Abstract
Background/purpose
A perfect sealing of root canal system is essential for preventing ingress of bacteria from the oral environment. The purpose of this study was to evaluate the apical sealing ability of bioceramic (EndoSequence BC Sealer®) and epoxy resin-based (AH Plus®) sealers at 24 h, 7 days and 4 weeks.
Materials and methods
Forty two extracted human upper anterior teeth were sectioned to leave the root 15-mm long, then all the roots were instrumented using a set of ProTaper® rotary instruments. Four roots were selected randomly as controls, and the remaining 38 roots were randomly divided into 2 groups of 19 roots each: group 1: EndoSequence BC Sealer® and gutta-percha, and group 2: AH Plus® and gutta-percha using a multiple wave condensation technique. The apical sealing ability of the filled root canal was measured using the fluid filtration method with 200 mmHg (26.67 KPa) above atmospheric pressure at 24 h, 7 days and 4 weeks. Scanning electron microscopy (SEM) was used to assess the adaptation and penetration of the sealers. The apical microleakage between 2 groups was compared using Student's t-test. P < 0.05 was considered statistically significant.
Results
EndoSequence BC Sealer® had significantly better sealing ability than AH Plus® at all test periods (P < 0.001). SEM showed EndoSequence BC Sealer® had better penetration into dentinal tubules.
Conclusion
Bioceramic sealer could promote proper sealing of root canals obturated with multiple wave condensation.
Keywords: Apical sealing ability, Fluid filtration, Bioceramic sealer, Epoxy resin-based sealer
Introduction
The success outcome of root canal treatment is the elimination of microorganisms from the root canal system; however, complete elimination of microorganisms cannot be achieved consistently due to anatomical complication of the root canal system.1,2 Therefore, a perfect sealing of the root canal system is essential for preventing ingress of bacteria from the oral environment and entombing any residual microorganisms.3 Root canal sealers serve as lubricants during the obturation process, seal the space between the dentinal wall and the root filling material and fill the accessory canals, voids and irregularities in the root canals.3 AH Plus® is epoxy resin-based sealer and has been commonly used as gold standard endodontic sealers due to its high bond strength to dentine, adequate radiopaque, flow, dimensional stability, low solubility and high resistance.3
EndoSequence BC Sealer® is a bioceramic sealer which has been introduced to the market in regarding of efficacious technology.4 The premixed ready-to-use injectable EndoSequence BC Sealer is composed of calcium phosphate, calcium silicates, calcium hydroxide, zirconium oxide, filler and thickening agents, which requires the presence of water to set and harden.5 EndoSequence BC Sealer® does not shrink during setting and demonstrates excellent physical properties with antibacterial property due to its highly alkaline pH.6,7 Dye penetration evaluation of the apical sealing ability of bioceramic sealer suggested that EndoSequence BC Sealer® sealed the root canal better compared to AH Plus® but cannot totally eliminate leakage in single rooted teeth obturated by continuous wave condensation technique.8 Whereas, EndoSequence BC Sealer® was not superior to AH Plus® in terms of resistance to bacteria leakage and 3D compaction in roundly-prepared canals using matched single-cone technique.9 However, limitations must be concerned in dye penetration test and bacteria leakage test.10,11
To date, the fluid filtration technique is commonly used to evaluate the apical sealing ability of root canal sealers because this technique allows quantitative measurements of leakage in the root canals and the root specimens could be repeatedly measured over period of time without the root specimen destructions.12,13 Furthermore, the sensitivity of the fluid filtration technique can be adjusted by altering the pressure used.12 Scanning electron microscopy enabled ultrastructural examination and assessment of the penetration of root canal sealer into the dentinal tubules, and investigation of the adaptation of sealer to the radicular dentine on the various levels of sectioning because it has large depth of field, higher resolution and better magnification at the interface.14 The SEM uses electromagnets rather than lenses allowing the researcher to have more control over the degree of magnification, thereby providing conspicuously clear images.15
Therefore, the objective of this study was to evaluate the apical sealing ability of bioceramic (EndoSequence BC Sealer®) and epoxy resin-based (AH Plus®) sealers at 24 h, 7 days and 4 weeks using the fluid filtration technique and scanning electron microscopy.
Materials and methods
Sample preparation
The experiments were carried out on 42 extracted maxillary anterior human teeth with single straight root canals and fully developed apices. This study was approved by the Ethics Committee of the Faculty of Dentistry and Faculty of Pharmacy at Mahidol University, Bangkok, Thailand (COA.No.MU-DT/PY-IRB 2015/004.0901). All teeth were extracted for periodontal reasons. Roots with resorptive defects, caries, cracks, or open apices were excluded.
All teeth were cleaned to remove attached debris and stored in 0.1% thymol solution at the room temperature until use. The crowns of all teeth were removed at the cemento-enamel junction using a low-speed saw (Isomet®, Buehler, Lake Bluff, IL, USA) with water coolant, before all the roots were adjusted to 15-mm length. Canal patency was determined by passing a size 20 K-file (Densply Maillefer, Ballaigues, Switzerland) through the apical foramen, but unable to passing a size 25 K-file through the apical foramen. Working lengths were established 0.5 mm short of the apical foramen, and the roots were instrumented by using Protaper® (Densply Maillefer, Ballaigues, Switzerland) NiTi rotary instruments. Coronal and middle portions of root canals were flared by SX, S1, and S2, before the apical portion of root canals were instrumented with S1, S2, F1, F2, F3, F4 and F5. Between the uses of each file use, the root canals were irrigated with 5 ml of 2.5% NaOCl solution.
On completion of instrumentation, the root canals were irrigated with 2 ml of 17% EDTA acid followed by 5 ml of 2.5% NaOCl to remove the smear layer. All irrigating solutions were delivered through via 25-gauge needles and the root canals dried with paper points (Diadent, Almere, Netherlands).
Four teeth were selected randomly as controls and the remaining 38 teeth were randomly divided into two experimental groups of 19 teeth each: EndoSequence BC Sealer® (Group 1) and AH Plus® (Group 2). The roots were filled as follows:
Group 1: The roots were filled with EndoSequence BC Sealer® (Brasseler USA, Savannah, Georgia, USA) and gutta-percha using a multiple wave condensation technique.16 Sealer was applied into the root canals with an F4 file. The F5 gutta-percha cone (Densply Maillefer) that fitted and gave tug-back at the working length was chosen. The apical section of the F5 gutta-percha cone was coated with sealer, and slowly inserted into the root canal until the working length was reached. A system B heat source (SybronEndo, Orange, CA, USA) was set at 200 °C and used for cutting the gutta percha at the orifice. The gutta percha was packed with Machtou hand plugger no. 3 (VDW, Munich, Germany). Next, system B heat source and multiple hand pluggers were used to down pack and condense gutta percha apically into the canal. After removing the coronal and middle portions of the fillings, the created space in the coronal and middle part of the canal was back-filled using heat-softened gutta percha (BeeFill catridges; VDW, Munich, Germany) and gutta percha obturation gun with 23-gauge needle (BeeFill device; VDW, Munich, Germany) at 160 °C and then the obturation material was packed with multiple pluggers in multiple waves of downpacking steps until the canal was completely filled with gutta-percha.
Group 2: The roots were filled with AH Plus® (Densply DeTrey, Konstanz, Germany) and gutta-percha (Densply Maillefer) using a multiple wave condensation technique.16 AH Plus® was mixed according to the manufacturer's instructions and the root canals were filled in the manner described for group 1.
Positive control group: The roots (n = 2) were filled with an F5 taper master gutta percha cone, using a multiple wave condensation technique without sealer.
Negative control group: The roots (n = 2) were filled with an F5 taper master gutta percha cone using the multiple wave condensation technique without sealer. The roots were then totally coated with two layers of nail polish, including the apical foramina, to ensure that there was no leak of fluid movement anywhere within the device.
After obturation of the root canals, all specimens were stored in gauze dampened with sterile saline,17 and enclosed in a humidifier at 37 °C and 100% humidity.5
Measurement of apical microleakage
Apical microleakage was measured using the fluid filtration method described by Asawaworarit et al.13 Each root was mounted in a cap and collar made from a plastic rod (ICI Plastic Division, Welwyn Garden City, U.K.), which had been sealed into a stainless steel tube (No.18 hypodermic needle). The tube was connected to a glass capillary (internal diameter 300 μm, Supracaps Cat No. 709007) to accommodate the rate of flow under different pressures. The pressure of the system was controlled by attaching the tubing from the capillary to a mercury manometer. Apical microleakage was detected by observing the movement of a small air bubble introduced into the capillary. The measurements of fluid flow were recorded with a positive pulpal pressure of 200 mmHg (26.67 KPa). Three consecutive measurements were taken to calculate the mean flow rate. Apical microleakage was defined as the fluid flow rate in nl/s.
Scanning electron microscopy
Two teeth from each experimental group at each test period were randomly selected for SEM analysis. Therefore, in each group, the teeth were selected at 24 h (n = 2), 7 days (n = 2) and 4 weeks (n = 2). All twelve roots were sectioned perpendicular to the long axis and divided into apical, middle and coronal sections. Subsequently, an impression was made using a vinyl polysiloxane impression material (Silagum light body, DMG; Hamburg, Germany) to create a replica for SEM analysis. Replicas were fabricated immediately using a self-curing epoxy material. After setting, they were stored in desiccator for 24 h. Replicas of the samples were mounted on an aluminum stub, sputter coated with gold and viewed under scanning electron microscope (SEM model JSM-5410 LV; JEOL LTD, Tokyo, Japan) to assess the adaptation and penetration of the sealers into the root canal walls from the apical to the coronal at a magnification between X500 and X1000. The use of an indirect method for scanning was favoured over a direct one to avoid potential damage to specimens due to over-drying.18
Statistical analysis
The mean apical microleakage values of each sealer at 24 h, 7 days and 4 weeks were compared using one way repeated-measures ANOVA and Tukey's test for pair-wise comparisons. P < 0.05 was considered significant. The apical microleakage values of the 2 sealers were compared using Student's t-test. P < 0.05 was considered significant.
Results
The apical microleakage of EndoSequence BC Sealer® and AH Plus® sealers at 24 h, 7 days and 4 weeks are presented in Table 1.
Table 1.
Mean and standard deviation (SD) apical microleakage values of both experimental groups in nl/s at 200 mmHg at 24 h, 7 days and 4 weeks.
| Test periods | Apical microleakage values (nl/s at 200 mmHg) |
|
|---|---|---|
| EndoSequence BC Sealer® | AH Plus® | |
| 24 h | 0.651 ± 0.097A,a | 4.013 ± 1.302C,b |
| 7 days | 0.325 ± 0.136B,c | 0.941 ± 0.045D,d |
| 4 weeks | 0.288 ± 0.092B,e | 0.880 ± 0.188D,f |
Within each column, the same superscripted capital letters (A, B, C, D) indicate statistically similar means (P > 0.05). Within each row, the different superscripted lower case letters (a, b, c, d, e, f) indicate significant differences among the groups (P < 0.001).
Apical microleakage values of control groups confirmed the consistency of the experimental fluid filtration model, with no fluid transport observed in the negative controls showing that there was no leakage in the device, and immeasurably rapid movement observed in the positive controls (no sealer) indicating very high leakage, or served as 100% leakage.
A one way repeated-measures ANOVA test showed a significant reduction in the apical microleakage of EndoSequence BC Sealer® and AH Plus® sealers after 7 days (P < 0.001). For both sealers, no significant difference between 7 days and 4 weeks was observed (P > 0.05). In comparisons between the two sealers, EndoSequence BC Sealer® had significantly better sealing than AH Plus® at all test periods (P < 0.001).
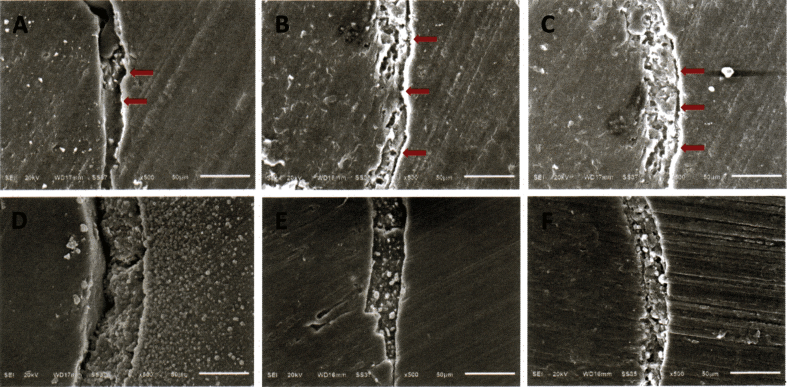
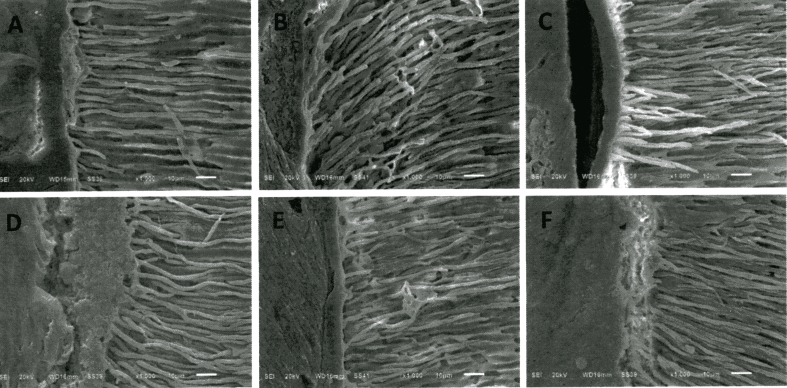
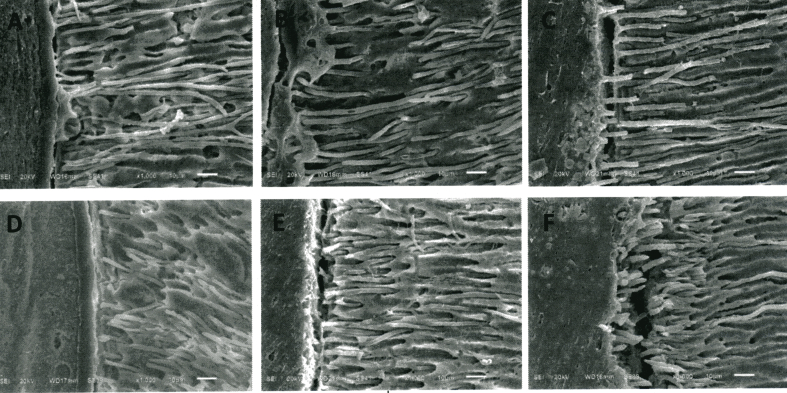
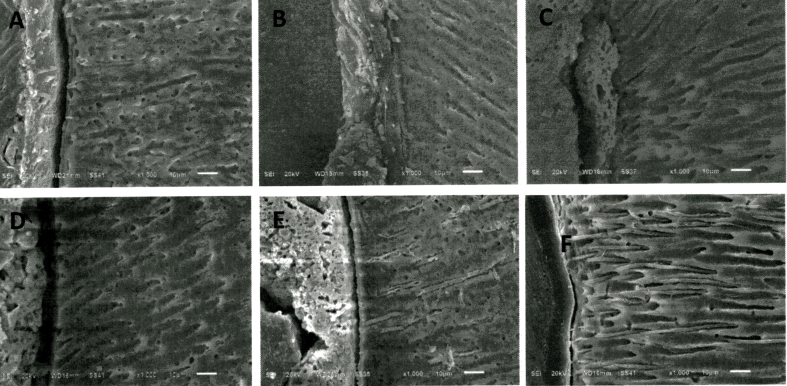
Scanning electron microscopy showed EndoSequence BC Sealer® had better adaptation to the root canal wall than AH Plus®, and both sealers demonstrated better sealing ability after 7 days as shown in Fig. 1. In the coronal and mid-root sections, both sealers completely penetrated into the dentinal tubules of root canal walls as shown in Figure 2, Figure 3. While in the apical region, sealer penetration was not observed in AH Plus® group. EndoSequence BC Sealer® had better sealer penetration into the dentinal tubules in the apical portion of root canals, compared to AH Plus® group as shown in Fig. 4.
Figure 1.
Scanning electron microscopy (SEM) images of the apical section of the obturated roots showing the adaptation to root canal wall (original magnification X500); A, B, C obturated root canals with GP/AH Plus® at 24 h, 7 days and 4 weeks, respectively; The resins were shown to have a good seal at the interface between gutta-percha and the sealer but their seal at the interface between the sealer and apical root dentine contained some interfacial gaps (red arrows); D, E, F obturated root canals with GP/EndoSequence BC Sealer® at 24 h, 7 days and 4 weeks, respectively; The formation of hydroxyapatite during the setting reaction of EndoSequence BC Sealer® resulted in a chemical bond to the canal wall and a better seal at the interface between the sealer and dentine.4 (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Figure 2.
SEM images of the coronal section of the obturated roots showing root canal sealer penetration into dentinal tubules (original magnification X1000); A, B, C obturated root canals with GP/AH Plus® at 24 h, 7 days and 4 weeks, respectively; D, E, F obturated root canals with GP/EndoSequence BC Sealer® at 24 h, 7 days and 4 weeks, respectively.
Figure 3.
SEM images of the middle section of the obturated roots showing root canal sealer penetration into dentinal tubules (original magnification X1000); A, B, C obturated root canals with GP/AH Plus® at 24 h, 7 days and 4 weeks, respectively; D, E, F obturated root canals with GP/EndoSequence BC Sealer® at 24 h, 7 days and 4 weeks, respectively.
Figure 4.
SEM images of the apical section of the obturated roots showing root canal sealer penetration into dentinal tubules (original magnification X1000); A, B, C obturated root canals with GP/AH Plus® at 24 h, 7 days and 4 weeks, respectively; D, E, F obturated root canals with GP/EndoSequence BC Sealer® at 24 h, 7 days and 4 weeks, respectively.
Discussion
Using fluid filtration method, the present study found EndoSequence BC Sealer® had significantly better apical sealing ability than AH Plus® at 24 h, 7 days, and 4 weeks. Both sealers had better sealing ability after 7 days. The results of SEM evaluation showed EndoSequence BC Sealer® has better adaptation and higher sealer penetration into the dentinal tubules than AH Plus®, especially in the apical third of root canals.
Apical microleakage in AH Plus® group could be due to the presence of gaps between sealer and root canal wall at all test period (Fig. 1). Epoxy resins sealers tends to shrink during setting, and results in disintegrate adaptation and de-bonding from root canal wall.19 The apical third of root dentine showed a smaller tubular density/area and a smaller tubular size than middle and cervical third of root dentine.20 Hence, the penetration of sealer into the dentinal tubules and the sealing ability to the root canal wall became less at the apical portion. The hydrophobic property of AH Plus® also prevents good adaptation to the incompletely dried canal wall.21 Whereas, EndoSequence BC Sealer® has higher flowability and smaller particle size, related to the dentinal tubule penetration property of sealer.7,19 EndoSequence BC Sealer® also has hydrophilic property and low contact angle which enable the sealer to spread easily over the dentinal wall and penetrate into the tubules and irregularities of radicular dentine.6 In addition, the moisture remaining in the dentinal tubules triggers its setting reaction with the production of hydroxyapatite, thereby creating the chemical bond with root dentine.22 This chemical bond might improve adaptation to root canal wall and help to prevent microleakage in EndoSequence BC Sealer® group. A significant expansion of 0.20% of EndoSequence BC Sealer® also leads to the formation of a gap free between the sealer and root canal wall which makes the sealer more effective.15,19
Both EndoSequence BC Sealer® and AH Plus® had significantly better sealing ability after 7 days. This may be related to the setting time of both sealers. Loushine et al.5 found EndoSequence BC Sealer® had setting time approximately 168–240 h (7–10 days), and its setting time depends upon the moisture in root canals. Thus, at 24 h, EndoSequence BC Sealer® had not complete setting of materials. This confirmed by SEM photographs (Fig. 1) which found more adaptation to root canal wall after 7 days. Meanwhile, AH Plus® had faster setting time approximately 18 h, although the manufacturer claim that AH Plus® had setting time period only 8 h.23 The fast setting time and shrinkage in the early stage of the setting reaction of AH Plus® may lead to higher microleakage at 24 h.
Under SEM evaluation, EndoSequence BC Sealer® showed higher sealer penetration into the dentinal tubules than AH Plus®, especially in the apical third of root canals at all test periods (Fig. 4). In accordance with the results of Hachem et al.,24 who found that EndoSequence BC Sealer® demonstrated better tubule penetration than AH Plus® in human maxillary central incisors filled with gutta-percha using a single-cone technique. Probably explanation could be that the different particle sizes between EndoSequence BC Sealer® and AH Plus® might affect the results. EndoSequence BC Sealer® has a smaller particle size on average of 0.2 μm, which might enhance the penetration of the particles into dentinal tubules, especially smaller tubules at the apical root area; whereas, AH Plus® contains larger calcium tungstate particles with an average size of 8 μm and zirconium oxide particles with a size of 1.5 μm, which might not enter easily into the smaller tubules at the apical root area.25 Sealer penetration in dentinal tubules has the benefit of improving the mechanical retention of sealer to the dentinal walls.26 This retention might operate as a physical barrier to prevent microleakage of the root canal system. Thus, SEM micrographs strongly confirmed the results of fluid filtration measurements that EndoSequence BC Sealer® had significantly better apical sealing ability than AH Plus® at 24 h, 7 days, and 4 weeks.
Within the limitations of the present study, EndoSequence BC Sealer® had significantly better apical sealing ability than AH Plus® at all test periods. Both sealers had better sealing ability after 7 days. SEM evaluation confirmed EndoSequence BC Sealer® had better adaptation to the root canal wall and higher sealer penetration into the dentinal tubules in the apical third of root canals when compared to AH Plus®. Hence, Bioceramic sealer could promote proper sealing of root canals obturated with multiple wave condensation.
Declaration of Competing Interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This study was supported by Faculty of Dentistry, Mahidol University.
References
- 1.Stoll R., Betke K., Stachniss V. The influence of different factors on the survival of root canal fillings: a 10-year retrospective study. J Endod. 2005;31:783–790. doi: 10.1097/01.don.0000158229.43298.a9. [DOI] [PubMed] [Google Scholar]
- 2.Wu M.K., van der Sluis L.W.M., Wesselink P.R. The capability of two hand instrumentation techniques to remove the inner layer of dentine in oval canals. Int Endod J. 2003;36:218–224. doi: 10.1046/j.1365-2591.2003.00646.x. [DOI] [PubMed] [Google Scholar]
- 3.Ørstavik D. Materials used for root canal obturation; technique, biological and clinical testing. Endod Top. 2005;12:25–38. [Google Scholar]
- 4.Koch K., Brave D. Bioceramic technology–the game changer in endodontics. Endod Pract. 2009;2:17–21. [Google Scholar]
- 5.Loushine B.A., Bryan T.E., Looney S.W. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J Endod. 2011;37:673–677. doi: 10.1016/j.joen.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H., Shen Y., Ruse N.D., Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009;35:1051–1055. doi: 10.1016/j.joen.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Candeiro G.T., Correia F.C., Duarte M.A., Ribeiro-Siqueira D.C., Gavini G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J Endod. 2012;38:842–845. doi: 10.1016/j.joen.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Pawar S.S., Pujar M.A., Makandar S.D. Evaluation of the apical sealing ability of bioceramic sealer, AH Plus & epiphany: an in vitro study. J Conserv Dent. 2014;17:579–582. doi: 10.4103/0972-0707.144609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanpiset K., Banomyong D., Chotvorrarak K., Srisatjaluk R.L. Bacterial leakage and micro-computed tomography evaluation in round-shaped canals obturated with bioceramic cone and sealer using matched single cone technique. Restor Dent Endod. 2018;43 doi: 10.5395/rde.2018.43.e30. e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu M.K., Wesselink P.R. Endodontic leakage studies reconsidered. Part I Methodology, application and relevance. Int Endod J. 1993;26:37–43. doi: 10.1111/j.1365-2591.1993.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 11.De-Deus G. Research that matters-root canal filling and leakage studies. Int Endod J. 2012;45:1063–1064. doi: 10.1111/j.1365-2591.2012.02104.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu M.K., Wesselink P.R., Boersma J. A 1-year follow-up study on leakage of four root canal sealers at different thickness. Int Endod J. 1995;28:185–189. doi: 10.1111/j.1365-2591.1995.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 13.Asawaworarit W., Yachor P., Kijsamanmith K., Vongsavan N. Comparison of apical sealing ability of calcium silicate-based sealer and resin-based sealer using the fluid filtration technique. Med Princ Pract. 2016;25:561–565. doi: 10.1159/000450577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eltair M., Pitchika V., Hickel R., Kühnisch J., Diegritz C. Evaluation of the interface between gutta-percha and two types of sealers using scanning electron microscopy (SEM) Clin Oral Investig. 2018;22:1631–1639. doi: 10.1007/s00784-017-2216-x. [DOI] [PubMed] [Google Scholar]
- 15.Shinde A., Kokate S., Hegde V. Comparative assessment of apical sealing ability of three different endodontic sealers: a scanning electron microscopic study. J Pierre Fauchard Acad (India) 2014;28:78–82. [Google Scholar]
- 16.Whitworth J. Methods of filling root canals: principles and practices. Endod Top. 2005;12:2–24. [Google Scholar]
- 17.Onay E.O., Ungor M., Orucoglu H. An in vitro evaluation of the apical sealing ability of a new resin-based root canal obturation system. J Endod. 2006;32:976–978. doi: 10.1016/j.joen.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Moinzadeh A.T., Mirmohammadi H., Veenema T. Effect of a two-step placement procedure on the dislocation resistance of a methacrylate resin-based root canal sealer: a proof of concept. J Adhes Dent. 2014;16:567–574. doi: 10.3290/j.jad.a33200. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H.M., Shen Y., Zheng W., Zheng Y., Haapasalo M. Physical properties of 5 root canal sealers. J Endod. 2013;39:1281–1286. doi: 10.1016/j.joen.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Xu H., Zheng Q., Shao Y. The effects of ageing on the biomechanical properties of root dentine and fracture. J Dent. 2014:305–311. doi: 10.1016/j.jdent.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Roggendorf M.J., Ebert J., Petschelt A., Frankenberger R. Influence of moisture on the apical seal of root canal fillings with five different types of sealer. J Endod. 2007;33:31–33. doi: 10.1016/j.joen.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Han L., Okiji T. Bioactivity evaluation of three calcium silicate-based endodontic materials. Int Endod J. 2013;46:808–814. doi: 10.1111/iej.12062. [DOI] [PubMed] [Google Scholar]
- 23.Vitti R.P., Prati C., Silva E.J. Physical properties of MTA Filapex sealer. J Endod. 2013;39:915–918. doi: 10.1016/j.joen.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Hachem R.E., Khalil I., Brun G.L. Dentinal tubule penetration of AH plus, BC sealer and a novel tricalcium silicate sealer: a confocal scanning microscopy study. Clin Oral Investig. 2019;23:1871–1876. doi: 10.1007/s00784-018-2632-6. [DOI] [PubMed] [Google Scholar]
- 25.Primus C.M. Products and distinctions. In: Camilleri J., editor. Mineral trioxide aggregate in dentistry: from preparation to application. Springer Berlin Heidelberg; London: 2014. pp. 151–156. [Google Scholar]
- 26.Osiri S., Banomyong D., Sattabanasuk V., Yanpiset K. Root reinforcement after obturation with calcium silicate–based sealer and modified gutta-percha cone. J Endod. 2018;44:1843–1848. doi: 10.1016/j.joen.2018.08.011. [DOI] [PubMed] [Google Scholar]