Abstract
Background
The incidence of idiopathic membranous nephropathy (IMN) has recently increased remarkably. Immune dysfunction caused by disordered intestinal flora might be an important factor affecting IMN. The Jian Pi Qu Shi Formula (JPQSF) shows promise in treating IMN. Here, we sequenced 16S rRNA genes to compare intestinal flora between patients with IMN and healthy persons. We also conducted a randomized controlled clinical trial to further compare the intestinal flora of patients with IMN treated with traditional Chinese medicine (TCM) and western medicine (WM).
Methods
Among 40 patients with IMN treated at Department of Nephrology in Xiyuan Hospital, Chinese Academy of Traditional Chinese Medicine between July 2016 and December 2018, we compared 30 of them with 10 healthy persons (controls). The IMN group was randomly assigned to receive JPQSF (TCM) or immunosuppressant WM therapy in (n = 15 per group) for 6 months. Intestinal microbiota diversity was analyzed using alpha diversity and beta diversity. Intestinal flora that significantly differed between the groups was analyzed using MetaStat. The effects and safety of the therapies were determined based on the values for plasma albumin, 24-h urine protein excretion, serum creatinine, urea nitrogen, estimate glomerular filtration rate (eGFR), complete blood count, and liver enzymes. All data were statistically analyzed using Statistical Package for the Social Sciences (SPSS) 20.0 statistical software.
Results
Baseline characteristics did not significantly differ between the IMN and healthy groups, or the TCM and WM groups. After six months of treatment, 24-h urinary protein significantly declined in the TCM and WM groups (before and after treatment: 3.24 ± 1.74 vs. 1.73 ± 1.85 g, P < 0.05 and 3.94 ± 1.05 vs. 1.91 ± 1.18 g, P < 0.05, respectively). Plasma albumin was significantly increased in the TCM group (before vs. after treatment: 32.44 ± 9.04 vs. 39.99 ± 7.03 g/L, P < 0.05), but did not significantly change in the WM group (31.55 ± 4.23 vs. 34.83 ± 9.14 g/L, P > 0.05). Values for urea nitrogen, serum creatinine, and eGFR did not significantly change in either group. The alpha diversity index for intestinal flora differed between the IMN and healthy groups, and the TCM and WM groups. Comparisons of multiple samples (beta diversity) revealed differences in intestinal flora between the IMN and healthy groups, and the TCM and WM groups. The Metastat analysis findings showed that the main genera that differed between the IMN group before treatment and the healthy group were Christensenellaceae_R-7_group, Bifidobacterium (77), Dorea, Escherichia-Shigella, Parabacteroides, Bifidobacterium, and Coprococcus_3. After TCM therapy, the main differential genera were Butyricimonas, Bacteroides, Alistipes, and Lachnospira, and after WM therapy, these were Ruminococcus_2, Lachnospiraceae_ND3007_group, Lachnospira, Bifidobacterium, Alistipes, and [Eubacterium]_ventriosum_group.
Conclusion
Patients with IMN might have disordered intestinal flora, and JPQSF can regulate intestinal flora in patients with IMN.
Keywords: Idiopathic membranous nephropathy, Jian Pi Qu Shi Formula, Intestinal flora, Randomized controlled trial
Introduction
Idiopathic membranous nephropathy (IMN) is a highly prevalent primary glomerular disease in adults, and its incidence in China has increased over the past 10 years. Recent studies have shown that IMN accounts for 24.1% of primary glomerular diseases, rendering it the second most prevalent pathological type.1
Hormone-associated cyclophosphamide (CTX) and hormone-associated calcineurin inhibitors (CNI) are mainly recommended to treat IMN in patients undergoing renal biopsy, according to the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines.2 The remission rate with this regimen is acceptable, but many patients experience recurrence. Complications associated with treatment include infection,3 renal dysfunction,4 and other adverse events that can lead to repeated hospitalization and even death. Kidney diseases have been treated with traditional Chinese medicine (TCM) for centuries in many countries and much clinical experience has accumulated.5,6 The results of a multicenter, randomized, controlled clinical trial of IMN treatment have shown comparable clinical remission rates between TCM and hormone + CTX at 48 weeks. Treatment with TCM significantly and safely reduced severe side effects.7
Jian Pi Qu Shi Formula (JPQSF) was developed to treat IMN at Xiyuan Hospital, which is affiliated with the China Academy of Chinese Medical Science. The 10 herbs that comprise JPQSF were selected based on TCM theory and the extensive experience of Professor Ren-Huan Yu, who has treated kidney diseases with TCM for several decades. He has applied JPQSF to invigorate the spleen and dispel dampness, thereby restoring harmony between Yin and Yang. We previously showed an 80% clinical remission rate of refractory membranous nephropathy without adverse reactions after JPQSF therapy.8
The chronic kidney disease (CKD)-colon axis might be associated with the development, progression, and prognosis of IMN. The intestinal microecology is altered in patients with CKD. When the intestinal structure changes, microorganisms and antigens can activate the host immune system across the intestinal barrier, causing inflammatory reactions.9 The intestinal flora can also activate immune cells via their metabolites and other specific components, thereby inducing inflammatory responses that further accelerate the progression of kidney disease.10 Immune dysfunction caused by disordered intestinal flora might be an important contributor to the development of IMN.
The 16S rRNA located on the ribosomal small subunit of prokaryotic cells has 10 conserved regions and 9 hypervariable regions. The conserved regions do not significantly differ among bacteria, whereas the hypervariable regions are genus- or species-specific and differ according to kinship. These specific 16S rDNA sequences can reveal biological species diversity and are thus considered the most suitable index for identifying bacterial phylogenies.11
We sequenced the 16S rRNA gene to compare intestinal microbiota between patients with IMN and healthy persons. We also measured the effects of JPQSF (TCM) and immunosuppressants on the intestinal flora of IMN patients in a randomized controlled trial (RCT).
Methods
Ethical approval
The Medical Committee at Xiyuan Hospital approved the study (No. 2016XLA127-2). All participants provided written informed consent before enrolling in the study.
Study design and participant selection
This study comprised concurrent and single-center randomized controlled trials that proceeded between July 2016 and December 2018 at the Department of Nephrology, Xiyuan Hospital, China Academy of Chinese Medical Sciences. This trial comprised 30 patients with IMN and 10 healthy persons. The patients with IMN were randomly assigned, using a random number table to groups that received either TCM therapy or immunosuppressant western medicine (WM) therapy (n = 15 per group). The inclusion criteria for the patients were as follows: renal biopsy and pathological findings that met IMN-compliant diagnostic criteria; met the diagnostic criteria of TCM spleen deficiency and dampness excess syndrome, namely primary symptoms of mental fatigue, waist pain, and edema; secondary symptoms of reduced appetite, fear of cold, loose stools, or diarrhea, pale complexion, frequent urination, or frequent urination at night; and tongue as well as pulse symptoms of enlarged, tooth-marked tongue with greasy coating and a slow and sunken pulse. Participants were diagnosed with a spleen deficiency and dampness excess syndrome if they were aged 18–70 years; had one primary symptom, one secondary symptom, and tongue or pulse symptoms; 24-h urine protein excretion >0.5 g; estimated glomerular filtration rate (eGFR) ≥ 60 mL/min/1.73 m2; and disease persisting for > 6 months; were not administered antibiotics and probiotics in the month before starting the trial; and had provided signed written informed consent. The exclusion criteria comprised secondary membranous nephropathy (including tumors, hepatitis B, lupus, drugs, toxins and other related membranous nephropathies; eGFR < 60 mL/min/1.73 m2; history of bacterial infection, or antibiotics or probiotic use within the last month; autoimmune diseases such as rheumatoid arthritis and Sjögren syndrome; metabolic diseases such as diabetes and hypertension; sexually transmitted diseases such as hepatitis B, hepatitis C, and AIDS; digestive tract diseases such as peptic ulcers; current or past malignant tumors; severe mental disease, or heart and cerebrovascular or liver disease; pregnant and lactating women; and other factors that might affect the determination of intestinal flora.
Inclusion criteria for healthy persons comprised the following: complete documentation submitted within 2 weeks before the trial regarding medical history, vital signs, physical examination, clinical laboratory test, and echocardiography (ECG) findings; age, 18–70 years; and females who were not presently or potentially pregnant. Exclusion criteria comprised a history of severe circulatory, endocrine, nervous, digestive, respiratory, hematological, immunological, or psychiatric diseases; recently prescribed or investigational medication, especially any drug that is known to harm a particular organ, within 3 months; drug or alcohol abuse; and drug allergies or an allergic constitution. Eligible persons were apprised of the risks of the study; they then read, understood, and signed written informed consent forms before enrolling in the study.
Intervention
All patients were given renin-angiotensin system (RAS) blockers as basic treatment to ensure that each patient received the maximum tolerated dose. Meanwhile, control blood pressure according to KDIGO guidelines (2012) for glomerulonephritis and hypertension. Patients who were randomly assigned to the TCM (n = 15) or WM (n = 15) groups were treated with JPQSF and immunosuppressants, respectively, for 6 months. The TCM department at Xiyuan Hospital prepared and supplied the JPQSF, which was administered orally once in the morning and once at night (Table 1).8 The WM group was treated with the current immunological inhibitors recommended by the KDIGO clinical practice guidelines,2 including hormone + cyclosporine A, hormone + tacrolimus or a calcium-regulating phosphatase inhibitor alone. An experienced nephrologist followed up all patients monthly at the hospital for 6 months.
Table 1.
Components of Jian Pi Qu Shi Formula.
| Latin Binomial Name | English Name | Part Used | Origin of Producta | Weight (g) |
|---|---|---|---|---|
| Astragalus membranaceus | Radix Astragali | Root | Gansu | 30 |
| Codonopsis pilosula | Radix Codonopsitis Pilosulae | Root | Gansu | 15 |
| Poria cocos | Sclerotium Poriae Cocos | Sclerotium | Anhui | 30 |
| Atractylodes macrocephala | Rhizoma Atractylodis Macrocephalae | Stem | Zhejiang | 20 |
| Angelica sinensis | Radix Angelicae sinensis | Root | Gansu | 15 |
| Coix lacryma-jobi | Semen coicis | Seed | Guizhou | 30 |
| Polyporus umbellatus | Sclerotium polypori Umbrellati | Sclerotium | Shanxi | 15 |
| Stephania tetrandra | Radix Stephaniae Tetrandrae | Root | Zhejiang | 20 |
| Dioscorea nipponica Makino | Dioscoreae Nipponicae Rhizoma | Root | Liaoning | 30 |
| Folium Perillae | Perillae Folium | Foliage | Shanxi | 15 |
Province. All products were supplied in raw dried form.
Outcome measures
We measured 24-h urine protein excretion, plasma albumin, serum creatinine, urea nitrogen, eGFR, complete blood counts, and liver enzymes monthly. Patients were asked to report any symptoms and adverse effects at each appointment or immediately when they occurred.
Detection of intestinal flora
The detection of intestinal microflora was completed as the following steps: fecal DNA extraction, PCR amplification and purification, library construction and sequencing. Fecal samples collected before breakfast were stored at −80 °C. The TCM and WM groups provided specimens again after 6 months of treatment. Detection of intestinal flora in the specimens was assisted by a commercial company (Beijing Zhongkangbo Biotechnology Co., Ltd., Beijing, China), then genomic DNA was extracted from each type of flora, using the cetyltrimethylammonium ammonium bromide (CTAB) method, and purified. Specific primers were prepared using Barcode (New England Biolabs Inc., Ipswich, MA, USA), with High-Fidelity Polymerase Chain Reaction (PCR) Master Mix and GC Buffer (New England Biolabs Inc.). High-Fidelity DNA Polymerase (New England Biolabs Inc.) was used for PCR amplification. The PCR products were diluted with sterile water at a concentration of 1:1 according to their concentrations, then resolved by electrophoresis on 2% agarose gels (Solarbio Science and Technology Co., Ltd., Beijing, China). Target strips were recovered using gel recovery kits (Qiagen GmbH, Hilden, Germany). A library constructed using DNA PCR-Free Sample Preparation Kit (Illumina Inc., San Diego, CA, USA) was quantified using Qubit and quantitative (Q)-PCR. The library was then sequenced using HiSeq2500 PE250 (Illumina Inc.).
Bioinformatics analysis of sequencing results
After obtaining the original data files of the intestinal flora, quality control, de-chimerization, and specific and statistical analyses of the sequencing parameters proceeded using scikit-bio™, an open-source, Berkeley Software Distribution (BSD) licensed, python package with Quantitative Insights Into Microbial Ecology (QIIME) modules and the Illumina platform.12 Sample abundance information was clustered at the species and sample levels and plotted as heat maps. Intestinal microbiota diversity was analyzed using alpha diversity. Mothur software (Version 1.35.1, The University of Michigan Department of Microbiology and Immunology, Ann Arbor, MI, USA) was used to determine abundance-based coverage (ACE; computed abundance) and the Shannon index (computed flora diversity). The composition of the intestinal flora was compared among samples using beta diversity and R software version 2.15.3 (MathSoft, Cambridge, MA. USA), including principal components analysis (PCA) and principal co-ordinates analysis (PCoA).
Significantly different species in intestinal flora between groups based on species abundance information in the samples were detected by Metastat analysis using Mothur software. The significance of differences was evaluated by testing multiple hypotheses and analyzing false discovery rates (FDR) of rare frequency data.
Statistical analyses
All data were analyzed using Statistical Package for the Social Sciences (SPSS) 20.0 statistical software (IBM Corp., Armonk, NY, USA). Measured data are shown as means ± standard deviation (SD). Abnormally distributed data were assessed using Mann–Whitney U tests. Normally distributed data were assessed using Levene tests to judge the homogeneity of variance, and t-tests for uniform variance. If the variance was non-uniform, Mann–Whitney U tests were applied and counts were analyzed using chi-squared tests. All P values are 2-sided. P values < 0.05 were considered statistically significant.
Results
Patient disposition and baseline data
This trial included 30 patients with IMN treated with TCM or WM (n = 15 per group) and 10 healthy persons. Five participants, who opted out of the trial, were from the WM group. Three refused western immunosuppressive therapy and two stopped complying due to concern about adverse reactions. Table 2 shows no significant differences between the IMN and the healthy group, or the TCM and the WM group (P > 0.05).
Table 2.
Baseline characteristics of healthy and IMN groups treated with TCM or WM.
| Characteristics | Healthy (n = 10) | IMN (n = 30) | χ2 or t | P | IMN |
χ2 or t | P | |
|---|---|---|---|---|---|---|---|---|
| TCM (n = 15) | WM (n = 10) | |||||||
| Female, n (%) | 5 (50) | 13 (43.3) | 0.00 | 1.00 | 6 (40.0) | 6 (60.0) | 0.33 | 0.57 |
| Age (years) | 44.10 ± 9.23 | 48.83 ± 11.31 | 1.20 | 0.24 | 48.60 ± 11.67 | 46.70 ± 11.72 | 0.40 | 0.69 |
| Height (cm) | 169.80 ± 5.49 | 167.37 ± 6.90 | −1.01 | 0.32 | 167.73 ± 7.41 | 165.30 ± 7.30 | 0.81 | 0.43 |
| Weight (kg) | 65.70 ± 6.99 | 66.87 ± 9.13 | 0.37 | 0.72 | 66.53 ± 9.26 | 66.10 ± 10.02 | 0.11 | 0.91 |
| Systolic pressure (mmHg) | 118.30 ± 8.55 | 123.23 ± 10.50 | −1.60 | 0.11 | 123.40 ± 11.39 | 122.80 ± 10.12 | −0.68 | 0.50 |
| Diastolic pressure (mmHg) | 74.10 ± 6.42 | 77.07 ± 7.61 | −1.19 | 0.24 | 78.33 ± 8.42 | 76.70 ± 7.39 | 0.50 | 0.62 |
Data are shown as means ± standard deviation (SD) or n (%). IMN: idiopathic membranous nephropathy; TCM: traditional Chinese medicine; WM: western medicine.
Index change after TCM and WM therapy for 6 months
Urinary protein excretion, serum albumin, urea nitrogen, serum creatinine, or eGFR did not significantly differ between the TCM and WM groups before therapy (P > 0.05). However, urinary protein excretion differed between them after therapy. Serum albumin values after treatment were higher in the TCM group (P < 0.05), but did not significantly differ in the WM group (P > 0.05). Urea nitrogen, serum creatinine and eGFR did not significantly differ between the groups after therapy (P > 0.05; Table 3).
Table 3.
Index changes after TCM and WM therapy.
| Index | TCM (n = 15) |
WM (n = 10) |
t | P | ||||
|---|---|---|---|---|---|---|---|---|
| Values | t | P | Values | t | P | |||
| Urinary protein excretion, g | ||||||||
| Before | 3.24 ± 1.74 | 3.94 ± 1.05 | −1.13 | 0.270 | ||||
| After | 1.73 ± 1.85 | −2.43 | 0.015a | 1.91 ± 1.18 | 4.05 | 0.001a | −0.72 | 0.471 |
| Serum albumin, g/L | ||||||||
| Before | 32.44 ± 9.04 | 31.55 ± 4.23 | 0.29 | 0.774 | ||||
| After | 39.99 ± 7.03 | −2.55 | 0.017a | 34.83 ± 9.14 | −1.03 | 0.317 | 1.60 | 0.124 |
| Urea nitrogen, mg/dL | ||||||||
| Before | 14.34 ± 6.23 | 11.42 ± 4.86 | 1.23 | 0.230 | ||||
| After | 17.13 ± 6.89 | −0.98 | 0.326 | 13.93 ± 4.92 | −1.15 | 0.266 | −1.39 | 0.165 |
| Serum creatinine, mg/dL | ||||||||
| Before | 0.80 ± 0.33 | 0.76 ± 0.16 | 0.45 | 0.659 | ||||
| After | 0.85 ± 0.30 | −0.81 | 0.418 | 0.70 ± 0.28 | −1.17 | 0.241 | −1.443 | 0.149 |
| eGFR, mL/min/1.73 m2 | ||||||||
| Before | 110.40 ± 47.36 | 97.30 ± 19.15 | 0.96 | 0.349 | ||||
| After | 99.20 ± 37.79 | 0.72 | 0.480 | 113.60 ± 35.69 | −1.27 | 0.219 | −0.95 | 0.350 |
Data are shown as means ± SD. eGFR: estimated glomerular filtration rate; IMN: idiopathic membranous nephropathy; SD: standard deviation; TCM: traditional Chinese medicine; WM: western medicine.
aP < 0.05 vs. before therapy.
Intestinal flora analysis
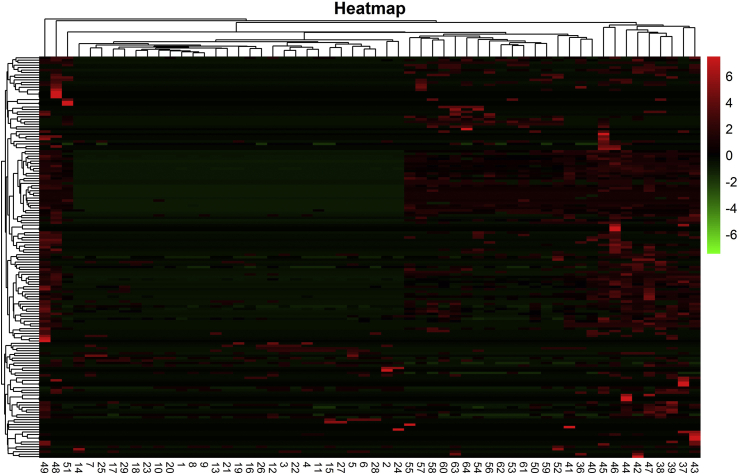
We analyzed 30 fecal samples from the IMN group before treatment (n = 30), 10 from the healthy group, then 15 samples after TCM, and 10 after WM. Among them, four in the IMN group, one in the healthy group, one after TCM treatment, and two after WM treatment did not return valid data and were excluded. The actual number of effective samples was 57. We then prepared a clustering heat map according to the abundance of species at the genus level in each sample (Fig. 1).
Fig. 1.
Cluster heat map of species abundance at genus level. Ordinate is sample information; abscissa is species annotation information; left cluster tree is species; upper cluster tree is between sample groups.
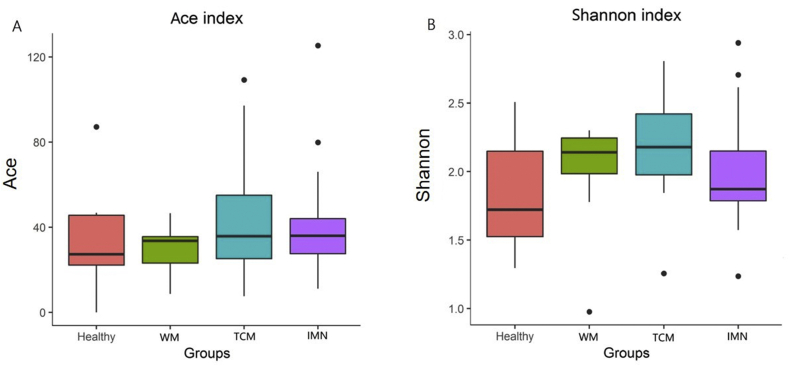
The alpha diversity index showed that intestinal flora differed between the IMN and healthy groups, and the TCM and WM groups (Fig. 2).
Fig. 2.
Differences in alpha diversity indices between groups. A: Abundance-based coverage (ACE) index; B: Shannon index. WM: western medicine; TCM: traditional Chinese medicine; IMN: idiopathic membranous nephropathy.
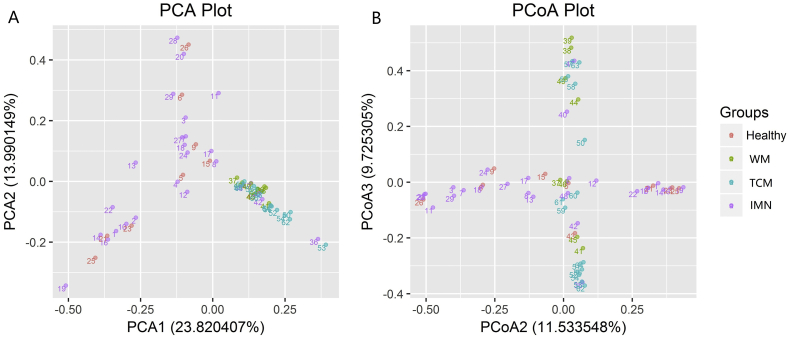
The comparison of multiple samples (beta diversity) also revealed differences in flora between IMN and healthy groups, and between the TCM and WM groups. The composition of intestinal flora was similar between the TCM and WM groups, but some differences were evident. Principal components analysis is a variance decomposition, reduced dimensionality and multidimensional method of extracting the most important elements and structures from complex mixtures.13 PCoA [also known as multidimensional (MDS) scaling] is a reduction method with which to explore and visualize similarities or dissimilarities of data, that is similar to PCA. The difference is that PCoA searches for main coordinates based on a distance matrix; that is, a shorter distance between genera indicated closer similarity. Samples with high and low structural similarity in terms of intestinal flora were respectively clustered close together and located far from each other, respectively (Fig. 3).
Fig. 3.
Findings of principal co-ordinates analysis. Samples with high and low structural similarity in terms of intestinal flora are respectively clustered together and located far from each other. A: Principal components analysis (PCA); B: Principal co-ordinates analysis (PCoA); WM: western medicine; TCM: traditional Chinese medicine; IMN: idiopathic membranous nephropathy.
Metastat analysis: The main genera that differed between the IMN group before TCM therapy and the healthy group were Christensenellaceae_R-7_group, Bifidobacterium (77), Dorea, Escherichia- Shigella, Parabacteroides, Bifidobacterium, and Coprococcus_3. The main differential genera after TCM were Butyricimonas, Bacteroides, Alistipes, and Lachnospira therapy, and Ruminococcus_2, Lachnospiraceae_ND3007_group, Lachnospira, Bifidobacterium, Alistipes, [Eubacterium]_ventriosum_group.
Discussion
The concept of the “CKD-colonic axis” proposed by Pahl in 2015, suggests that patients with CKD develop intestinal micro-ecological disorders during disease progression. Such disorders include the increased production of uremic toxins and reduced probiotics that exacerbate the accumulation of intestinal-derived urinary toxins in the bloodstream that cannot be removed by damaged kidneys. Renal function is further reduced, eventually forming a vicious circle between the intestines and kidneys. Dysfunctional intestinal bacteria also destroy the intestinal epithelial barrier function, causing intestinal-derived urinary toxins and pathogens to shift enter the circulation, activate the intestinal mucosal immune system, induce systemic micro-inflammatory reactions, and thus aggravate kidney damage. The intestinal mucosal immune system consists of intestinal-associated lymphoid tissues. Abnormal microorganisms and antigens activate the host immune system via the intestinal barrier in an altered intestinal structure, causing inflammatory responses.9 Intestinal microbes can activate immune cells through their metabolites and other specific components, and induce inflammatory responses.10
The present study found that intestinal flora differed between patients with IMN and healthy individuals, suggesting that the intestinal flora is disordered in such patients. The main genera that differed between the IMN and healthy groups were Christensenellaceae_R-7_group, Bifidobacterium (77), Dorea, Escherichia-Shigella, Parabacteroides, Bifidobacterium, and Coprococcus_3. However, the physiological and pathological effects of these genera have not been investigated in detail, leaving their identities unclear. As far as we understand, Bifidobacterium is the most representative intestinal probiotic organism, and it is abundant in the adult human gut. Bifidobacteria play important roles in the metabolism of nutrients, inhibiting the growth of intestinal pathogens, maintaining the balance of flora, improving digestion and immunity, promoting development, and delaying aging, among other functions.14 Bifidobacteria also exert anti-allergic effects when acetic and butyric acids are the main fermentation products,15 possibly contributing to the immunomodulatory effects of intestinal short-chain fatty acids.16 Differences in the genera of intestinal flora between patients with IMN and healthy individuals might provide clues to the pathogenesis of IMN, in which Bifidobacteria might plays a role.
Both JPQSF and the immunosuppressants decreased the index of 24-h urinary protein. Plasma albumin levels were increased by JPQSF, but not by immunosuppressants. Urea nitrogen, serum creatinine and eGFR not significantly differ between the groups before and after therapy. Among TCM syndromes, the proportion of IMN is probably the highest in spleen deficiency syndrome.17 The most prevalent syndromes associated with IMN are damp-heat and blood stasis. Chronic nephritis is commonly treated with JPQSF.18
Expression of the marker proteins nephrin and podocalyxin increased in damaged podocytes containing JPQSF serum cultured in vitro indicating that the damaged glomerular filtration barrier is repaired. This was achieved by JPQSF serum through inhibiting mTOR activation in injured podocytes, reducing P–P70S6K and P-4EBP1 synthesis, elevating microtubule-associated protein 1A/1B-light chain 3 (LC3)-II expression and restoring the autophagy of damaged podocytes. The mechanism by which JPQSF reduced IMN urinary protein was associated with the protection of glomerular podocytes.19 Astragalus membranaceus has an active ingredient that protects against adriamycin-induced nephropathy. It reduces levels of urinary protein, mainly by elevating the expression of the podocyte-associated proteins nephrin and podocin.20 Radix Stephaniae Tetrandrae and its alkaloids might exert anti-inflammatory effects by inhibiting the release of nitric oxide (NO) and the cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6.21 Tetrandrine, the active ingredient of Radix Stephaniae Tetrandrae, can reduce β-glucan-induced inflammatory responses in macrophages.22 While JPQSF effectively treats IMN, it also significantly improves gastrointestinal symptoms. Shenling Baizhu Formula, which invigorates the spleen and removes dampness, can regulate intestinal flora unbalanced by antibiotics.23 Sijunzi Decoction, which invigorates the spleen, can increase intestinal flora diversity in rats with a spleen deficiency.24 Thus, TCM with spleen strengthening effects can regulate and increase the diversity of intestinal flora. Probiotics can protect the kidneys, and JPQSF is suitable for patients with IMN who have a deficient spleen and dampness-heat syndrome. Its main effects are spleen invigoration, qi replenishment, dampness removal and clearing heat. These effects are similar to those of the two prescriptions described above. Data from national health and nutrition surveys and studies of the relationship between probiotics and kidney parameters have shown that probiotics can reduce the risk of proteinuria. Thus, JPQSF might protect the kidneys by increasing ratios of intestinal probiotics.25
The main intestinal flora that differed after JPQSF therapy comprised Butyricimonas, Bacteroides, Alistipes, and Lachnospira. Bacteroides is a dominant intestinal strain that plays a pivotal role in improving nutrient utilization,26 accelerating intestinal mucosal angiogenesis,27 developing the immune system, improving immunity,28 and maintaining the intestinal flora balance.29,30 Bacteroides can produce short-chain fatty acids in the colon,31 and significantly enhance the growth of colonic lamina propria Treg cells after colonization.32 Such enhancement further exerts immunomodulatory effects. Lachnospira triggers intestinal inflammation and is the main genus of the Lachnospiraceae family.33 Alistipes is a gram-negative, anaerobic bacterium, and a facultative pathogen that can cause various diseases.34 The differences between the probiotic genus Bacteroides, the pathogenic bacterium Alistipes shahii and Lachnospira before and after JPQSF therapy might be associated with the ability of this TCM to treat IMN.
The main differential intestinal flora that changed after immunosuppressive therapy was Ruminococcus_2, Lachnospiraceae_ND3007_group, Lachnospira, Bifidobacterium, Alistipes, and Eubacterium_ventriosum_group. The differences in the probiotic Bifidobacterium and the pathogenic bacteria Alistipes and Lachnospira between before and after JPQSF therapy might result from immunosuppression.
In conclusion, patients with IMN might have disordered intestinal flora. The finding that intestinal flora differs after therapy with JPQSF implies that this TCM could regulate intestinal flora. Immunosuppressants can also alter intestinal flora. The effects of JPQSF on IMN might also be associated with improving the balance of intestinal flora.
Funding
This study was supported by grants from the National Key R&D Plan of China (No. 2019YFC1708500), the National Natural Science Foundation of China (No. 81374036), and the Natural Science Foundation of Wannan Medical College under Grant (No. WYRCQD2018009).
Conflicts of interest
None.
Edited by Yan-Gang Ren and Yi Cui
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Hou J.H., Zhu H.X., Zhou M.L. Changes in the spectrum of kidney diseases: an analysis of 40,759 biopsy-proven cases from 2003 to 2014 in China. Kidney Dis (Basel) 2018;4:10–19. doi: 10.1159/000484717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofstra J.M., Fervenza F.C., Wetzels J.F.M. Treatment of idiopathic membranous nephropathy. Nat Rev Nephrol. 2013;9:443–458. doi: 10.1038/nrneph.2013.125. [DOI] [PubMed] [Google Scholar]
- 3.Ahlmann M., Hempel G. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol. 2016;78:661–671. doi: 10.1007/s00280-016-3152-1. [DOI] [PubMed] [Google Scholar]
- 4.Shah S.R., Altaf A., Arshad M.H. Use of cyclosporine therapy in steroid resistant nephrotic syndrome (SRNS): a review. Glob J Health Sci. 2015;8:136–141. doi: 10.5539/gjhs.v8n4p136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y., Cai G., Sun X., Chen X. Treatment of chronic kidney disease using a traditional Chinese medicine, Flos Abelmoschus manihot (Linnaeus) Medicus (Malvaceae) Clin Exp Pharmacol Physiol. 2016;43:145–148. doi: 10.1111/1440-1681.12528. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Y., Deng Y., Chen Y., Chuang P.Y., Cijiang He J. Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney Int. 2013;84:1108–1118. doi: 10.1038/ki.2013.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y., Deng Y., Ni Z. Efficacy and safety of traditional Chinese medicine (Shenqi particle) for patients with idiopathic membranous nephropathy: a multicenter randomized controlled clinical trial. Am J Kidney Dis. 2013;62:1068–1076. doi: 10.1053/j.ajkd.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Shi B., Zhang R.R., Liang Y., Wang X.H., Lang R., Yu R.H. Efficacy of traditional Chinese medicine regimen Jian Pi Qu Shi Formula for refractory patients with idiopathic membranous nephropathy: a retrospective case-series study. Evid Based Complement Alternat Med. 2018;2018:5854710. doi: 10.1155/2018/5854710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pahl M.V., Vaziri N.D. The chronic kidney disease - colonic Axis. Semin Dial. 2015;28:459–463. doi: 10.1111/sdi.12381. [DOI] [PubMed] [Google Scholar]
- 10.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 11.Caporaso J.G., Lauber C.L., Walters W.A. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 2011;108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caporaso J.G., Kuczynski J., Stombaugh J. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avershina E., Frisli T., Rudi K. De novo semi-alignment of 16S rRNA gene sequences for deep phylogenetic characterization of next generation sequencing data. Microb Environ. 2013;28:211–216. doi: 10.1264/jsme2.me12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Olden C., Groen A.K., Nieuwdorp M. Role of intestinal microbiome in lipid and glucose metabolism in diabetes mellitus. Clin Ther. 2015;37:1172–1177. doi: 10.1016/j.clinthera.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz L., Delgado S., Ruas-Madiedo P., Sánchez B., Margolles A. Bifidobacteria and Their Molecular Communication with the Immune System. Front Microbiol. 2017;8:2345. doi: 10.3389/fmicb.2017.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugahara H., Odamaki T., Fukuda S. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci Rep. 2015;5:13548. doi: 10.1038/srep13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M.D., Guo Y.F., Wang Y.F., Zhao W.J. Rules of traditional Chinese medicine in treating idiopathic membranous nephropathy based on syndrome differentiation and a literature analysis (in Chinese) Global Traditi Chin Med. 2019;12:1834–1840. doi: 10.3969/j.issn.1674-1749.2019.12.012. [DOI] [Google Scholar]
- 18.Zhan Y.L., Yu R.H., Wei Z.N. Differentiation and treatment of common accompanied syndromes in chronic kidney disease with traditional Chinese medicine (in Chinese) Chin J Kidney Dis Investig. 2013;2:232–236. doi: 10.4028/www.scientific.net/AMR.901.53. [DOI] [Google Scholar]
- 19.Wang X.H., Zhao W., Li P. Effect of Jianpi qushi Heluo Formula containing serum on the expression of podocyte marker protein Nephrin,Podocalyxin and mTOR (in Chinese) Chin J Integr Tradit West Med. 2018;38:1106–1111. doi: 10.7661/j.cjim.20180814.223. [DOI] [Google Scholar]
- 20.Wang N., Wei R.B., Li Q.P., Yang X., Chen X.M. Protective effects of astragaloside in rats with adriamycin nephropathy and underlying mechanism. Chin J Nat Med. 2016;14:270–277. doi: 10.1016/s1875-5364(16)30027-9. [DOI] [PubMed] [Google Scholar]
- 21.Guo C., Wang M., Li J. Effect of inflammatory cytokines in the LPS induced RAW264.7 cells by the decoction and its split components from stephania tetrandra S.moore (in Chinese) Acta Chin Med Pharmacol. 2015;43:33–36. doi: 10.19664/j.cnki.1002-2392.2015.04.011. [DOI] [Google Scholar]
- 22.Xu J., Liu D., Yin Q., Guo L. Tetrandrine suppresses β-glucan-induced macrophage activation via inhibiting NF-κB, ERK and STAT3 signaling pathways. Mol Med Rep. 2016;13:5177–5184. doi: 10.3892/mmr.2016.5187. [DOI] [PubMed] [Google Scholar]
- 23.Dong K.Z., Gao Y.S., Qin N.N.J., Su L. Effects of senlin Baishu san on the dysbacteriosis induced by antibiotics in mices (in Chinese) Chin J Exper Tradit Med Formul. 2015;21:154–157. doi: 10.13422/j.cnki.syfjx.2015010154. [DOI] [Google Scholar]
- 24.Huang W.W., Peng Y., Wang M.Y., Peng Z.S., Li X.B. Regulatory effect of Sijunzi Tang and its single herbs on intestinal flora in rats with spleen deficiency (in Chinese) Chin J Experl Tradit Med Formul. 2019;25:8–15. doi: 10.13422/j.cnki.syfjx.20190847. [DOI] [Google Scholar]
- 25.Yacoub R., Kaji D., Patel S.N. Association between probiotic and yogurt consumption and kidney disease: insights from NHANES. Nutr J. 2016;15:10. doi: 10.1186/s12937-016-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stappenbeck T.S., Hooper L.V., Gordon J.I. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bäckhed F., Ding H., Wang T. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooper L.V. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004;12:129–134. doi: 10.1016/j.tim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Sears C.L. A dynamic partnership: celebrating our gut flora. Anaerobe. 2005;11:247–251. doi: 10.1016/j.anaerobe.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Hooper L.V., Wong M.H., Thelin A., Hansson L., Falk P.G., Gordon J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 31.Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 32.Lathrop S.K., Bloom S.M., Rao S.M. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai Y.M., Zhang L.L., Li Y. Characterization of Trichococcus paludicola sp. nov. and Trichococcus alkaliphilus sp. nov., isolated from a high-elevation wetland, by phenotypic and genomic analyses. Int J Syst Evol Microbiol. 2018;68:99–105. doi: 10.1099/ijsem.0.002464. [DOI] [PubMed] [Google Scholar]
- 34.Sharma S., Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes (Lond) 2013;37:382–389. doi: 10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]