Abstract
COVID-19 has been declared a pandemic by the World Health Organization on March 11th and since then more than 3 million cases and a quarter million deaths have occurred due to it. The urge to find a resultful treatment or cure is now pressing more than any other time since the outbreak of the pandemic. Researchers all over the world from different fields of expertise are trying to find the most suitable drugs, that are already known to treat other diseases, and could tackle the process of SARS-CoV2 through which it invades and replicates in human cells. Here, we discuss five of the most promising drugs that can potentially play a major role in the treatment of COVID-19. While nicotine and ivermectin may be blocking transport abilities of the virus or its components, famotidine, remdesivir and chloroquine in combination with zinc ions can deactivate important enzymes needed for the replication of the virus. While clinical trials for some of these drugs have already started, it is common knowledge that lack of organization between countries, institutes and hospitals might slow down the whole process for an official treatment based in wide, randomized, placebo controlled trials.
Keywords: Chloroquine, Coronavirus, COVID-19, Famotidine, Ivermectin, Nicotine, Remdesivir, SARS-Cov2
Introduction
The SARS-CoV-2 virus emerged in December 2019 and then spread rapidly worldwide, starting China, Japan, and South Korea and then to Europe and North America, while the World Health Organization on March 11th declared the rapidly spreading novel coronavirus outbreak a pandemic, acknowledging that the virus will likely spread to all countries on the globe.1 As of May 2nd 2020, more than 3.5 million confirmed cases of coronavirus disease 2019 (COVID-19) and almost 250.000 deaths have been reported, with one third of the cases and more than one fifth of the deaths to have occurred in the United States (John Hopkins Coronavirus Resource Center statistics). In response to the most serious global health threat in more than a century, the world's biomedical establishment has unleashed an unprecedented response to the Covid-19 pandemic, rapidly increasing resources aimed at finding safe and effective treatments for the disease, comprehensively reviewed in.2 Research for treatments has emerged from different medical backgrounds, both pharmacologically with the use of well known drugs for other diseases3, 4, 5, 6, 7 or corticosteroids8 and immunologically from the serum/antibodies of former patients against other coronaviruses or from patients that have recovered from COVID-199, 10, 11 or even with the use of revolutionary ideas such as the combination of CRIPR tool with Cas13.12, 13, 14 Another tremendous effort from NIH (http://ClinicalTrials.gov Identifier: https://clinicaltrials.gov/ct2/show/NCT04283461) and all countries around the globe focuses on the successful development of a vaccine that would prevent the emergence of COVID-19 through the years and the creation of a repeating cycle of spreading, like the influenza virus.15,16
In this review, we are going to focus on small molecules and drugs that are already in use and FDA approved for other diseases, and show promising results for treatment of COVID-19 (all except nicotine as of May 2020) and are all under further investigation with large numbers of patients and some of them with randomised, placebo controlled studies, which is considered the golden standard in medicine for the use of a certain compound for a treatment in a novel disease.
Background
In order to better understand the mechanisms through which these drugs are working, it is vital to elucidate the basic steps through which the SARS-CoV2 virus is infecting a human cell (more often alveolar epithelial type II cells17) and how it replicates inside the human organism.
Coronaviruses (CoVs) are the largest RNA viruses identified so far and belong to the Coronaviridae family. They are divided into 4 groups (α-, β-, γ- and δ-), while the β-coronaviruses are further divided into A, B, C, and D lineages. SARS-CoV and SARS-CoV-2 are members of β-coronaviruses lineage B.18 Particularly, SARS-CoV and SARS-CoV-2 have 89.8% sequence identity in their spike (S) protein. S2 subunits mediate the membrane fusion process, and both of their S1 subunits utilize human angiotensin-converting enzyme 2 (hACE2) as the receptor to infect human cells.19 Most importantly, the ACE2-binding affinity of the of S protein of SARS-CoV-2 is 10- to 20-fold higher than that of SARS-CoV,15 which contributes to the higher infectivity of SARS-CoV-2 as compared to SARS-CoV.20
After binding of the S protein on the virion to the ACE2 receptor on the target cell, the heptad repeat 1 (HR1) and 2 (HR2) domains in its S2 subunit of S protein interact with each other to form a six-helix bundle (6-HB) fusion core,21 bringing viral and cellular membranes into close proximity for fusion and infection.22 Therefore, the S-protein–receptor interaction is the primary determinant for a coronavirus to infect a host cell and also governs the tissue specificity of the virus. The virus gains access to the host cell cytosol by acid-dependent proteolytic cleavage of S protein by a cathepsin protease, followed by fusion of the viral and cellular membranes. S protein cleavage occurs at two sites within the S2 subunit of the protein.23 Fusion occurs within acidified endosomes and the formation of the bundle after fusion allows for the mixing of viral and cellular membranes, resulting in the release of the viral genome into the cytoplasm.22
The next step in the coronavirus lifecycle is the translation of the most vital gene of the virus, the RNA-dependent RNA polymerase or replicase gene, from the virion genomic RNA. The replicase gene encodes two large ORFs that produce two polyproteins, pp1a and pp1ab.24,25 These can be expressed by overlapping reading frames through ribosomal frameshifting from the rep1a into the rep1b ORF reading frame. In vitro studies predict the incidence of ribosomal frameshifting is as high as 25%.26 Polyproteins of coronaviruses are furtherly cleaved by a group of proteases, either two or three proteases that cleave the replicase polyproteins. These are the papain-like proteases (PLpro) and a serine type protease.27,28
Many non-structural proteins (nsps) are assembling the replicase–transcriptase complex (RTC) to create an environment suitable for RNA synthesis, while specifically nsp12 encodes the RNA-dependent RNA polymerase (RdRp) domain. This is the enzyme that will elongate new positive sense RNA molecules from the original RNA of the virion when conditions will allow this to happen.29
Viral RNA synthesis produces both genomic and sub-genomic RNAs (sgRNAs). SgRNAs serve as mRNAs for the structural and accessory genes of the virus. In this stage, coronaviruses are known for their ability to recombine using both homologous and nonhomologous recombination.30,31 This ability is considered to play a prominent role in viral evolution.32
After replication and sgRNA synthesis, the viral structural proteins, S, E, and M are translated and inserted into the endoplasmic reticulum (ER). These proteins move to the endoplasmic reticulum–Golgi intermediate compartment (ERGIC)33,34 and are encapsulated by N protein buds into membranes of the ERGIC containing viral structural proteins, forming mature virions.35 The M protein is the most abundant of the three and directs most protein–protein interactions required for assembly of coronaviruses, while the E protein functions as a chaperone to the M protein.36 Lastly, The S (spike) protein is incorporated into virions at this step by interacting with the M protein, but is not required for assembly. As already stated, the trimeric S protein make up the distinctive spike structure on the surface of the virus37,38 and acts as a class I fusion protein22 that mediates attachment to the host receptor. Following assembly, virions are transported to the cell surface in vesicles and released by exocytosis.39
Potential drugs for COVID-19
There more than a dozen already known drugs or small molecules that are potentially able to help in tackling the novel coronavirus’ infectious process. Here, we discuss five of the most promising compounds and describe how they are able to intervene in one of the many steps that SARS-CoV2 virus needs to do in order to be replicated successfully in a human cell.
Zinc and chloroquine
Zinc will shut down RNA dependent RNA polymerase or replicase. The problem is how to get zinc inside the cell because zinc is a 2+ ion and ions cannot get through the cellular membrane unless there is a transporter that allows it to come in. This has been already tested in vitro with an ionophore which allows zinc to come into the cell so they could see that the activity of this RNA dependent RNA polymerase was reduced. Thus, zinc inhibited coronavirus RNA polymerase activity in vitro and zinc ionophores blocked the replication of these viruses in cell culture when they looked at the SARS virus.40 As the zinc concentration inside the cell went up, the by-product of the RNA dependent RNA polymerase (Rd RP) went down clearly demonstrating that zinc intracellularly is blocking this very important enzyme of the virus.
Zinc supplements get absorbed into the body in the blood into the extracellular space and the problem is how to get that zinc from the extracellular space into the intracellular space in the cytosol where it needs to work on infected cells and the viral proteins. Again, what is needed is some sort of ionophore or a gated mechanism to open and to allow that zinc to come into the cell, thus increasing the concentration of zinc into the cell so it can block Rd RP.
An, irrelevant of SARS infection research, publication of 2014 has showed that zinc may help some cancer cells to be led to autophagocytosis in the lysosomes and that by giving chloroquine they could have these cancer cells disappear in a far more superior rate. What they show is that they were able to detect much higher intracellular zinc by increasing concentrations of chloroquine. In fact, small amount of chloroquine at 10 μM will increase the intracellular concentration of zinc ten-fold.41 Chloroquine is a medication that has been around for decades end is used to treat malaria. While it is pretty inexpensive to produce and widely available, one does need a prescription to use it and it doesn't come without side effects.42
Under the pressure of finding a treatment that could possibly ease the COVID-19 implications in serious cases, a Chinese expert consensus published an article in February 2020 that states that chloroquine phosphate or chloroquine has a wide range of antiviral effects including anti-coronavirus effects and in patients diagnosed with novel coronavirus pneumonia might improve the success rate of treatment and improve patient outcome in 500 mg twice per day for 10 days for patients diagnosed as mild moderate and severe cases of coronavirus pneumonia.43 Surely, further investigation needs to be done with this combination of drugs to prove or reject their relevance with blocking SARS-CoV2's infection process.
Remdesivir
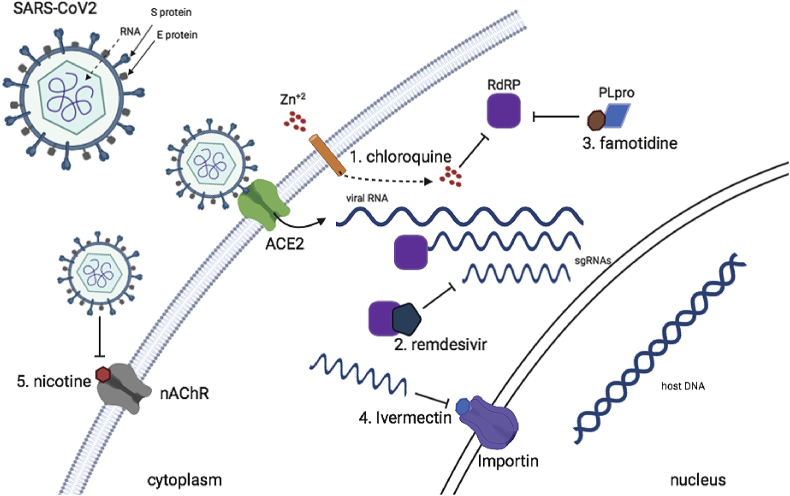
Remdesivir, developed by Gilead Sciences Inc., is an investigational broad-spectrum antiviral treatment. It was previously tested in humans with Ebola virus disease and so far it has shown promise in animal models for treating Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). Remdesivir triphosphate (RDV-TP) is a direct antagonist of Adenosine triphosphate (ATP) and blocks the function of RdRP when it takes its place upon elongation of the positive sense RNA of the virus by RdRP6,44 (Fig. 1).
Figure 1.
The five different drugs mentioned in this review and how they can block vital enzymes of SARS-CoV2 or the transport of its components.
In a letter to editor in Nature journal, M. Wang et al proved for the first time that remdesivir is highly effective in the control of COVID-19 infection in vitro, at a stage post virus entry.45 After that, many clinics start using remdesivir in a compassionate use for COVID-19 patients with severe symptoms, with the general feeling that it is helpful. Although, only with a randomized, unbiased trial, remdesivir could be used in as an official treatment for COVID-19.46, 47, 48, 49, 50
While a randomized trial with remdesivir took place in China with 237 patients who were in ICU for 12 days, found no difference in time to recovery or mortality between the two groups,51, 52, 53 very recently (April 2020) in an American trial with 1063 patients, remdesivir showed the most optimistic results so far for treatment of COVID-19 in a randomized clinical trial where patients with serious case of the illness from SARS-CoV2 treated with remdesivir had a 4–5 days faster relief from symptoms and were able to exit ICU in comparison with patients treated with a placebo. Due to the positive and statistically significant result of this study, all patients in placebo were treated with remdesivir as well after a Data Safety Monitoring Board (DSMB) which showed significantly positive results even before the study had finished. This is the norm for such studies, because it would be unethical for patients on the placebo to not receive a treatment that would help them recover faster and easier (https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19).
Famotidine
Another very interesting compound that could have positive effects on the prevention of the SARS-CoV2 infection is this of Famotidine. This started when dr. Michael Callahan from Massachusetts General Hospital visited China and reviewed about 6212 charts of patients who survived or died by COVID-19. Some of these patients had heartburn, which is one of the most common problems to have in the general population, and some of them were taking a medication called famotidine (known by its trade name as pepcid), while another group of patients were taking a more expensive and newer medication for suppressing acid with totally different structure, omeprazole (known trade name: prilosec or nexium). What was interestingly observed was that those patients that who were taking the cheaper medication (pepcid) had a mortality rate of in only 14%, whereas in the group of patients with omeprazole the mortality was 27%. Despite that, when they looked at the statistical significance of that they found that the p-value was greater than 0.05 which means that they couldn't tell whether or not there was a greater than 95 percent chance of confidence that this was random or not (https://www.sciencemag.org/news/2020/04/new-york-clinical-trial-quietly-tests-heartburn-remedy-against-coronavirus).
In an unrelated study with these observation, famotidine was one of the three mostly fitted protein to be predicted computationally as possible targets ofviral protein of SARS-CoV2, important for its successful infection and replication. Important targets such as 3-chymotrypsin-like protease (3CLpro), Spike, RNA-dependent RNA polymerase (RdRp), and papain like protease (PLpro) were matched structurally with already known compounds, with famotidine having one of the highest scores against PLpro.54 PLpro is responsible for the cleavages of N-terminus of the replicase poly-protein to release Nsp1, Nsp2 and Nsp3, which is essential for correcting virus replication55 and can antagonize the host's innate immunity56, 57, 58 (Fig. 1). As an indispensable enzyme in the process of coronavirus replication and infection of the host, PLpro is a popular target for coronavirus inhibitors and famotidine is structurally shown to be a very plausible candidate.
Taken together with the results from patients taking pepcid as opposed to patients taking isomeprazole for common heartburn, on 14 April the U.S. Biomedical Advanced Research and Development Authority (BARDA) has funded scientists in the United States who have now started a randomized placebo controlled trial for intravenously given famotidine in the effort to a successful treatment for COVID-19 (https://www.sciencemag.org/news/2020/04/new-york-clinical-trial-quietly-tests-heartburn-remedy-against-coronavirus).
Ivermectin
Ivermectin is, like hydroxychloroquine, an FDA approved medication that already serves a purpose for some other infections but might be helpful with COVID-19 as well. Ivermectin is usually used for parasites and it has been investigated in other viruses like West Nile virus and influenza but now several research groups are looking into its use for treatment from the novel coronavirus.59,60 Several viral proteins are believed to be imported in the nucleus of the human cells, upon infection with SARS-CoV2. There's a specific target in the host's nuclear membrane for this transporting which is conveniently called Importin, which comes in versions alpha and beta, both important for the viral infection. What ivermectin does is that it shuts down this ability to transport viral proteins through importins into the nucleus and thus diminishes the ability of the virus to cause harm to the cell (Fig. 1). On April 2020, scientists from Australia showed that Ivermectin is an inhibitor of the virus SARS-CoV-2 in vitro, when a single addition to Vero cells with signaling lymphocytic activation molecule expression (Vero-hSLAM cells) 2 h post infection with SARS-CoV-2 it was able to create a ~5000-fold reduction in viral RNA at 48 h.61
Nicotine
Maybe the most recent small molecule (as of May 2020) to be considered as a potential tool against COVID-19, is nicotine. Two independent groups, one from France and one from Greece,62 have found that except ACE2 which is the main receptor of SARS-Cov2, a secondary receptor for the virus could be the nicotinic acetylcholine receptor (nAChR).63 This notion is firstly possible due to the fact that about a third of the patients experience anosmia and dysgeusia (smell and taste impairment) as an early symptom of COVID-19 which is connected with nAChR mechanisms.64 Secondly and maybe more importantly, even if a high smoking prevalence among patients with COVID-19 would be expected due to smokers’ higher rate of cardiovascular and respiratory defects, a 3 fold less from the expected population of smokers was identified with the disease in a Chinese study62,65. Finally, it has lately been reported that nicotine is a direct mediator of ACE2 receptor.66
Nicotine is a cholinergic agonist and acts as an important inhibitor of pro-inflammatory cytokines acting through the cholinergic anti-inflammatory pathway via α7-nAChRs (image 1). Nicotine inhibits TNF, IL-1, IL-6 and HMGB1, pro-inflammatory cytokines which are known to be elevated among COVID-19 patients,67 creating a Cytokine storm which leads to acute respiratory distress syndrome (ARDS) and multi-organ failure, very usually seen in severe COVID-19 cases68,69. While undoubtedly smoking cannot be a possible solution for COVID-19 prevention, it seems really interesting to further investigate the function of nicotine on restoring the function of the cholinergic anti-inflammatory system in combination to SARS-CoV19 infection and replication mechanism in future studies.
Conclusions
Unfortunately, a clear and confirmed treatment for COVID-19 remains yet to be found. Still, the most successful way to prevent the spread of SARS-CoV2 so far is quarantine for affected individuals and physical distancing measures, while it is of outmost need to generate a drug or a combination of drugs in order to add another tool against this international pandemic, until the development of a vaccine against the novel coronavirus. In figure (1) we show how some of the most promising known drugs for other diseases can block the novel coronavirus' transport or replication into the host cell. Currently, more than 200 studies with different compounds or other potential treatments with various ways are ongoing in the United States and more than 1000 worldwide (source: https://clinicaltrials.gov/ct2/results/map?cond=COVID-19&map = ), but due to the rapid emergence for research many of the efforts lack organizing and randomized clinical trials and remain on the level of anecdotal evidence about their ability to fight COVID-19. Even with researchers working around-the-clock there still seems to be lack of a centralized national strategy, and small-scale trials do not lead to definitive answers for which society is in need now of all times. This seems to be stabilized week to week and collaborations among research groups from all over the world and from different fields of expertise give place to small scale efforts, such as the Remdesivir clinical trial stated above. We hope that with efforts like this, we will be able to produce solid results in the near future where physicians all over the world will put into practice as soon as possible. Except for remdesivir, we believe that more wide range randomized clinical trials must be done for the compounds stated or other drugs that we didn't discuss in this review, in order to have alternative ways to treat patients that inevitably will not be responsive to a certain treatment or this treatment will be prohibited for them due to other conditions that they might have.
Conflict of Interest
The author declares no conflict of interest.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheahan T.P., Sims A.C., Leist S.R. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao J., Hu S. Update on use of chloroquine/hydroxychloroquine to treat coronavirus disease 2019 (COVID-19) Biosci Trends. 2020 doi: 10.5582/bst.2020.03072. [DOI] [PubMed] [Google Scholar]
- 8.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulangu S., Dodd L.E., Davey R.T., Jr. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Group Convalescent Plasma Study The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan K., Liu B., Li C. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S., Yu X., Guo D. Viruses; 2018. CRISPR-Cas Targeting of Host Genes as an Antiviral Strategy; p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knott G.J., East-Seletsky A., Cofsky J.C. Guide-bound structures of an RNA-targeting A-cleaving CRISPR-Cas13a enzyme. Nat Struct Mol Biol. 2017;24:825–833. doi: 10.1038/nsmb.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbott T.R., Dhamdhere G., Liu Y. Cell; 2020. Development of CRISPR as an Antiviral Strategy to Combat SARS-CoV-2 and Influenza. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV-a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sungnak W., Huang N., Becavin C. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020 doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier H.J., Bickerton E., Britton P. Preface. Coronaviruses. Methods Mol Biol. 2015;1282 doi: 10.1007/978-1-4939-2438-7. [DOI] [PubMed] [Google Scholar]
- 20.Keep S.M., Bickerton E., Britton P. Transient dominant selection for the modification and generation of recombinant infectious bronchitis coronaviruses. Methods Mol Biol. 2015;1282:115–133. doi: 10.1007/978-1-4939-2438-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubo H., Yamada Y.K., Taguchi F. Localization of neutralizing epitopes and the receptor-binding site within the amino-terminal 330 amino acids of the murine coronavirus spike protein. J Virol. 1994;68:5403–5410. doi: 10.1128/jvi.68.9.5403-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosch B.J., Martina B.E., Van Der Zee R. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc Natl Acad Sci U S A. 2004;101:8455–8460. doi: 10.1073/pnas.0400576101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci U S A. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baranov P.V., Henderson C.M., Anderson C.B. Programmed ribosomal frameshifting in decoding the SARS-CoV genome. Virology. 2005;332:498–510. doi: 10.1016/j.virol.2004.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brierley I., Digard P., Inglis S.C. Characterization of an efficient coronavirus ribosomal frameshifting signal: requirement for an RNA pseudoknot. Cell. 1989;57:537–547. doi: 10.1016/0092-8674(89)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araki K., Gangappa S., Dillehay D.L. Pathogenic virus-specific T cells cause disease during treatment with the calcineurin inhibitor FK506: implications for transplantation. J Exp Med. 2010;207:2355–2367. doi: 10.1084/jem.20100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J Gen Virol. 2000;81:853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- 28.Mielech A.M., Chen Y., Mesecar A.D., Baker S.C. Nidovirus papain-like proteases: multifunctional enzymes with protease, deubiquitinating and deISGylating activities. Virus Res. 2014;194:184–190. doi: 10.1016/j.virusres.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snijder E.J., Bredenbeek P.J., Dobbe J.C. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J Mol Biol. 2003;331:991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keck J.G., Makino S., Soe L.H., Fleming J.O., Stohlman S.A., Lai M.M. RNA recombination of coronavirus. Adv Exp Med Biol. 1987;218:99–107. doi: 10.1007/978-1-4684-1280-2_11. [DOI] [PubMed] [Google Scholar]
- 31.Keck J.G., Stohlman S.A., Soe L.H., Makino S., Lai M.M. Multiple recombination sites at the 5'-end of murine coronavirus RNA. Virology. 1987;156:331–341. doi: 10.1016/0042-6822(87)90413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai M.M., Baric R.S., Makino S. Recombination between nonsegmented RNA genomes of murine coronaviruses. J Virol. 1985;56:449–456. doi: 10.1128/jvi.56.2.449-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krijnse-Locker J., Ericsson M., Rottier P.J., Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J Cell Biol. 1994;124:55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac- cells: determination of the first site of budding of progeny virions. Eur J Cell Biol. 1984;33:281–293. [PubMed] [Google Scholar]
- 35.de Haan C.A., Rottier P.J. Molecular interactions in the assembly of coronaviruses. Adv Virus Res. 2005;64:165–230. doi: 10.1016/S0065-3527(05)64006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye Y., Hogue B.G. Role of the coronavirus E viroporin protein transmembrane domain in virus assembly. J Virol. 2007;81:3597–3607. doi: 10.1128/JVI.01472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beniac D.R., Andonov A., Grudeski E., Booth T.F. Architecture of the SARS coronavirus prefusion spike. Nat Struct Mol Biol. 2006;13:751–752. doi: 10.1038/nsmb1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delmas B., Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J Virol. 1990;64:5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins A.R., Knobler R.L., Powell H., Buchmeier M.J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell-cell fusion. Virology. 1982;119:358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.te Velthuis A.J., van den Worm S.H., Sims A.C. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue J., Moyer A., Peng B., Wu J., Hannafon B.N., Ding W.Q. Chloroquine is a zinc ionophore. PloS One. 2014;9 doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mejia Torres R.E., Banegas E.I., Mendoza M. Efficacy of chloroquine for the treatment of uncomplicated Plasmodium falciparum malaria in Honduras. Am J Trop Med Hyg. 2013;88:850–854. doi: 10.4269/ajtmh.12-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Multicenter collaboration group of Department of, Science, Province Technology of Guangdong, and pneumonia Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia. Zhonghua Jiehe He Huxi Zazhi. 2020;43:185–188. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9:100128. doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bastola A., Sah R., Rodriguez-Morales A.J. The first 2019 novel coronavirus case in Nepal'. Lancet Infect Dis. 2020;20:279–280. doi: 10.1016/S1473-3099(20)30067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai J.H., Wang X.S., Ge Y.L. First case of 2019 novel coronavirus infection in children in Shanghai. Zhonghua Er Ke Za Zhi. 2020;58:86–87. doi: 10.3760/cma.j.issn.0578-1310.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Hao W., Li M., Huang X. First Atypical case of 2019 novel coronavirus in Yan'an, China. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao Y.C., Deng Q.X., Dai S.X. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Trav Med Infect Dis. 2020:101647. doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ledford H. Hopes rise for coronavirus drug remdesivir. Nature. 2020 Apr 29 doi: 10.1038/d41586-020-01295-8. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 53.Ko W.C., Rolain J.M., Lee N.Y. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020;55:105933. doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C., Liu Y., Yang Y. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harcourt B.H., Jukneliene D., Kanjanahaluethai A. Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain-like protease activity. J Virol. 2004;78:13600–13612. doi: 10.1128/JVI.78.24.13600-13612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X., Yang X., Zheng Y. SARS coronavirus papain-like protease inhibits the type I interferon signaling pathway through interaction with the STING-TRAF3-TBK1 complex. Protein Cell. 2014;5:369–381. doi: 10.1007/s13238-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baez-Santos Y.M., St John S.E., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antivir Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matthews K., Schafer A., Pham A., Frieman M. The SARS coronavirus papain like protease can inhibit IRF3 at a post activation step that requires deubiquitination activity. Virol J. 2014;11:209. doi: 10.1186/s12985-014-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mercorelli B., Palu G., Loregian A. Drug repurposing for viral infectious diseases: how far are we? Trends Microbiol. 2018;26:865–876. doi: 10.1016/j.tim.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azeem S., Ashraf M., Rasheed M.A., Anjum A.A., Hameed R. Evaluation of cytotoxicity and antiviral activity of ivermectin against Newcastle disease virus. Pak J Pharm Sci. 2015;28:597–602. [PubMed] [Google Scholar]
- 61.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farsalinos K., Niaura R., Le Houezec J. Editorial: nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol Rep. 2020 doi: 10.1016/j.toxrep.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bencherif M., Lippiello P.M., Lucas R., Marrero M.B. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci. 2011;68:931–949. doi: 10.1007/s00018-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giacomelli A., Pezzati L., Conti F. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 66.Leung J.M., Yang C.X., Sin D.D. COVID-19 and nicotine as a mediator of ACE-2. Eur Respir J. 2020 doi: 10.1183/13993003.01261-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 69.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]