Abstract
Background/purpose
Pediatric obstructive sleep apnea (OSA) might be a serious cause of neurocognitive deficits, behavioral changes, and craniofacial disharmony in children at very young age with mild type of OSA. This study aims to examine the effect of mild OSA on craniofacial morphology as well as dental arch morphology and characteristics in preschool children.
Materials and methods
The test group comprised 16 preschool children (11 boys, 5 girls; mean age: 5.14 years old; mean AHI: 2.02) with confirmed polysomnographic diagnosis of mild OSA. Ten control subjects also underwent polysomnography (5 boys, 5 girls; mean age: 5.18 years old; median AHI: 0.43). Lateral cephalometric radiographs and dental arch impressions were obtained and measured. A survey on characteristics and quality of life (OSA-18) was filled out by study participants' caregivers.
Results
For craniofacial morphology, a significant increase in ANB angle, a decrease in SNB angle, and larger overjet size were seen in the group with mild OSA, compared with the control group. More frequent sleep disturbances and mood swing were also found in children with mild OSA, based on the OSA-18 assessment.
Conclusion
Preschool children with mild OSA present the following: skeletal Class II pattern with a more retrognathic mandible, increased overjet size, and more pronounced symptoms in the domains of sleep and emotion. Dental arch constriction is not a typical feature in our sample of Asian preschool children with mild OSA.
Keywords: Cephalometric analysis, Dental arch morphology, Obstructive sleep apnea, OSA-18, Preschool children
Introduction
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder characterized by partial or complete episodes of upper airway obstruction. OSA occurs in all ages of children, most commonly in preschool children,1,2 with a prevalence of approximately 2%.2, 3, 4 Unlike the adult OSA, only a small percentage of OSA children experience sleepiness during the day. Instead, they are more prone to neurocognitive deficits, behavioral changes, or attention-deficit/hyperactivity disorder.5,6
A number of risk factors are associated with childhood OSA. Adenotonsillar hypertrophy is considered a major risk factor.7 Other potential risk factors include craniofacial abnormalities, dental arch morphology, tongue size, soft palate size, and childhood obesity. For example, previous studies have found craniofacial abnormalities such as high angle face, greater mandibular plane angle, larger anterior facial height, and retrognathic mandible in preschool children with OSA.8, 9, 10 Only one study has investigated dental arch morphology during the primary dentition period as a predisposing factor for OSA among preschoolers. Lofstrand et al. found that children with sleep obstruction and OSA demonstrated significantly narrower maxillary arch and deeper palatal vault.8
Currently, overnight polysomnography (PSG) is considered the gold standard for the diagnosis of OSA.11 The Apnea-Hypopnea-Index (AHI) is the most commonly used parameter to classify the severity of OSA. The OSA-18 developed by Franco et al. is a disease-specific questionnaire commonly used for evaluating the characteristics and quality of life among children with OSA.12 It has been suggested that clinicians should consider both the OSA severity and its impact on quality of life when managing pediatric OSA.13
To the best of our knowledge, no previous studies have focused on the effect of mild obstructive sleep apnea (OSA) on craniofacial morphology as well as dental arch morphology and characteristics in preschool children with mild OSA during the primary dentition period. Hence, the objectives of the present investigation were:
-
1.
To use PSG as a diagnostic tool to recruit preschool children with mild OSA (1/hour < AHI ≤ 5/hour), and children with AHI < 1 as normal controls;
-
2.
To compare the characteristics between children diagnosed with mild OSA and normal controls by using the OSA-18 questionnaire; and
-
3.
To compare the dental arch and craniofacial morphology during the primary dentition period between the preschool children diagnosed with mild OSA and normal controls.
Materials and methods
This study (104-9308A3) and the protocol (201601757A3C501) were approved by the Institutional Review Board of the Chang Gung Memorial Hospital, Taiwan. Informed consent was obtained from participating children's parents.
Subjects: The subjects in the test group were referred to the Department of Pediatric Dentistry from the Department of Pediatric Psychiatry in the Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan. The chief complaints were snoring and symptoms of obstructive sleep disorder. The control group consisted of siblings of the test group and they showed no symptoms of obstructive sleep disorder. The weight and height were measured for all subjects and the body mass index (BMI) was calculated (kg/m2).
The inclusion criteria for the test group were: 1) children with primary dentition, and 2) children with a diagnosis of mild OSA confirmed by overnight PSG. The criteria were the same for the control group, except that the AHI was less than 1 for overnight PSG. Exclusion criteria for both groups were: 1) children with the craniofacial syndrome or a history of adenotonsillectomy surgery, and 2) children with missing teeth.
Study model measurements: All subjects had upper and lower dental impressions. Plaster models of dental impressions were scanned using a 3D scanning system (3shape Dental System D640, 3shape A/S, Copenhagen, Denmark) with an accuracy of 15 μm. The casts were measured digitally using Simplant O&O software (Materialise Dental NV, Leuven, Belgium) by the same investigator (the first author of this study) who did not know the group allocation of the models.
The following were the parameters measured (Fig. 1):
-
1.
Molar relation: Molar relations are classified as mesial step, flush, and distal step by the terminal plane of lower primary second molar in relation to that of the upper primary second molar;
-
2.
Intercanine width: Distance between the midpoints of the canine cusp;
-
3.
Intermolar width: Distance between the centroid of the second primary molars;
-
4.
Upper arch length: Distance from incisive papilla to a line connecting the extreme distal points of the upper second primary molars;
-
5.
Lower arch length: Distance from the tip of gingival papilla between lower central incisors to a line connecting the extreme distal points of the lower second primary molars; and
-
6.
Palatal height: Palatal height was defined as the distance from the midpoint of palatal vault between primary molars to the occlusal plane. An occlusal plane was defined by incisal edges of central incisors and the tip of palatal cusp of primary second molars.
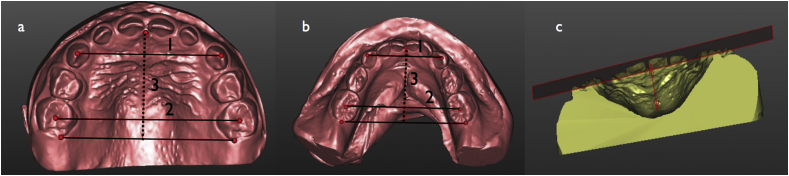
Figure 1.
a & b: 1) Intercanine width. 2) Intermolar width. 3) Arch length (dotted line). c: Study model measurements of palatal height.
Lateral cephalometric measurements: Lateral cephalometric radiographs were obtained for every subject with their head in natural position and teeth in centric occlusion. These lateral cephalometric radiographs were measured digitally by the same investigator (the first author of this study) using the PACS (Picture Archiving & Communication System) software. The investigator was blinded to the group allocation of the subjects. The landmarks and measurements were shown in Fig. 2.
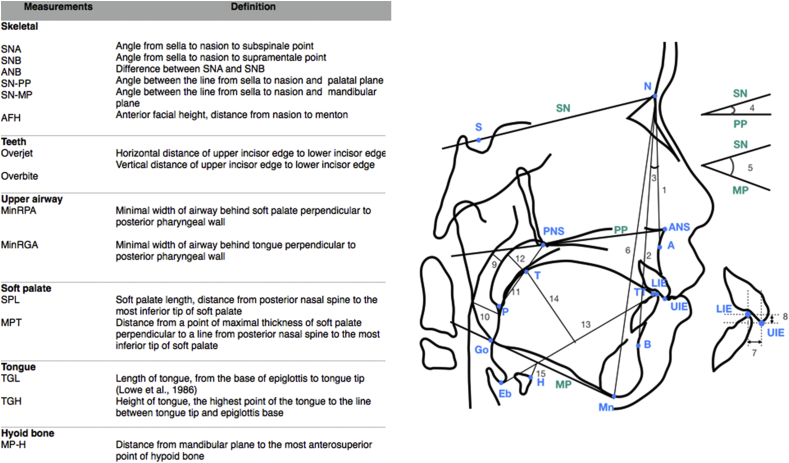
Figure 2.
A diagrammatic representation and definition of landmarks, reference lines, and variables used to identify facial skeleton, teeth, upper airway, soft palate, tongue, and hyoid bone. A: Subspinale. ANS: Anterior nasal spine. B: Supramentale. Eb: Epiglottis base. Go: Gonion. H: The most anterosuperior point of the hyoid bone. LIE: Lower incisor edge. Mn: Menton, the most inferior point on symphyseal outline. MP: Mandibular plane. N: Nasion. P: The most inferior tip of soft palate. PNS: Posterior nasal spine. PP: Palatal plane. S: Sella. SN: Line from sella to nasion. T: The highest point of tongue dorsum. TT: Tongue tip. UIE: Upper incisor edge. 1. SNA 2. SNB 3. ANB 4. SN-PP 5. SN-MP 6. AFH 7. Overjet 8. Overbite 9. MinRPA 10. MinRGA 11. SPT 12. MPT 13. TGL 14. TGH 15. MPH.
Characteristics and quality of life survey: The caregivers of all subjects were asked to complete the Chinese version of OSA-18 which is divided into two parts. Part one: a questionnaire on characteristics. The questionnaire consists of 18 items along five domains (sleep disturbance, physical symptom, emotional symptom, daytime function, and caregiver concern). Each item is graded on a seven-point scale ranging from one (none of the time) to seven (all of the time) to evaluate the severity of the problem. The total score, the domain score, and the item score were recorded. Part two: quality of life. The caregiver used a 10-point visual analogue scale with specific semantic anchors to provide a direct global rating of OSA-related quality of life (QOL) of participating children.
Measurement error: To calculate the error of method, 10 randomly selected models and radiographs were measured again by one investigator (the first author of this study) one month after the initial measurements under the same condition.
Statistical analysis: The statistical analysis was performed using the SPSS statistical package (version 20, IBM Corp., Armonk, N.Y.). Intra-rater reliability was measured by the intraclass correlation coefficients for linear and angular measurements. Descriptive statistics were presented as mean and standard deviation. Linear and angular measurements for the two study groups were compared using the Mann–Whitney U test, and for categorical variables, the Fisher's exact test was used. A p value less than 0.05 was considered statistically significant.
Results
The intraclass correlation coefficients for intra-rater reliability ranged from 0.964 to 0.997 for the study model measurements and from 0.846 to 0.998 for the lateral cephalometric measurements.
As shown in Table 1, the test group consists of eleven boys and five girls while the control group consists of five boys and five girls. The age, BMI, and sex distributions did not differ significantly between the two groups.
Table 1.
Demographic data of study subjects.
| Test group (n = 16) |
Control group (n = 10) |
p-value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (year) | 5.14 (0.79) | 5.18 (0.62) | 0.730 |
| BMI | 15.38 (2.24) | 15.19 (1.39) | 0.979 |
| AHI | 2.02 (0.85) | 0.43 (0.22) | <0.001* |
*p-value < 0.05, which is deemed statistically significant.
BMI: Body-mass-index; AHI: Apnea-hypopnea-index.
Study model measurements: No significant difference was found in the distribution of molar relation between the two groups (Table 2). There was also no statistically significant difference in linear measurements between the groups (Table 3).
Table 2.
The frequency and distribution of molar relation in the test group and the control group.
| Test group (n = 16) |
Control group (n = 10) |
p-value | |
|---|---|---|---|
| number (%) | number (%) | ||
| Mesial step | 3 (18.75) | 2 (20) | 0.446 |
| Flush terminal plane | 11 (68.75) | 7 (70) | |
| Distal step | 2 (12.5) | 1 (10) |
Table 3.
Study model and lateral cephalometric measurements in the test group and the control group.
| Test group (n = 16) |
Control group (n = 10) |
p-value |
|
|---|---|---|---|
| Mean (SD) |
Mean (SD) |
||
| Study model measurements | |||
| Upper arch | |||
| Inter-canine width | 29.84 (3.55) | 28.20 (1.75) | 0.356 |
| Inter-molar width | 38.82 (3.40) | 39.47 (1.52) | 0.833 |
| Arch length | 24.99 (1.64) | 24.23 (1.40) | 0.126 |
| Palatal height | 13.30 (1.36) | 13.49 (1.30) | 0.772 |
| Lower arch | |||
| Inter-canine width | 21.70 (1.83) | 21.89 (1.08) | 0.812 |
| Inter-molar width | 34.37 (2.99) | 34.64 (1.43) | 0.874 |
| Arch length |
22.37 (1.42) |
22.16 (1.31) |
0.712 |
| Lateral cephalometric measurements | |||
| Skeletal | |||
| SNA (degree) | 80.64 (2.31) | 81.03 (2.05) | 0.712 |
| SNB (degree) | 75.05 (1.96) | 76.97 (2.45) | 0.045* |
| ANB (degree) | 5.60 (1.60) | 3.83 (1.57) | 0.015* |
| SN-PP (degree) | 6.59 (2.28) | 7.24 (2.20) | 0.673 |
| SN-MP (degree) | 39.22 (3.28) | 37.04 (4.80) | 0.162 |
| AFH (mm) | 103.24 (4.57) | 100.48 (3.65) | 0.162 |
| Teeth | |||
| Overjet (mm) | 3.11 (1.04) | 1.87 (1.18) | 0.023* |
| Overbite (mm) | 1.66 (0.86) | 1.88 (1.31) | 0.291 |
| Upper airway | |||
| MinRPA (mm) | 6.11 (3.19) | 5.49 (1.25) | 0.792 |
| MinRGA (mm) | 8.21 (2.82) | 7.66 (2.29) | 0.635 |
| Soft palate | |||
| SPL (mm) | 26.95 (1.93) | 27.76 (2.96) | 0.317 |
| MPT (mm) | 5.72 (1.10) | 5.37 (1.46) | 0.874 |
| Tongue | |||
| TGL (mm) | 58.12 (3.20) | 58.03 (4.83) | 0.461 |
| TGH (mm) | 27.46 (2.98) | 26.05 (3.08) | 0.370 |
| Hyoid bone | |||
| MPH (mm) | 7.97 (4.09) | 9.45 (4.59) | 0.292 |
*p-value < 0.05, which is deemed statistically significant.
AFH: Anterior facial height; ANB: Anteroposterior maxilla and mandible discrepancy; MinRGA: Minimal retroglossal airway; MinRPA: Minimal retropalatal airway; MPH: Vertical position of hyoid bone; MPT: Thickness of soft palate; SNA: Position of maxilla relative to cranial base; SNB: Position of mandible relative to cranial base; SN-PP: Palatal plane angle; SN-MP: Mandibular plane angle; SPL:Soft palate length; TGH: Height of tongue; TGL: Length of tongue.
Lateral cephalometric measurements: In the mild OSA group, a significantly larger anteroposterior maxilla and mandible discrepancy (ANB angle, p = 0.015), a more reduced SNB angle (p = 0.045), and larger teeth overjet (p = 0.028) were seen (Table 3).
Characteristics and quality of life survey: Children diagnosed with mild OSA experienced loud snoring during sleep and mood swing or tantrums more frequently (Table 4).
Table 4.
Characteristics and quality of life based on the OSA-18 survey.
| Test group (n = 16) |
Control group (n = 10) |
p-value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Sleep disturbance | 10.71 (2.92) | 9.67 (4.92) | 0.227 |
| Loud snoring | 3.50 (0.86) | 2.44 (1.33) | 0.037* |
| Breath- holding/Pauses | 1.93 (0.91) | 2.33 (1.41) | 0.597 |
| Choking or gasping | 2.50 (1.34) | 2.22 (1.48) | 0.445 |
| Fragmented sleep | 2.79 (1.19) | 2.67 (1.00) | 1.000 |
| Physical symptoms | 12.14 (4.37) | 11.00 (2.55) | 0.465 |
| Mouth breathing | 3.57 (1.40) | 2.67 (1.11) | 0.105 |
| Frequent colds or URIs | 3.43 (1.40) | 3.33 (0.70) | 0.870 |
| Rhinorrhea | 3.07 (1.33) | 3.22 (0.67) | 0.480 |
| Dysphagia | 2.07 (1.14) | 1.78 (1.09) | 0.522 |
| Emotional symptoms | 9.64 (2.85) | 7.33 (3.60) | 0.076 |
| Mood swing or tantrums | 3.57 (0.85) | 2.56 (0.88) | 0.017* |
| Aggressive-ness or hyperactivity | 2.93 (1.27) | 2.11 (1.36) | 0.129 |
| Discipline problems | 3.14 (1.23) | 2.67 (1.48) | 0.271 |
| Daytime function | 8.14 (3.44) | 6.56 (2.60) | 0.280 |
| Daytime drowsiness | 1.64 (0.84) | 1.44 (0.73) | 0.588 |
| Poor attention span | 3.21 (1.80) | 2.44 (1.33) | 0.319 |
| Difficulty in awakening | 3.29 (1.64) | 2.67 (1.00) | 0.432 |
| Caregiver concern | 12.29 (6.11) | 9.44 (6.59) | 0.137 |
| Over child's health | 3.93 (1.39) | 3.00 (1.87) | 0.219 |
| Over child not getting enough air | 2.93 (1.69) | 2.44 (2.13) | 0.315 |
| Missing daily activities | 2.50 (1.74) | 2.00 (1.41) | 0.476 |
| Feeling frustrated | 2.93 (1.97) | 2.00 (1.80) | 0.192 |
| Total OSA-18 Score | 52.93 (14.14) | 44.00 (17.39) | 0.088 |
| QOL | 6.92 (1.68) | 7.56 (1.76) | 0.452 |
*p-value < 0.05, which is deemed statistically significant.
Discussion
The present study found no significant difference in dental arch dimensions between children diagnosed with mild OSA and healthy children. This finding contrasts with those in previous studies. Lofstrand et al. found that four-year-old obstructed children had narrower maxillary arch width, shorter lower arch length, and higher palatal vault.8 Pirliia et al. (1995) also found a tendency to narrower maxillary and shorter lower arch length among seven-year-old OSA children during the mixed dentition period, but no significant difference in palatal height between OSA children and normal controls.15
This discrepancy could be the function of disease severity and the age of the subjects in the current study:
Disease severity: It has been suggested that children with OSA tend to have mouth breathing and lower tongue position which cause an imbalance in muscular forces of cheek and tongue.16 The tongue position has also been shown to correlate with the severity of OSA.17 The altered functioning of muscles induced by the morphological change in the dental arch renders the maxillary arch high and narrow. Furthermore, according to Maeda et al., there was an inverse correlation between narrow upper dentition and the AHI.18 In the present investigation, the study subjects were mild OSA children whose AHI ranged from 1.1 to 4.2, unlike the children in previous studies who suffered from more severe OSA and presented a higher range of AHI (3.5–17.3).8,15 Thus, we postulate that the mild OSA among children in the current study causes less alteration of muscle functioning and results in less change in the dental arch morphology.
Age of the subjects: The effect of mouth breathing and altered tongue position on dental arch morphology takes time to manifest. The children included in this study were still relatively young.
There was also no significant difference in the distribution of molar relation between the test and control groups in the present study. No previous studies have investigated the molar relation during the primary dentition period. Pirila et al. found that OSA children during the mixed dentition period had a tendency of class II molar relation.15 The differential growth of the mandible relative to the maxilla is an important factor as the molar relation transitions from the primary dentition to permanent dentition periods.19 Flush terminal plane was found in most children in the present study. To transition from flush terminal plane to class I molar relation requires the mesial movement of mandibular molar and the differential growth of the mandible.20 Children with OSA have been found to have abnormal growth hormone secretion causing deficient ramus growth and oral-facial hypotonia which lead to secondary changes in maxillary-mandibular growth.16,21,22 Thus a long-term study will be needed to investigate whether unfavorable mandibular growth may hinder the flush terminal in OSA children from transitioning to class I molar relation and the flush terminal transitions to class II molar relation, instead.
Several cephalometric characteristics have been found to correlate significantly with mild OSA. In the present study, children with mild OSA were more likely to have a skeletal class II malocclusion (larger ANB angle) and a more retrognathic mandible (decreased SNB angle). These findings mirror those in previous studies,9,23,24 suggesting the existence of certain genetic predisposition to the disease. The retrognathic mandible may result in a decreased space between the cervical column and the mandible, leading to a posteriorly postured tongue and soft palate and increasing the chance of impaired respiratory function.25 A larger overjet was another key cephalometric finding among mild OSA preschoolers in the current study. This is in agreement with the study results by Pirila et al.(2008) who found that older children diagnosed with OSA tended to have significantly larger overjet and reduced overbite.15 This may be attributed to increased overjet as resulted from the mouth breathing of OSA children.
The dimensions of the upper airway, soft palate and tongue, and the position of the hyoid bone were not significantly different between the preschool children with mild OSA and controls in this study. In contrast to this finding, previous studies have reported a narrower upper airway and more inferiorly positioned hyoid bone in children diagnosed with OSA.9,10,26,27 It has been suggested that the lower position of the hyoid bone might have served as a compensatory mechanism to alleviate the increase in airway resistance caused by a reduced airway space.28 According to Ozedemir et al., there was an inverse correlation between the minimal posterior airway space and the AHI, and a positive correlation between the position of the hyoid bone and the AHI.29 The lack of a significant difference in the changes in the airway dimension and the position of the hyoid bone in the current study could be the function of the relatively mild OSA among the participating children.
The current study examined the characteristics and quality of life using the OSA-18 questionnaire. In this study, the preschool children with mild OSA experienced more loud snoring and mood swings. An earlier study also showed that OSA children suffered more behavioral and emotional problems.30 Moreover, the present study showed that day time drowsiness was not a common characteristic in preschool children with mild OSA, which echoes the findings in previous investigations.6
To the best of our knowledge, this is the first study that uses PSG as the diagnostic tool for both the test and control groups to investigate the dental arch morphology as well as the craniofacial structure and characteristics in preschool children with OSA. In current research, we excluded the children with most common etiology (i.e. hypertrophic tonsils and adenoids) to focus on the effect of craniofacial and dental arch morphology.
The remaining samples are distinctive and valuable due to the cooperation from young patients. There are, however, some limitations of the study. First, the sample size is relatively small, because of the low prevalence of the disease and the challenge in getting patients of young age to cooperate. It is more difficult to reach a level of difference that is statistically significant. Second, 2-D cephalometric measurements have their inherent structural limitations, such as error of projection and error of identification.31
Clinical implications: Among one-third of children with mild OSA, the natural history is a worsening of disease.32 Early intervention may be needed to prevent the condition from deteriorating. Many forms of oral appliance have been proposed for the treatment of OSA, such as rapid maxillary expansion, modified monobloc appliance, and oral myofunctional therapy device.23,33 Our study results showed that, for children with mild OSA during the primary dentition period, there's an anteroposterior discrepancy between the maxilla and the mandible without the narrowing of dental arch. The use of functional appliances such as tongue training or mandibular repositioning devices in these patients may be promising and worth further investigations.
We conclude that even young children with a mild form of OSA experience more loud snoring and emotional swings. These children have a significantly larger discrepancy between the anteroposterior maxilla and mandible, more retrognathic mandible, and larger teeth overjet. The dental arch morphology is not significantly different between the OSA children and normal children.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This study was supported by the Chang Gung Memorial Hospital (Grant Number: CMRPG3G1951 and CMRPG3H1691; Grant Recipient: Li-Chuan Chuang).
References
- 1.Jeans W.D., Fernando D.C., Maw A.R., Leighton B.C. A longitudinal study of the growth of the nasopharynx and its contents in normal children. Br J Radiol. 1981;54:117–121. doi: 10.1259/0007-1285-54-638-117. [DOI] [PubMed] [Google Scholar]
- 2.Section on Pediatric Pulmonology SoOSASAAoP Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–712. doi: 10.1542/peds.109.4.704. [DOI] [PubMed] [Google Scholar]
- 3.Ali N.J., Pitson D.J., Stradling J.R. Snoring, sleep disturbance, and behaviour in 4-5 year olds. Arch Dis Child. 1993;68:360–366. doi: 10.1136/adc.68.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redline S., Tishler P.V., Schluchter M., Aylor J., Clark K., Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 5.Dehlink E., Tan H.L. Update on paediatric obstructive sleep apnoea. J Thorac Dis. 2016;8:224–235. doi: 10.3978/j.issn.2072-1439.2015.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padmanabhan V., Kavitha P.R., Hegde A.M. Sleep disordered breathing in children–a review and the role of a pediatric dentist. J Clin Pediatr Dent. 2010;35:15–21. doi: 10.17796/jcpd.35.1.u2010g22480145u4. [DOI] [PubMed] [Google Scholar]
- 7.Arens R., Marcus C.L. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 8.Lofstrand-Tidestrom B., Thilander B., Ahlqvist-Rastad J., Jakobsson O., Hultcrantz E. Breathing obstruction in relation to craniofacial and dental arch morphology in 4-year- old children. Eur J Orthod. 1999;21:323–332. doi: 10.1093/ejo/21.4.323. [DOI] [PubMed] [Google Scholar]
- 9.Zucconi M., Caprioglio A., Calori G. Craniofacial modifications in children with habitual snoring and obstructive sleep apnoea: a case-control study. Eur Respir J. 1999;13:411–417. doi: 10.1183/09031936.99.13241199. [DOI] [PubMed] [Google Scholar]
- 10.Zettergren-Wijk L., Forsberg C.M., Linder-Aronson S. Changes in dentofacial morphology after adeno-/tonsillectomy in young children with obstructive sleep apnoea a 5-year follow-up study. Eur J Orthod. 2006;28:319–326. doi: 10.1093/ejo/cji119. [DOI] [PubMed] [Google Scholar]
- 11.Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866–878. doi: 10.1164/ajrccm.153.2.8564147. American Thoracic Society. [DOI] [PubMed] [Google Scholar]
- 12.Franco R.A., Jr., Rosenfeld R.M., Rao M. First place–resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;123:9–16. doi: 10.1067/mhn.2000.105254. [DOI] [PubMed] [Google Scholar]
- 13.Ishman S.L., Yang C.J., Cohen A.P. Is the OSA-18 predictive of obstructive sleep apnea: comparison to polysomnography. The Laryngoscope. 2015;125:1491–1495. doi: 10.1002/lary.25098. [DOI] [PubMed] [Google Scholar]
- 15.Pirila-Parkkinen K., Pirttiniemi P., Nieminen P., Tolonen U., Pelttari U., Lopponen H. Dental arch morphology in children with sleep-disordered breathing. Eur J Orthod. 2009;31:160–167. doi: 10.1093/ejo/cjn061. [DOI] [PubMed] [Google Scholar]
- 16.Harvold E.P., Tomer B.S., Vargervik K., Chierici G. Primate experiments on oral respiration. Am J Orthod. 1981;79:359–372. doi: 10.1016/0002-9416(81)90379-1. [DOI] [PubMed] [Google Scholar]
- 17.Friedman M., Soans R., Gurpinar B., Lin H.C., Joseph N.J. Interexaminer agreement of Friedman tongue positions for staging of obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2008;139:372–377. doi: 10.1016/j.otohns.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Maeda K., Tsuiki S., Fukuda T., Takise Y., Inoue Y. Is maxillary dental arch constriction common in Japanese male adult patients with obstructive sleep apnoea? Eur J Orthod. 2014;36:403–408. doi: 10.1093/ejo/cjt058. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y.E., Nanda R.S., Sinha P.K. Transition of molar relationships in different skeletal growth patterns. Am J Orthod Dentofacial Orthop. 2002;121:280–290. doi: 10.1067/mod.2002.119978. [DOI] [PubMed] [Google Scholar]
- 20.Proffit W.R., Fields H.W., Sarver D.M. 5th ed. Mosby: Elsevier; 2013. Contemporary orthodontics; pp. 90–91. [Google Scholar]
- 21.Peltomaki T. The effect of mode of breathing on craniofacial growth–revisited. Eur J Orthod. 2007;29:426–429. doi: 10.1093/ejo/cjm055. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y.S., Guilleminault C. Pediatric obstructive sleep apnea and the critical role of oral-facial growth: evidences. Front Neurol. 2012;3:184. doi: 10.3389/fneur.2012.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cozza P., Gatto R., Ballanti F., Prete L. Management of obstructive sleep apnoea in children with modified monobloc appliances. Eur J Paediatr Dent. 2004;5:24–29. [PubMed] [Google Scholar]
- 24.Pirila-Parkkinen K., Pirttiniemi P., Paakko E., Tolonen U., Nieminen P., Lopponen H. Pharyngeal airway in children with sleep-disordered breathing in relation to head posture. Sleep Breath. 2012;16:737–746. doi: 10.1007/s11325-011-0569-y. [DOI] [PubMed] [Google Scholar]
- 25.Ozbek M.M., Miyamoto K., Lowe A.A., Fleetham J.A. Natural head posture, upper airway morphology and obstructive sleep apnoea severity in adults. Eur J Orthod. 1998;20:133–143. doi: 10.1093/ejo/20.2.133. [DOI] [PubMed] [Google Scholar]
- 26.Kawashima S., Niikuni N., Chia-hung L. Cephalometric comparisons of craniofacial and upper airway structures in young children with obstructive sleep apnea syndrome. Ear Nose Throat J. 2000;79:499–502. 5–6. [PubMed] [Google Scholar]
- 27.Behlfelt K., Linder-Aronson S., McWilliam J., Neander P., Laage-Hellman J. Dentition in children with enlarged tonsils compared to control children. Eur J Orthod. 1989;11:416–429. doi: 10.1093/oxfordjournals.ejo.a036014. [DOI] [PubMed] [Google Scholar]
- 28.Deng J., Gao X. A case-control study of craniofacial features of children with obstructed sleep apnea. Sleep Breath. 2012;16:1219–1227. doi: 10.1007/s11325-011-0636-4. [DOI] [PubMed] [Google Scholar]
- 29.Ozdemir H., Altin R., Sogut A. Craniofacial differences according to AHI scores of children with obstructive sleep apnoea syndrome: cephalometric study in 39 patients. Pediatr Radiol. 2004;34:393–399. doi: 10.1007/s00247-004-1168-x. [DOI] [PubMed] [Google Scholar]
- 30.Gozal D., Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13:505–509. doi: 10.1097/MCP.0b013e3282ef6880. [DOI] [PubMed] [Google Scholar]
- 31.Nalcaci R., Ozturk F., Sokucu O. A comparison of two-dimensional radiography and three- dimensional computed tomography in angular cephalometric measurements. Dentomaxillofac Radiol. 2010;39:100–106. doi: 10.1259/dmfr/82724776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li A.M., Au C.T., Ng S.K. Natural history and predictors for progression of mild childhood obstructive sleep apnoea. Thorax. 2010;65:27–31. doi: 10.1136/thx.2009.120220. [DOI] [PubMed] [Google Scholar]
- 33.Guilleminault C., Monteyrol P.J., Huynh N.T., Pirelli P., Quo S., Li K. Adeno- tonsillectomy and rapid maxillary distraction in pre-pubertal children, a pilot study. Sleep Breath. 2011;15:173–177. doi: 10.1007/s11325-010-0419-3. [DOI] [PubMed] [Google Scholar]