Introduction
MET gene amplification is an established mechanism of acquired resistance to all classes of EGFR tyrosine kinase inhibitors (TKIs) in EGFR-mutated lung cancer. Novel strategies to target EGFR-mutated MET-amplified tumors in clinical trials have reported promising results, with a clear activity of the combination of osimertinib and savolitinib reported from the TATTON trial (NCT02143466) in 2020.1 Savolitinib is a type IB–selective MET kinase inhibitor in the same class as capmatinib and tepotinib (Table 1).2,3 There is a paucity of data on the mechanisms of acquired resistance to combination therapy with osimertinib and savolitinib. Here, we report a case of EGFR-mutated and MET-amplified lung adenocarcinoma that developed resistance to a combination therapy of osimertinib and savolitinib owing to MET-dependent mechanisms. The clinical, genomic, radiographic, and retrospective outcome data used for this case report have been compiled under an ongoing institutional review board–approved protocol at our institution with an appropriate waiver of consent.
Table 1.
MET TKI Activity Against MET Wild-Type and Activating Loop Mutations
| Type | Name | MET Wild-Type | MET-D1228H | MET-D1228N | MET-D1228V | MET-Y1230C | MET-D1230H |
|---|---|---|---|---|---|---|---|
| Ia | Crizotinib | Sensitive | Resistant | Resistant | Resistant | Resistant | Resistant |
| Ib | Savolitinib | Sensitive | Resistant | Resistant | Resistant | Resistant | Resistant |
| Ib | Capmatinib | Sensitive | Resistant | Resistant | — | Resistant | Resistant |
| II | Cabozantinib | Sensitive | — | Sensitive | Sensitive | — | Sensitive |
| II | Glesatinib | Sensitive | Sensitive | Sensitive | — | Sensitive | Sensitive |
Note: References numbers 2 to 6 were used to generate the modified heat map. Green indicates sensitivity whereas red indicates resistance, with the intensity of color indicating higher or lower preclinical inhibitory patterns. —, indicates data not available.
TKI, tyrosine kinase inhibitors.
Case Presentation
A 61-year-old Asian man with a previous 5-pack-year history of smoking initially presented with early stage node-positive lung adenocarcinoma. After initial management with lobectomy and adjuvant platinum doublet chemotherapy, within 1 year he developed widespread metastatic recurrence harboring EGFR delL747_T751 mutation. He was treated with oral erlotinib daily for 14 months before developing disease progression in his liver. Targeted biopsy revealed metastatic lung adenocarcinoma with EGFR-delL747_T751 (exon 19 deletion) and MET amplification (FoundationOne, Foundation Medicine). The MET:centrome_probe_SE7 ratio was 7.73 (cMET FISH Kreatech assay, Integrated Genetics, LabCorp), confirming high-level amplification.
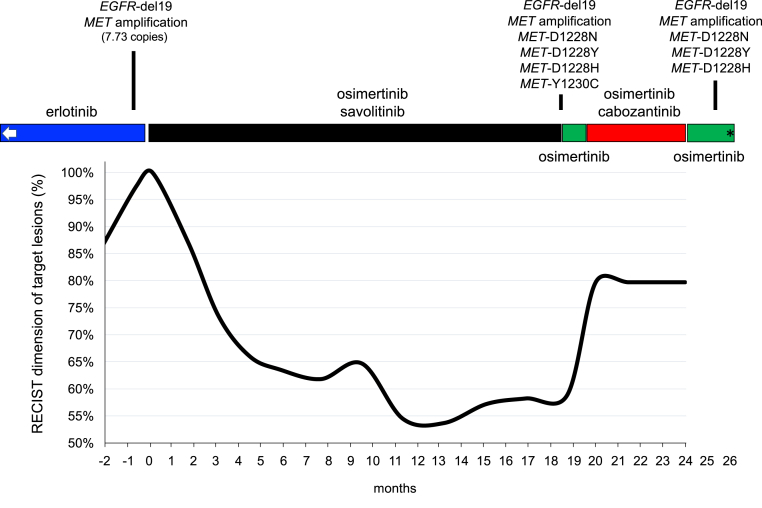
Suspecting MET amplification as the mechanism of resistance to the EGFR TKI, he was transitioned to oral osimertinib 80 mg daily in combination with oral savolitinib 300 mg daily under the clinical trial TATTON;1 he achieved partial response for 18 months before developing progressive disease (Fig. 1). Tissue biopsy confirmed adenocarcinoma, and next-generation sequencing of circulating tumor DNA (FoundationOne Liquid, Foundation Medicine) revealed EGFR-delL747_T751 mutation (allele frequency [AF] 3.3%), lack of EGFR-T790M and EGFR-C797S, MET amplification, and MET mutations (D1228H AF 15.6%, D1228N AF 1.8%, D1228Y AF 1.6%, and Y1230C AF 0.98%). Off-label oral cabozantinib 60 mg daily was added to oral osimertinib 80 mg daily in an effort to target these MET mutations (Table 1). Although he achieved stable disease (Fig. 1), he developed considerable fatigue, gastrointestinal toxicity, and cytopenias on dual-inhibitor therapy, thus requiring cessation of cabozantinib. A repeat liquid biopsy revealed the original truncal EGFR mutation, MET amplification, and the known MET-D1228X mutations (Fig. 1). He was continued on osimertinib but died after 3 months.
Figure 1.
The clinical, radiographic, and genomic course of EGFR-mutant NSCLC with MET-mediated resistance before, during, and after osimertinib plus savolitinib therapy. Graphical representation of RECIST target lesion measurements in percentage (baseline set at 100% at the time before the commencement of osimertinib plus savolitinib) over the course of therapy. Noted are different types of kinase inhibitors targeting EGFR (erlotinib and osimertinib) or targeting MET (savolitinib and cabozantinib). The results of tissue-based and liquid biopsy–based comprehensive genomic profiling results are highlighted in accordance with the course of therapy. The tissue-based comprehensive genomic profiling test (FoundationOne) at the time of erlotinib resistance also revealed additional genomic aberrations, including TP53 N239I, FGF14 amplification, and IRS2 amplification. The liquid biopsy–based (FoundationOne Liquid) tests after osimertinib and savolitinib also identified the TP53 N239I mutation. EGFR-del19 indicates EGFR exon 19 deletion (delL747_T751). The left white arrow indicates previous periods of erlotinib use; ∗ indicates the time of death. A note is made that this occurred during the COVID-19 pandemic in Massachusetts and no autopsy or SARS-CoV-2 testing was performed. COVID-19, coronavirus disease 2019; RECIST, Response Evaluation Criteria in Solid Tumor; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.
Discussion
MET amplification is a frequently observed (5%–20% of cases) mechanism of resistance to EGFR TKIs. The EGFR plus MET coinhibition strategy of osimertinib plus savolitinib is undergoing final regulatory approval for EGFR-mutated plus MET-amplified advanced lung cancer in the SAVANNAH trial (NCT03778229). Resistance to combined EGFR and MET inhibition is poorly understood but can be expected to occur should this strategy gain wide appeal. Our report highlights MET-dependent resistance mechanisms (rather than EGFR-dependent mechanisms) mediated by MET kinase mutations in a pattern similar to the one seen in a previous case report.4 Single-agent savolitinib has been associated with MET-D1228X and Y1230X resistance mutations in other MET-amplified tumor types.5 Cabozantinib is a multikinase type II MET kinase inhibitor with putative preclinical activity against type I MET inhibitor resistance mutations, including MET-D1228X and Y1230X activation loop mutations (Table 1)4,6 and offers a potential treatment strategy as seen in a previous case treated with oral erlotinib 100 mg daily plus oral cabozantinib 60 mg daily4 but has significant toxicity that may limit widespread combinatory use as seen in our case report. Understanding the true frequency of MET mutations as a mechanism of resistance to osimertinib plus savolitinib will help define the need to develop novel MET inhibitors with broader activity against MET mutations and additional therapies to delay or prevent the heterogeneity of on-target and off-target resistance to EGFR TKIs.
Acknowledgments
This work was funded in part through the National Institutes of Health/ National Cancer Institute grants R37 CA218707 (Dr. Costa), R01 CA169259 (Dr. Kobayashi), R01 CA240257 (Dr. Kobayashi) plus Department of Defense LC170223 (Dr. Kobayashi).
Footnotes
Drs. Piper-Vallillo and Halbert contributed equally to this work.
Disclosure: Dr. Costa reports receiving personal fees (consulting fees and honoraria) and nonfinancial support (institutional research support) from Takeda/Millennium Pharmaceuticals, AstraZeneca, and Pfizer; and nonfinancial support (institutional research support) from Merck Sharp & Dohme Corporation, Merrimack Pharmaceuticals, Bristol-Myers Squibb, Clovis Oncology, Spectrum Pharmaceuticals, and Tesaro outside of the submitted work. Dr. Rangachari reports receiving nonfinancial support (institutional research support) from Bristol-Myers Squibb, Novocure, and AbbVie/Stemcentrx, all outside of the submitted work. Dr. Kobayashi reports receiving research support from Boehringer Ingelheim, MiNA Therapeutics, and Taiho Therapeutics; and personal fees (honoraria) from Boehringer Ingelheim, Bristol-Myers Squibb, and Takeda Pharmaceuticals outside of the submitted work. The remaining authors declare no conflict of interest.
References
- 1.Sequist L.V., Han J.Y., Ahn M.J. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020;21:373–386. doi: 10.1016/S1470-2045(19)30785-5. [DOI] [PubMed] [Google Scholar]
- 2.Engstrom L.D., Aranda R., Lee M. Glesatinib exhibits antitumor activity in lung cancer models and patients harboring MET exon 14 mutations and overcomes mutation-mediated resistance to type I MET inhibitors in nonclinical models. Clin Cancer Res. 2017;23:6661–6672. doi: 10.1158/1078-0432.CCR-17-1192. [DOI] [PubMed] [Google Scholar]
- 3.Collie G.W., Koh C.M., O’Neill D.J. Structural and molecular insight into resistance mechanisms of first generation cMET inhibitors. ACS Med Chem Lett. 2019;10:1322–1327. doi: 10.1021/acsmedchemlett.9b00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahcall M., Sim T., Paweletz C.P. Acquired METD1228V mutation and resistance to MET inhibition in lung cancer. Cancer Discov. 2016;6:1334–1341. doi: 10.1158/2159-8290.CD-16-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frigault M.M., Markovets A., Nuttall B. Mechanisms of acquired resistance to Savolitinib, a selective MET inhibitor in MET-amplified gastric cancer. JCO Precis Oncol. 2020;4:222–232. doi: 10.1200/PO.19.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li A., Yang J.J., Zhang X.C. Acquired MET Y1248H and D1246N mutations mediate resistance to MET inhibitors in non-small cell lung cancer. Clin Cancer Res. 2017;23:4929–4937. doi: 10.1158/1078-0432.CCR-16-3273. [DOI] [PubMed] [Google Scholar]