significantly different with PRF at p < 0.05, by paired t-test).
Table 1.
Comparison of the retaining growth factors in PRF and CGF fibrin clots, as well as their remaining sera, including TGF, PDGF, and EGF.
| Protein concentration |
Protein amount |
|||||
|---|---|---|---|---|---|---|
| PRF | CGF | P value | PRF | CGF | P value | |
| Fibrin clots | ||||||
| TGF-β | 84.7 ± 57.9 | 84.4 ± 62.9 | 0.952 | 31.8 ± 25.2 | 31.4 ± 26.1 | 0.896 |
| PDGF | 3.2 ± 2.9 | 4.6 ± 3.6 | <0.003∗ | 1.1 ± 1.0 | 1.6 ± 1.1 | 0.006∗ |
| EGF | 4.7 ± 2.4 | 6.1 ± 3.0 | <0.001∗ | 1.7 ± 1.1 | 2.2 ± 1.1 | 0.002∗ |
| Serum | ||||||
| TGF-β | 8.1 ± 5.7 | 9.1 ± 3.5 | 0.136 | |||
| PDGF | 0.3 ± 0.5 | 0.3 ± 0.3 | 0.218 | |||
| EGF | 0.2 ± 0.2 | 0.3 ± 0.2 | <0.03∗ | |||
Data presented as mean and standard deviation.
∗Significant difference between that in PRF and CGF at p < 0.05.
Unit of 103 pg/mL and 103 pg for the protein concentration and amount, respectively.
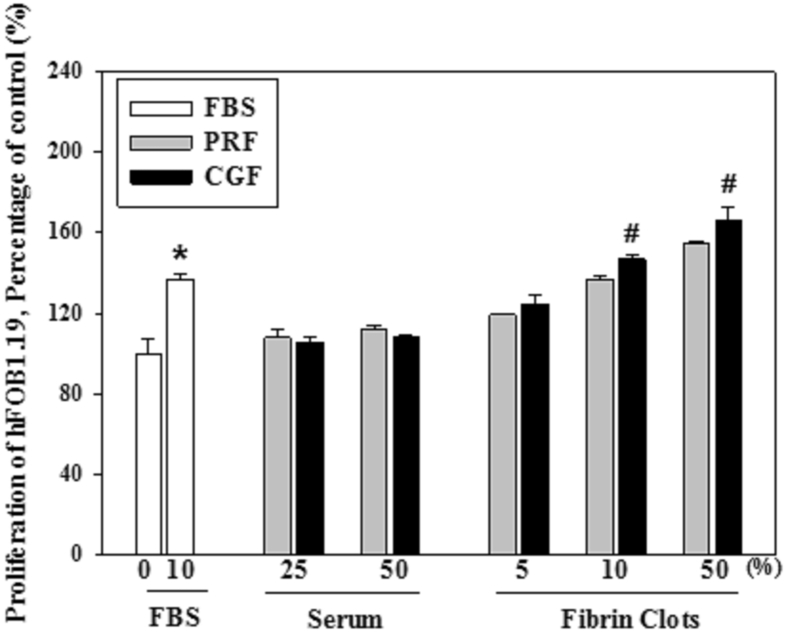
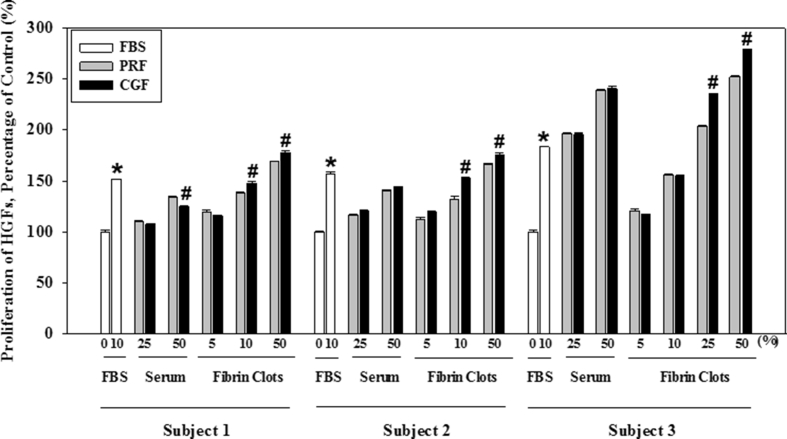
The osteoblast cell number was significantly less in the control dish without FBS when compared with that with 10% FBS, the positive control. The osteoblast number in those with fibrin clots from CGF with the high concentration of 10% or 50% was significantly greater than that from PRF (Fig. 2). Similar findings were observed for gingival fibroblasts, among the various subjects (Fig. 3).
Figure 2.
Effect of the serum or the fibrin clots prepared by PRF and CGF on the proliferation of hFOB1.19 osteoblasts (∗ and #: significantly different to those dishes with 0% FBS and that with PRF, respectively, at p < 0.05 by Duncan's post hoc test).
Figure 3.
Effect of the serum or the fibrin clots prepared by PRF and CGF on the proliferation of human gingival fibroblasts obtained from three subjects. (∗ indicates significant difference vs 0% FBS and # presents the difference vs that of PRF, respectively, at p < 0.05 by Duncan's post hoc test).
Discussion
In this study, the tensile strengths and growth factor contents of PRF and CGF were evaluated and compared (Fig. 1 and Table 1). Although the exact mechanism related to the mechanical and biological properties was still uncertain, the mode of polymerization of the final fibrin matrix in the absence of anticoagulant has considerable influence on these properties.3 The three-dimensional organization of a fibrin network, polymerizing naturally and slowly during centrifugation, could be crucial.15 Recently, the finding that a thicker and a more regular pattern of fibrin was observed in CGF than that in PRF16 might partly explain our result of the greater tensile strength and growth factor content observed in CGF compared to those in PRF. Our results showed that the contents of the growth factors, despite the amount and concentration, were greater in CGF than those in PRF (Table 1). When compared with PRP, a greater level of growth factors was observed in PRF and its derivatives, such as CGF and advanced PRF (A-PRF).17,18 However, the levels of growth factors in CGF and A-PRF were similar. Nevertheless, certain factors such as rotation duration or speed19 and shape and material of the blood collection tube20 may influence the growth factor contents in the preparation of the platelet concentrates.
The in vitro effects of PRF or CGF on cell proliferation, including the osteoblasts and the gingival fibroblasts, were also examined in this study. Our result showed that the cell number, regardless of fibroblasts or osteoblasts, was significantly increased in the dish with any preparation of the PRF/CGF fibrin clots, especially in the groups with high concentrations of 10% and 50% when compared to that in the control dish without FBS (Figure 2, Figure 3). A similar result of enhanced proliferation of periodontal ligament and osteoblast cells was observed after PRP stimulation in a previous study.21 The present study further demonstrated that the cell numbers in the dishes with CGF were significantly greater than those with PRF. The greater content of growth factors in CGF than in PRF might explain the results of enhanced cell proliferation (Table 1). In a recent experiment using mice calvaria, three-times condensed PRP (freeze-dried and then rehydrated) induced more bone formation after onlay grafting of tricalcium phosphate scaffold than that with normal concentration of PRP alone.22 The authors, therefore, suggested that the enhancement might be attributed to the greater contents of growth factors in the condensed PRP.
Recently, it has been shown that certain cells, including the inflammatory cells of monocytes and macrophages and the stem cells, are retained in PRF fibrins.23 Although the role of stem cells in the fibrin clots is still unknown, the hypothesis of using PRP as a delivery system (in providing the growth factors and fibrin scaffold) for the stem cells has been tested and evaluated in the treatment of tendon injuries and periodontal diseases.24, 25, 26 While the effects of platelet concentrates on periodontal and oral maxillary healing/regeneration are under investigation,4,27, 28, 29, 30, 31, 32 the clinical efficacy of platelet concentrates is still controversial, and a consensus has yet to be completely reached.2,33,34
In conclusion, in the absence of anticoagulant, the platelet concentrates of blood fibrin clots rich in growth factors can be successfully collected by centrifuging either at a constant speed or at varying speeds. However, speed switching may produce fibrin clots with a stronger tensile strength, richer growth factors, and higher ability to induce the proliferation of osteoblasts and gingival fibroblasts.
Declaration of competing interest
The authors report no conflicts of interest related to this study.
Acknowledgments
This study was supported by the grant from Taipei Tzu Chi Hospital (Buddhist Tzu Chi Medical Foundation, TCRD-TPE-104-RT-3), Taiwan, ROC.
References
- 1.Albanese A., Licata M.E., Polizzi B., Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing. 2013;10:23. doi: 10.1186/1742-4933-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawase T. Platelet-rich plasma and its derivatives as promising bioactive materials for regenerative medicine: basic principles and concepts underlying recent advances. Odontology. 2015;103:126–135. doi: 10.1007/s10266-015-0209-2. [DOI] [PubMed] [Google Scholar]
- 3.Dohan D.M., Choukroun J., Diss A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Castro A.B., Meschi N., Temmerman A. Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J Clin Periodontol. 2017;44:67–82. doi: 10.1111/jcpe.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panda S., Doraiswamy J., Malaiappan S., Varghese S.S., Del Fabbro M. Additive effect of autologous platelet concentrates in treatment of intrabony defects: a systematic review and meta-analysis. J Investig Clin Dent. 2016;7:13–26. doi: 10.1111/jicd.12117. [DOI] [PubMed] [Google Scholar]
- 6.Eren G., Kantarci A., Sculean A., Atilla G. Vascularization after treatment of gingival recession defects with platelet-rich fibrin or connective tissue graft. Clin Oral Investig. 2016;20:2045–2053. doi: 10.1007/s00784-015-1697-8. [DOI] [PubMed] [Google Scholar]
- 7.Suttapreyasri S., Leepong N. Influence of platelet-rich fibrin on alveolar ridge preservation. J Craniofac Surg. 2013;24:1088–1094. doi: 10.1097/SCS.0b013e31828b6dc3. [DOI] [PubMed] [Google Scholar]
- 8.Jeong S.M., Lee C.U., Son J.S. Simultaneous sinus lift and implantation using platelet-rich fibrin as sole grafting material. J Craniomaxillofac Surg. 2014;42:990–994. doi: 10.1016/j.jcms.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Martinez C.E., Smith P.C., Palma Alvarado V.A. The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Front Physiol. 2015;6:290. doi: 10.3389/fphys.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodella L.F., Favero G., Boninsegna R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech. 2011;74:772–777. doi: 10.1002/jemt.20968. [DOI] [PubMed] [Google Scholar]
- 11.Yu B., Wang Z. Effect of concentrated growth factors on beagle periodontal ligament stem cells in vitro. Mol Med Rep. 2014;9:235–242. doi: 10.3892/mmr.2013.1756. [DOI] [PubMed] [Google Scholar]
- 12.Honda H., Tamai N., Naka N., Yoshikawa H., Myoui A. Bone tissue engineering with bone marrow-derived stromal cells integrated with concentrated growth factor in Rattus norvegicus calvaria defect model. J Artif Organs. 2013;16:305–315. doi: 10.1007/s10047-013-0711-7. [DOI] [PubMed] [Google Scholar]
- 13.Choukroun J., Diss A., Simonpieri A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56–e60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Kuo P.J., Tu H.P., Chin Y.T. Cyclosporine-A inhibits MMP-2 and -9 activities in the presence of Porphyromonas gingivalis lipopolysaccharide: an experiment in human gingival fibroblast and U937 macrophage co-culture. J Periodontal Res. 2012;47:431–438. doi: 10.1111/j.1600-0765.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- 15.Mosesson M.W., Siebenlist K.R., Meh D.A. The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:11–30. doi: 10.1111/j.1749-6632.2001.tb03491.x. [DOI] [PubMed] [Google Scholar]
- 16.Park H.C., Kim S.G., Oh J.S. Early bone formation at a femur defect using CGF and PRF grafts in adult dogs: a comparative study. Implant Dent. 2016;25:387–393. doi: 10.1097/ID.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 17.Masuki H., Okudera T., Watanebe T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF) Int J Implant Dent. 2016;2:19. doi: 10.1186/s40729-016-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi E., Fluckiger L., Fujioka-Kobayashi M. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20:2353–2360. doi: 10.1007/s00784-016-1719-1. [DOI] [PubMed] [Google Scholar]
- 19.Eren G., Gurkan A., Atmaca H., Donmez A., Atilla G. Effect of centrifugation time on growth factor and MMP release of an experimental platelet-rich fibrin-type product. Platelets. 2016;27:427–432. doi: 10.3109/09537104.2015.1131253. [DOI] [PubMed] [Google Scholar]
- 20.Bonazza V., Borsani E., Buffoli B. How the different material and shape of the blood collection tube influences the Concentrated Growth Factors production. Microsc Res Tech. 2016;79:1173–1178. doi: 10.1002/jemt.22772. [DOI] [PubMed] [Google Scholar]
- 21.Okuda K., Kawase T., Momose M. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74:849–857. doi: 10.1902/jop.2003.74.6.849. [DOI] [PubMed] [Google Scholar]
- 22.Nakatani Y., Agata H., Sumita Y., Koga T., Asahina I. Efficacy of freeze-dried platelet-rich plasma in bone engineering. Arch Oral Biol. 2017;73:172–178. doi: 10.1016/j.archoralbio.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Ghanaati S., Booms P., Orlowska A. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40:679–689. doi: 10.1563/aaid-joi-D-14-00138. [DOI] [PubMed] [Google Scholar]
- 24.Voss A., McCarthy M.B., Allen D. Fibrin scaffold as a carrier for mesenchymal stem cells and growth factors in shoulder rotator cuff repair. Arthrosc Tech. 2016;5:e447–e451. doi: 10.1016/j.eats.2016.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J.H., Nirmala X. Application of tendon stem/progenitor cells and platelet-rich plasma to treat tendon injuries. Oper Tech Orthop. 2016;26:68–72. doi: 10.1053/j.oto.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z.S., Feng Z.H., Wu G.F. The use of platelet-rich fibrin combined with periodontal ligament and jaw bone mesenchymal stem cell sheets for periodontal tissue engineering. Sci Rep. 2016;6:28126. doi: 10.1038/srep28126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal A., Gupta N.D., Jain A. Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: a randomized split mouth clinical trail. Acta Odontol Scand. 2016;74:36–43. doi: 10.3109/00016357.2015.1035672. [DOI] [PubMed] [Google Scholar]
- 28.Aydemir Turkal H., Demirer S., Dolgun A., Keceli H.G. Evaluation of the adjunctive effect of platelet-rich fibrin to enamel matrix derivative in the treatment of intrabony defects. Six-month results of a randomized, split-mouth, controlled clinical study. J Clin Periodontol. 2016;43:955–964. doi: 10.1111/jcpe.12598. [DOI] [PubMed] [Google Scholar]
- 29.Chadwick J.K., Mills M.P., Mealey B.L. Clinical and radiographic evaluation of demineralized freeze-dried bone allograft versus platelet-rich fibrin for the treatment of periodontal intrabony defects in humans. J Periodontol. 2016;87:1253–1260. doi: 10.1902/jop.2016.160309. [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee A., Pradeep A.R., Garg V. Treatment of periodontal intrabony defects using autologous platelet-rich fibrin and titanium platelet-rich fibrin: a randomized, clinical, comparative study. J Investig Clin Dent. 2017;8:e1231. doi: 10.1111/jicd.12231. [DOI] [PubMed] [Google Scholar]
- 31.Kanoriya D., Pradeep A.R., Garg V., Singhal S. Mandibular degree II furcation defects treatment with platelet-rich fibrin and 1% Alendronate gel combination: a randomized controlled clinical trial. J Periodontol. 2017;88:250–258. doi: 10.1902/jop.2016.160269. [DOI] [PubMed] [Google Scholar]
- 32.Sohn D.S., Heo J.U., Kwak D.H. Bone regeneration in the maxillary sinus using an autologous fibrin-rich block with concentrated growth factors alone. Implant Dent. 2011;20:389–395. doi: 10.1097/ID.0b013e31822f7a70. [DOI] [PubMed] [Google Scholar]
- 33.Mihaylova Z., Mitev V., Stanimirov P. Use of platelet concentrates in oral and maxillofacial surgery: an overview. Acta Odontol Scand. 2017;75:1–11. doi: 10.1080/00016357.2016.1236985. [DOI] [PubMed] [Google Scholar]
- 34.Bajaj P., Pradeep A.R., Agarwal E. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: a randomized controlled clinical trial. J Periodontal Res. 2013;48:573–581. doi: 10.1111/jre.12040. [DOI] [PubMed] [Google Scholar]