Abstract
Background
Tocilizumab was approved for chimeric antigen receptor T–cell therapy induced cytokine release syndrome and it may provide clinical benefit for selected COVID–19 patients.
Methods
In this retrospective cohort study, we analyzed hypoxic COVID–19 patients who were consecutively admitted between March 13, 2020 and April 19, 2020. Patients with lung infiltrates and elevated inflammatory markers received a single dose of tocilizumab if no contraindication was present. Systemic steroid, hydroxychloroquine, and azithromycin were concomitantly used for majority of the patients.
Findings
Of the 51 patients included for analysis, 28 (55%) received tocilizumab and 23 (45%) did not receive tocilizumab. Tocilizumab cohort required more invasive ventilation (68% vs. 22%) at baseline and during entire hospitalization (75% vs. 48%). The median time to clinical improvement in tocilizumab vs. no tocilizumab cohorts was 8 days (Interquartile range [IQR]: 6·25 – 9·75 days) vs. 13 days (IQR: 9·75 – 15·25 days) among patients who required mechanical ventilation at any time (Hazard ratio for clinical improvement: 1·83, 95% confidence interval [CI]: 0·57 – 5·84) and 6·5 days vs. 7 days among all patients (Hazard ratio for clinical improvement: 1·14, 95% CI: 0·55 – 2·38), respectively. The median duration of vasopressor support and invasive mechanical ventilation were 2 days (IQR: 1·75 – 4·25 days) vs. 5 days (IQR: 4 – 8 days), p = 0.039, and 7 days (IQR: 4 – 14 days) vs. 10 days (IQR: 5 – 15 days) in tocilizumab vs. no tocilizumab cohorts, p = 0.11, respectively. Similar rates of hospital–acquired infections occurred in both cohorts (18% in tocilizumab and 22% in no tocilizumab cohort).
Interpretation
In patients with severe COVID-19, tocilizumab was associated with significantly shorter duration of vasopressor support. Although not statistically significant, tocilizumab also resulted in shorter median time to clinical improvement and shorter duration of invasive ventilation. These findings require validation from ongoing clinical trials of Tocilizumab in COVID–19 patients.
Keywords: Coronavirus, SARS–CoV–2, Interleukin 6, Tocilizumab, Cytokine release syndrome
Research in context.
Evidence before this study
There has been increasing evidence suggesting that extensive release of pro–inflammatory cytokines may contribute to poor outcomes of COVID–19 patients. We searched PubMed for previous studies published until May 22, 2020 and reported outcomes of COVID–19 patients treated with tocilizumab using the keywords “COVID–19″, “SARS–CoV–2″ AND “tocilizumab”. We found a few small retrospective studies compared tocilizumab with a control cohort and reported conflicting survival outcomes. These studies did not report other clinically important outcome measures.
Added value of this study
In this retrospective cohort study, treatment of severe COVID–19 patients with a single intravenous infusion of low–dose tocilizumab (8 mg/kg up to 400 mg) resulted in favorable outcomes such as shorter time to clinical improvement, shorter duration of invasive ventilation, and shorter duration of vasopressor support compared to a control group. These findings require cautious interpretation because a statistically significant difference was seen only for the duration of vasopressor support.
Implications of all available evidence
The use of tocilizumab in severe COVID–19 may shorten the duration of critical resource use and facilitate institutions across the world to allocate intensive care unit staff and resources in COVID–19 outbreak.
Alt-text: Unlabelled box
1. Introduction
Coronavirus disease 2019 (COVID–19), caused by severe acute respiratory syndrome coronavirus 2 (SARS–CoV–2), was initially detected in China and has been declared a global pandemic by World Health Organization on March 11, 2020 [1]. The clinical spectrum of this illness ranges from asymptomatic infection to acute respiratory distress syndrome (ARDS), circulatory shock, multiorgan failure, and ultimately death [2,3]. Massive demand for invasive ventilation and intensive care unit beds in a short period of time overwhelmed the healthcare systems around the world [4,5]. Currently, the standard of care is supportive therapy and there is an urgent need for effective treatment against COVID–19.
ARDS is the leading cause of mortality in COVID–19 patients and extensive release of pro–inflammatory cytokines is suspected to contribute to poor outcomes in some patients [6,7]. Overactivation of immune system, like in secondary hemophagocytic lymphohistiocytosis (sHLH), can be triggered by viral infections [8,9]. Similarities between clinical presentation of sHLH and severe COVID–19 such as multiorgan involvement, cytopenia, coagulopathy, high levels of ferritin and transaminases were previously noted [8,10]. Elevated pro–inflammatory cytokines and inflammatory biomarkers such as interleukin 6, C–reactive protein (CRP), and ferritin have been shown to be higher in patients with severe COVID–19 and predictors of mortality [11,12]. In one study, the level of interleukin 6 was noted to be 10 times higher in critically ill COVID–19 patients who required mechanical ventilation or vasopressor support compared to those with milder disease and interleukin 6 level correlated with the detection of SARS–CoV–2 RNAaemia [13]. These findings suggest that anti–cytokine targeted therapies might be of benefit for patients with severe COVID–19. Tocilizumab, a monoclonal antibody against interleukin 6 receptor, was previously approved by Food and Drug Administration for the treatment of cytokine release syndrome [14] and may provide clinical benefit for selected COVID–19 patients with high inflammatory biomarkers. We hereby report our experience with using tocilizumab in hospitalized patients with severe COVID–19.
2. Methods
2.1. Study design and patients
In this cohort study, we retrospectively reviewed all patients who were diagnosed with COVID–19 by reverse–transcriptase–polymerase–chain–reaction assay (RT–PCR) and consecutively admitted to Cleveland Clinic Fairview Hospital between March 13, 2020 and April 19, 2020. This study timeframe was not predetermined and represents the time between first COVID–19 admission in our hospital and initiation of data collection. Fairview Hospital has 50 intensive care unit beds and has been designated as a referral center among Cleveland Clinic facilities for COVID–19 admissions in the west side of Cleveland. Our inclusion criteria were patients aged more than 18 years and diagnosed with severe COVID–19, defined as requiring supplemental oxygen for saturation of less than 94% while on ambient air. We have excluded patients who did not require supplemental oxygen during hospitalization. This study was conducted according to the Declaration of Helsinki and approved by the institutional review board of Cleveland Clinic.
2.2. Treatment for COVID–19
On admission, all COVID–19 patients were started on Hydroxychloroquine with a loading dose of 400 mg twice daily followed by 200 mg per day twice daily for additional five days and Azithromycin 500 mg per day for five days, unless contraindicated. An electrocardiogram was obtained for each patient to check corrected QT interval at baseline. Tocilizumab was given to COVID–19 patients in the presence of hypoxia, lung infiltrates on chest radiography, elevated inflammatory biomarkers (either CRP >3 g/dl or ferritin >400 ng/ml), concern for clinical deterioration, and if none of the following contraindications were present; confirmed or suspected bacterial or fungal infections, platelet count <100,000/mm3, neutrophil count <2000/mm3, and alanine aminotransferase (ALT) or aspartate aminotransferase (AST) more than five times the upper limit of the normal range (50 U/L for ALT and 40 U/L for AST). Interleukin 6 level was not part of the tocilizumab criteria because it did not necessarily result in an actionable time frame. Consultation of an infectious disease specialist was required to ensure patients were eligible to receive tocilizumab based on these criteria. The predetermined dose of tocilizumab was 4 to 8 mg/kg with a maximum dose of 400 mg [15,16]. All patients in our cohort were dosed based on 8 mg/kg and received 400 mg tocilizumab as a 60 min single intravenous infusion. Patients were not screened for latent tuberculosis or hepatitis viruses prior to tocilizumab infusion. There is no standard protocol in place for use of corticosteroid in patients with COVID–19 at our hospital.
2.3. Study variables and assessments
We have retrospectively collected data on patients’ demographics, comorbidities, symptoms, oxygen–support category, ratio of partial pressure of arterial oxygen over fraction of inspired oxygen (PaO2/FiO2 ratio), laboratory values, diagnostic workup, therapies, complications, and outcomes. Oxygen–support categories, defined as an ordinal scale of 1 (ambient air), 2 (low–flow oxygen), 3 (high–flow oxygen), and 4 (invasive mechanical ventilation), were collected daily since admission for each patient. Change in oxygen requirement was considered improved if the ordinal scale of oxygen–support category was decreased at last follow–up or remained same for patients discharged from the hospital. Clinical improvement was defined as live discharge from the hospital without worsening of oxygen–support category compared to initial admission or at least 2 points decrease in the six–point scale as follows; 1) not hospitalized, 2) hospitalized but not requiring supplemental oxygen, 3) hospitalized and requiring supplemental oxygen, 4) hospitalized and requiring nasal high–flow oxygen therapy, noninvasive mechanical ventilation, or both, 5) hospitalized and requiring invasive mechanical ventilation, extracorporeal membrane oxygenation, or both, and 6) death. These criteria were used in prior COVID–19 clinical trials [[17], [18], [19]] but we simplified the original eight–point scale into a six–point scale by combining two non–hospitalized categories into one category and by combining two non–hypoxic categories into one category [18]. Main outcomes of interest were time to clinical improvement, duration of invasive ventilation, and duration of circulatory shock requiring vasopressor support in tocilizumab vs. no tocilizumab cohorts. We have regarded hospital–acquired infectious complications and trend of AST and ALT for both cohorts as safety measures.
2.4. Statistical analysis
No sample size calculations were performed. Descriptive analysis was performed to compare baseline characteristics of patients in tocilizumab and no tocilizumab cohorts. Continuous variables were reported as median with interquartile range (IQR) and medians of two groups were compared using Wilcoxon-Mann-Whitney test. Categorical variables were compared using chi–square or Fisher's exact test. We have used Kaplan–Meier analysis and log–rank test to compare cumulative incidence of clinical improvement among patients in tocilizumab and no tocilizumab cohorts. Cox proportional hazards analysis was performed to test for association between baseline characteristics and clinical improvement. This analysis did not include a provision for correcting for multiple comparisons in tests for association between baseline variables and outcomes and thus, results are reported as point estimates and 95% confidence intervals. The confidence intervals should not be used to infer definitive associations with outcomes because the widths of confidence intervals have not been adjusted for multiple comparisons. All reported p values are two–sided and a p value of less than 0·05 was considered statistically significant. All statistical calculations were made using R statistical software version 3·4·0 (R Foundation for Statistical Computing, Vienna, Austria).
2.5. Role of funding
This study was not funded by any source. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Baseline characteristics of the patients
We have identified 58 COVID–19 patients, seven of them were excluded due to absence of hypoxia during their hospitalization and remaining 51 were included for analysis. Twenty–eight (55%) patients received a single dose of tocilizumab after a median of 2 days (IQR: 1 – 3) from the day of hospitalization and 23 (45%) patients did not receive tocilizumab. Table 1 summarize the baseline characteristics of the patients. Median age and number of comorbidities were 65 years (IQR: 53 – 74) and 2 (IQR: 1 – 3), respectively. Most common symptoms at presentation were shortness of breath (88%), chills (73%), and cough (65%). Objective fever was documented in 31 (61%) patients within 24 h of presentation. Individual patient data is available for demographics and comorbidities in table S1 and for vitals and symptoms on presentation in table S2.
Table 1.
Baseline characteristics of the patients.
| Characteristic | All patients(N = 51) | Tocilizumab(N = 28) | No Tocilizumab(N = 23) |
|---|---|---|---|
| Median age (IQR) – year | 65 (53 – 74) | 62 (53 – 71) | 70 (55 – 75) |
| Age category– no. (%) | 18 (35) | 8 (29) | 10 (43) |
| <50 year | 8 (16) | 4 (14) | 4 (17) |
| 50 to 70 year | 25 (49) | 16 (57) | 9 (39) |
| >70 year | 18 (35) | 8 (29) | 10 (43) |
| Male sex – no. (%) | 31 (61) | 20 (71) | 11 (48) |
| European American ethnicity – no. (%) | 36 (71) | 22 (79) | 14 (61) |
| Total number of comorbidities – median (IQR) | 2 (1 – 3) | 2 (1·75 – 3) | 2 (1 – 3) |
| Comorbid conditions – no. (%) | |||
| Hypertension | 36 (71) | 19 (68) | 17 (74) |
| Former tobacco smoker | 21 (41) | 14 (50) | 7 (30) |
| Diabetes mellitus | 19 (37) | 11 (39) | 8 (35) |
| Coronary artery disease | 9 (18) | 2 (7) | 7 (30) |
| Asthma | 9 (18) | 5 (18) | 4 (17) |
| Obstructive sleep apnea | 9 (18) | 6 (21) | 3 (13) |
| Chronic kidney disease | 8 (16) | 5 (18) | 3 (13) |
| Chronic obstructive pulmonary disease | 6 (12) | 3 (11) | 3 (13) |
| Rheumatoid arthritis | 2 (4) | 2 (7) | 0 (0) |
| Systemic lupus erythematosus | 1 (2) | 1 (4) | 0 (0) |
| Ulcerative colitis | 1 (2) | 0 (0) | 1 (4) |
| Kidney transplant recipient | 1 (2) | 1 (4) | 0 (0) |
| Lung transplant recipient | 1 (2) | 1 (4) | 0 (0) |
| Symptoms at presentation – no. (%) | |||
| Shortness of breath | 45 (88) | 27 (96) | 18 (78) |
| Cough | 35 (65) | 21 (75) | 14 (61) |
| Fatigue | 21 (41) | 11 (39) | 10 (43) |
| Diarrhea | 11 (22) | 6 (21) | 5 (22) |
| Nasal congestion | 8 (16) | 4 (14) | 4 (17) |
| Nausea / vomiting | 7 (14) | 2 (7) | 5 (22) |
| Sputum production | 4 (8) | 2 (7) | 2 (9) |
| Sore throat | 3 (6) | 3 (11) | 0 (0) |
| Vitals on admission – no. (%) | |||
| Highest temperature within 24 h >100·4°F or 38 °C | 31 (61) | 19 (68) | 12 (52) |
| Heart rate >100 beats per min | 14 (27) | 4 (14) | 10 (43) |
| Respiratory rate ≥20 breaths per min | 35 (69) | 22 (79) | 13 (57) |
| Highest oxygen–support category – no. (%) | |||
| Invasive mechanical ventilation | 32 (63) | 21 (75) | 11 (48) |
| High–flow oxygen | 2 (4) | 1 (4) | 1 (4) |
| Low–flow oxygen | 17 (33) | 6 (21) | 11 (48) |
| Ambient air | 0 (0) | 0 (0) | 0 (0) |
| Highest level of care – no. (%) | |||
| Medical intensive care unit | 40 (78) | 24 (86) | 16 (70) |
| Regular nursing floor | 11 (22) | 4 (14) | 7 (30) |
In the control cohort, 17 (74%) patients had at least one predetermined contraindication for tocilizumab. The reasons of withholding tocilizumab for each patient were summarized in table S3. Although patient demographics did not influence the decision of giving tocilizumab, patients in tocilizumab cohort were younger (median age: 62 vs. 70 years, p = 0·32), more likely to be male (71% vs. 48%, p = 0·09), European American (79% vs. 61%, 0·17), and former tobacco smoker (50% vs. 30%, p = 0·16) compared to control cohort. Median body mass index was 30 (IQR: 26 – 36) and 31 (IQR: 27 – 37) in tocilizumab and no tocilizumab cohorts, respectively (p = 0·54). Tocilizumab cohort also had higher prevalence of obstructive sleep apnea (21% vs. 13%, p = 0·43) and rheumatologic conditions (11% vs. 0%, p = 0·49). Prevalence of hypertension (74% vs. 68%, p = 0·76) and coronary artery disease (30% vs. 7%, p = 0·06) were higher in patients who did not receive tocilizumab.
Notably, 2 (8%) patients with solid organ transplantation received tocilizumab. Patient–8 received a kidney transplant 5 years before the presentation and was on chronic immunosuppression with tacrolimus 1 mg twice daily, mycophenolate mofetil 250 mg twice daily, and prednisone 5 mg once daily. On admission, tacrolimus and mycophenolate mofetil were held and she was started on hydrocortisone 50 mg every six hours. Tacrolimus was resumed on day–13 of admission at home dose after serum tacrolimus level dropped below the goal range (5 – 7). Steroid was tapered down to her home regimen by day–14 of admission, and mycophenolate mofetil was restarted with home dose at discharge. Patient–14 received left lung transplant six years prior to presentation and was on chronic immunosuppression with tacrolimus 2·5 mg twice daily and prednisone 5 mg once daily. On admission, steroid was resumed at home dose. Tacrolimus was held due to high serum level and remained held for three days after the tocilizumab infusion. Three days after discontinuation, tacrolimus was resumed at lower dose of 1 mg twice daily and patient was discharged on this dose. Drug interactions between tocilizumab and tacrolimus could not be assessed because they were not given concomitantly.
3.2. Laboratory, radiologic and microbiologic findings
On admission, patients in tocilizumab cohort had lower median lymphocyte count (720/mm3 vs. 860/mm3, p = 0·57), lower median interleukin 6 (14 vs. 35 pg/mL, p = 0·53), higher median CRP (16·1 vs. 5·1 mg/dL, p = 0·003), higher median ferritin (1451 vs. 334 ng/mL, p = 0·09), and higher serum creatinine (1·27 vs. 1·05 mg/dL, p = 0·1) levels compared to no tocilizumab cohort. Both cohorts had similar levels of AST, ALT, and total bilirubin on presentation (Table 2). Laboratory findings of each patient are shown in table S4. We have observed a downward trend in CRP and ferritin and an upward trend in lymphocyte count after administration of tocilizumab, while these values fluctuated in no tocilizumab cohort (Figure S1).
Table 2.
Diagnostic work–up, treatment, and outcomes of patients.
| Characteristic | All patients(N = 51) | Tocilizumab(N = 28) | No Tocilizumab(N = 23) |
|---|---|---|---|
| Laboratory values on admission | |||
| White–cell count, median (IQR) – per mm3 | 6750 (5365 – 9710) | 7180 (5520 – 9142) | 6740 (4285 – 9710) |
| Lymphocyte count, median (IQR) – per mm3 | 790 (550 – 1230) | 720 (452 – 1175) | 860 (705 – 1265) |
| <1500/mm3 – positive/total no. (%) | 41/49 (84) | 22/26 (85) | 19/23 (83) |
| Platelet count, median (IQR) – per 109/liter | 230 (174 – 287) | 242 (180 – 291) | 201 (168 – 262) |
| Serum creatinine, median (IQR) – mg/dL | 1·19 (0·83 – 1·77) | 1·27 (0·95 – 2·17) | 1·05 (0·82 – 1·62) |
| Interleukin–6 level, median (IQR) – pg/mL * | 25 (11 – 56) | 14 (8 – 59) | 35 (16 – 55) |
| C–reactive protein, median (IQR) – mg/dL † | 14·4 (8·7 – 23·5) | 16·1 (12·5 – 25·1) | 5·1 (1·4 – 17·7) |
| Ferritin, median (IQR) – ng/mL ‡ | 873 (336 – 1644) | 1451 (845 – 2256) | 334 (147 – 708) |
| Fibrinogen, median (IQR) – mg/dL § | 621 (476 – 717) | 686 (585 – 767) | 490 (393 – 640) |
| Aspartate aminotransferase, median (IQR) – U/L | 37 (29 – 70) | 41 (30 – 62) | 34 (26 – 71) |
| Alanine aminotransferase, median (IQR) – U/L | 31 (17 – 44) | 36 (20 – 51) | 21 (14 – 37) |
| Total bilirubin, median (IQR) – mg/dL | 0·6 (0·35 – 1) | 0·7 (0·4 – 0·9) | 0·6 (0·3 – 1·1) |
| Highest troponin >0·06 ng/mL – positive/total no. (%) | 6/43 (14) | 3/23 (13) | 3/20 (15) |
| Infection analyses on admission – positive/total no. (%) | |||
| Blood cultures ‖ | 1/45 (2) | 0/25 (0) | 1/20 (5) |
| Influenza A or B | 0/41 (0) | 0/23 (0) | 0/18 (0) |
| Respiratory syncytial virus | 1/41 (2) | 1/23 (4) | 0/18 (0) |
| Chest radiography findings on admission – no. (%) | |||
| Bilateral infiltrates | 49 (96) | 28 (100) | 21 (91) |
| Clear | 2 (4) | 0 (0) | 2 (9) |
| Therapies – no. | |||
| Tocilizumab | 28 (55) | 28 (100) | 0 (0) |
| Systemic steroid ¶ | 31 (61) | 20 (71) | 11 (48) |
| Hydroxychloroquine and azithromycin ** | 48 (94) | 26 (93) | 22 (96) |
| Complications during hospitalization – no. (%) | |||
| Hospital–acquired infectious complications | 10 (20) | 5 (18) | 5 (22) |
| Circulatory shock requiring vasopressor support | 25 (49) | 16 (57) | 9 (39) |
| Acute kidney injury without need of renal replacement therapy | 19 (37) | 8 (29) | 11 (48) |
| Acute kidney injury requiring renal replacement therapy | 9 (18) | 7 (25) | 2 (9) |
| Venous thromboembolism | 5 (10) | 3 (11) | 2 (9) |
| Outcomes | |||
| Median hospital length of stay, median (IQR) – day | 10 (5·5 – 17) | 11 (6 – 22·25) | 7 (5 – 13·5) |
| Median intensive care unit length of stay, median (IQR) – day | 8·5 (4 – 16) | 8·5 (6·75 – 17) | 8·5 (3·75 – 12) |
| Median duration of vasopressor support, median (IQR) – day | 3 (2 – 5) | 2 (1·75 – 4·25) | 5 (4 – 8) |
| Median duration of invasive ventilation, median (IQR) – day | 8·5 (4·75 – 15) | 7 (4 – 14) | 10 (5 – 15) |
| Improvement in oxygen–support category – no. (%) | 31 (61) | 18 (64) | 13 (57) |
| Discharged from hospital – no. (%) | 24 (47) | 11 (39) | 13 (57) |
| Died in hospital – no. (%) | 5 (10) | 3 (11) | 2 (9) |
*Interleukin–6 level was missing for 2 and 12 patients in tocilizumab and no tocilizumab cohorts, respectively.
†C–reactive protein level was missing for 2 patients in no tocilizumab cohort.
‡Ferritin level was missing for 3 patients in no tocilizumab cohort.
§Fibrinogen level was missing for 6 and 8 patients in tocilizumab and no tocilizumab cohorts, respectively.
‖Cultures from two separate peripheral blood samples showed methicillin–susceptible Staphylococcus aureus.
¶Among patients who required vasopressor and/or mechanical ventilation support, systemic steroid was given to 17 of 21 (81%) patients in tocilizumab cohort and 9 of 11 (82%) patients in no tocilizumab cohort.
**Hydroxychloroquine and azithromycin were not given to 3 patients due to prolonged corrected QT interval.
All patients underwent a chest radiograph on admission, 49 (96%) had bilateral pulmonary opacities and two (9%) patients in the no tocilizumab cohort did not have any pathological finding at initial presentation. A computed tomographic scan of the chest was obtained for six (12%) patients and all showed bilateral ground–glass opacities.
Nasopharyngeal swabs were obtained from 41 (80%) patients to test for respiratory syncytial virus and influenza A and B by RT–PCR. Of which, only one patient in tocilizumab cohort was concomitantly diagnosed with respiratory syncytial virus, influenza A or B were not detected in any patients. Blood cultures were obtained from 45 (88%) patients on admission. Of which, all were negative except both peripheral blood samples of one patient in no tocilizumab cohort showed methicillin–susceptible Staphylococcus aureus.
3.3. Clinical course and other therapies
Tocilizumab cohort was sicker because more patients required admission to intensive care unit (86% vs. 70%, p = 0·19), invasive mechanical ventilation (75% vs. 48%, p = 0·046), vasopressor support (57% vs. 39%, p = 0·2), and renal replacement therapy (25% vs. 9%, p = 0·16) during hospitalization as compared to patients in no tocilizumab cohort. The median lowest PaO2/FiO2 ratio within 24 h of intubation was 148 (IQR: 107 – 195) in tocilizumab cohort and 207 (IQR: 127 – 231) in no tocilizumab cohort (p = 0·13).
Forty–eight (94%) patients were started on hydroxychloroquine and azithromycin after COVID–19 diagnosis was confirmed. Of the remaining patients, two (7%) patients in tocilizumab and one (4%) patient in no tocilizumab cohort did not receive this regimen due to prolonged corrected QT interval (p = 1). Among patients who required vasopressor and/or mechanical ventilation support at any time, systemic steroid was given to 17 of 21 (81%) patients in tocilizumab cohort and nine of 11 (82%) patients in no tocilizumab cohort (p = 1). In patients who did not require invasive oxygen–support, three of seven (43%) patients in tocilizumab cohort and two of 12 (17%) patients in no tocilizumab cohort received systemic steroid (p = 0·3). Median daily dose and median duration of systemic steroid in tocilizumab vs. no tocilizumab cohorts were 50 mg equivalent of prednisone (IQR: 50 – 75) vs. 50 mg equivalent of prednisone (IQR: 50 – 50), p = 0·34, and 2·5 days (IQR: 2 – 4·25 days) vs. 2 days (IQR: 2 – 5), p = 0·93, respectively.
3.4. Outcomes
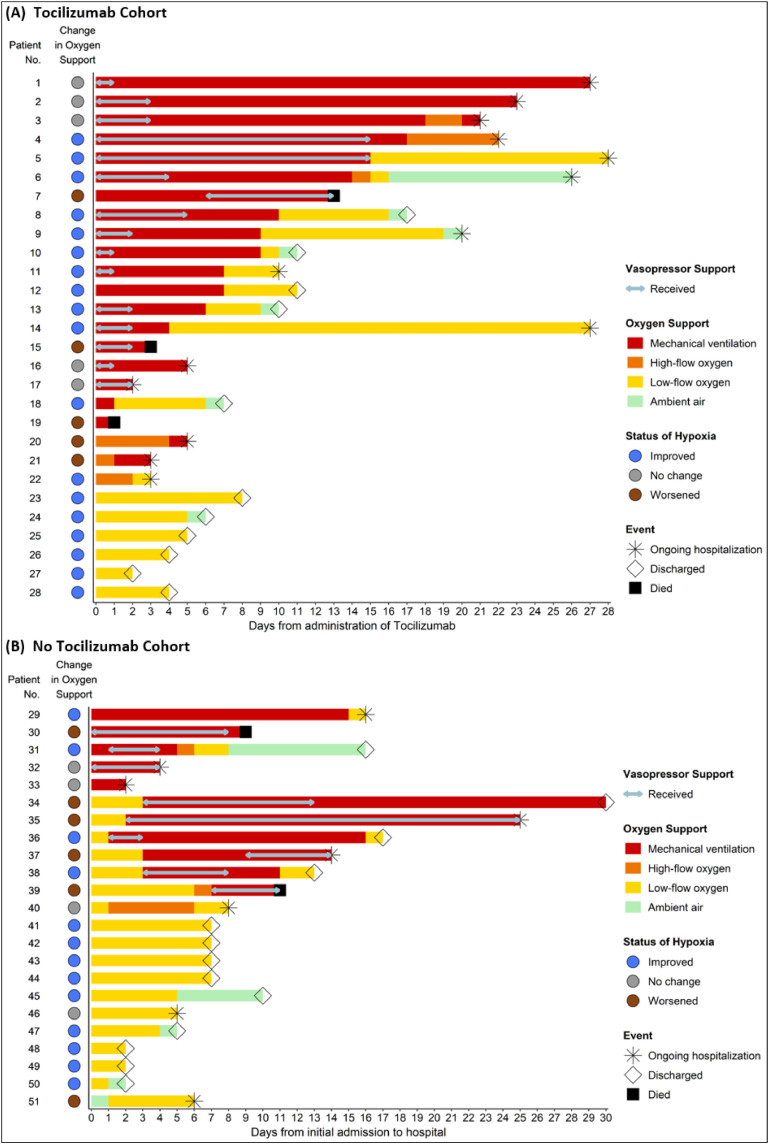
Fig. 1 shows the outcomes of individual patients. Median follow–up of the patients was 10 days (IQR: 6 – 17). Outcomes of intubated patients in tocilizumab (N = 21) vs. no tocilizumab (N = 11) cohorts were as follows; 11 (52%) vs. four (36%) had improvement in oxygen–support category (p = 0·47), five (24%) vs. four (36%) were discharged from the hospital (p = 0·68), 13 (62%) vs. five (45%) were still hospitalized (p = 0·37), and three (14%) vs. two (18%) died (p = 1), respectively. Among patients who required non–invasive oxygen support in tocilizumab (N = 7) vs. no tocilizumab (N = 12) cohorts, no patients died, seven (100%) vs. nine (75%) had improvement in oxygen–support category (p = 0·26) and six (86%) vs. nine (75%) were discharged from the hospital (p = 1), respectively.
Fig. 1.
Changes in Oxygen and Vasopressor Requirements for Individual Patients. (A) Tocilizumab cohort (B) No Tocilizumab cohort. Each horizontal line represents a patient. Arrows within the lines show the duration of vasopressor support for patients who required vasopressor. The colored circles near the patient number indicate the improvement status of oxygen–support category between baseline (Day 0) and last follow–up. A discharged patient was considered “improved” if the oxygen–support category at discharge was not worse than initial admission. The shapes of star, open diamond, and solid square at the end of each line mean ongoing hospitalization, discharge, and death for the corresponding patient, respectively.
Cumulative incidence of clinical improvement at 21 days in tocilizumab vs. no tocilizumab cohorts were 76·5% (95% CI: 57·3 – 95·6) vs. 79·4% (95% CI: 56·0 – 100) among all patients (p = 0·7) and 67·9 (95% CI: 43·2 – 92·7) vs. 61·9 (95% CI: 21·9 – 100) among patients required mechanical ventilation (p = 0·3), respectively (Fig. 2). Median time to clinical improvement in tocilizumab vs. no tocilizumab cohorts were 6·5 days (IQR: 4 – 9 days) vs. 7 days (IQR: 5 – 10 days) among all patients (Hazard ratio for clinical improvement: 1·14, 95% CI: 0·55 – 2·38, p = 0·72) and 8 days (IQR: 6·25 – 9·75 days) vs. 13 days (IQR: 9·75 – 15·25 days) among patients required mechanical ventilation at any time (Hazard ratio for clinical improvement: 1·83, 95% confidence interval [CI]: 0·57 – 5·84, p = 0·31), respectively. Table S5 shows the association between baseline characteristics and clinical improvement. The median duration of vasopressor support and invasive mechanical ventilation were 2 days (IQR: 1·75 – 4·25 days) vs. 5 days (IQR: 4 – 8 days), p = 0·039, and 7 days (IQR: 4 – 14 days) vs. 10 days (IQR: 5 – 15 days), p = 0·40, in tocilizumab vs. no tocilizumab cohorts, respectively. Overall, median length of stay in intensive care unit was 8·5 days in both cohorts. The median length of stay in hospital was 11 days (IQR: 6 – 22·5 days) in tocilizumab cohort and 7 days (IQR: 5 – 13·5 days) in no tocilizumab cohort (p = 0·11) (Table 2).
Fig. 2.
Cumulative incidence of clinical improvement. Day 0 (baseline) represents the day on which tocilizumab was given for tocilizumab cohort and represents the first day of admission to hospital for no tocilizumab cohort.
The age difference between both cohorts is an important confounding factor. Thus, we also compared the outcomes among patients aged ≥65 years and required invasive ventilation. In this subset of patients, tocilizumab cohort still had higher rate of clinical improvement; 40% (four of 10 patients) vs. 13% (one of eight patients), p = 0·20, shorter median time to clinical improvement; 8 days (IQR: 5 – 14·5) vs. 12·5 (IQR: 7·75 – 17·5), p = 0·53, and shorter median duration of vasopressor support; 2·5 days (IQR: 1·75 – 3·25) vs. 6·5 days (IQR: 4·25 – 9·5), p = 0·011, compared to no tocilizumab cohort, respectively.
3.5. Safety
Minor changes in AST and ALT were observed for patients in both tocilizumab and no tocilizumab cohorts during hospitalization (Figure S2). Five (18%) patients in tocilizumab cohort and five (22%) patients in no tocilizumab cohort were diagnosed with hospital–acquired infections (p = 0·74). Details of these infections are shown in Table S6.
4. Discussion
In this study, tocilizumab cohort had a higher rate of improvement in oxygen–support category for both subsets of patients who required invasive and non–invasive oxygen support. Among intubated patients, tocilizumab cohort had 5 days shorter median time to clinical improvement despite concomitant use of systemic steroid (81% vs. 82%) and combination of hydroxychloroquine and azithromycin (93% vs. 96%) were similar in both cohorts. The median duration of vasopressor support and invasive mechanical ventilation were both 3 days shorter in tocilizumab cohort compared to no tocilizumab cohort. Overall, median length of stay in hospital was 4 days longer in tocilizumab cohort but importantly higher proportion of these patients required admission to intensive care unit. In general, older patients are more fragile and tend to have poor outcomes given the higher prevalence of other coexisting medical comorbidities [3,10]. Importantly, tocilizumab cohort was younger in our study but had similar total number of comorbidities compared to control cohort. This may, in part, explain the reason for more severe disease in tocilizumab cohort despite being younger than the control cohort. We have not observed significantly higher adverse events in tocilizumab cohort with regards to similar rates of hospital–acquired infection and minor changes in AST and ALT for both cohorts.
Previous studies suggested that SARS–CoV–2 can cause over–activation of immune system and clinicians need to be vigilant against cytokine release syndromes [6]. High prevalence of multiorgan failure along with high interleukin 6 and other inflammatory biomarkers may support the role of cytokine storm in clinical picture of our patients. In addition to our study, respiratory failure and circulatory shock were previously reported to be most common causes of intensive care unit admission [20]. Although corticosteroid treatment was previously shown to cause delay in clearance of viral RNA for other coronavirus species [21], balanced immunosuppression that would allow immune system to clear the virus but prevent the harmful effects on the body, might be of benefit. Measurement of inflammatory biomarkers may guide clinicians to select appropriate patients for immunosuppressive therapy [6]. Although there is no established cut–off value for interleukin 6 level to predict a higher likelihood of cytokine release syndrome, the median interleukin 6 level was roughly five times the upper limit of normal in our study. The trend of serum interleukin 6 level after tocilizumab infusion is not reported in this study because we did not perform serial measurements of interleukin 6 level after the baseline assessment. We have demonstrated the consistent downtrend of CRP and ferritin after administration of tocilizumab, it is unclear yet whether downtrend of these acute phase reactants correlates with clinical improvement.
A few case series previously noted quicker fever resolution, decrease in inflammatory biomarkers, and improvement in oxygenation after tocilizumab for COVID–19 patients who required mostly non–invasive ventilation [15,22]. In a study from Italy, authors reported 100 COVID–19 patients who were treated with tocilizumab. Improvement in oxygenation was seen in 32 (74%) of 43 patients who were on invasive ventilation and 37 (65%) of 57 patients who were on non–invasive ventilation [23]. These studies did not have a control cohort. In a propensity–matched case–control study from Italy, 21 COVID–19 patients received tocilizumab and had similar rates of intensive care unit admission and 7–day mortality compared to those treated with a combination of hydroxychloroquine and azithromycin [24]. Changes in oxygenation status of the patients were not reported in this study. On the contrary, another study in Italy reported significantly greater survival benefit among 62 COVID–19 patients treated with tocilizumab compared to 23 patients treated with a combination of hydroxychloroquine, lopinavir, and ritonavir [16]. Similar to our study, patients were given 400 mg tocilizumab as a single intravenous infusion in this study [16].
Our study has several limitations that require careful interpretation of the findings and may affect the generalizability of the results. First, the study design was retrospective in nature with a non–randomized control group. Second, this single–center cohort study with a small sample size was not feasible to perform propensity score matched analysis. Although tocilizumab cohort was younger, we have shown that favorable outcomes of tocilizumab cohort persisted among patients aged ≥65 years as well. Third, we had a relatively short follow–up duration. As a result, clinical outcomes of 9 patients did not improve or worsen at last follow–up. Fourth, although similar proportion of intubated patients in both cohorts received hydroxychloroquine, azithromycin, and systemic steroid, concomitant use of these drugs may have influenced the outcomes. In a recent multinational registry analysis, hydroxychloroquine and azithromycin were not associated with any clinical benefit [25]. As such, these drugs were unlikely to contribute to the favorable outcomes in our cohort. Other potentially beneficial agents were also concomitantly used in previous reports of tocilizumab for COVID–19 patients.
Despite the limitations, our findings have important clinical implications. Higher mortality rates in certain countries such as Italy, Spain, and France were in part due to surge of patients requiring high level of care [26,27]. The quicker recovery with decreased duration of vasopressor and invasive ventilation support in our cohort is a promising finding that would help hospitals across the world to accommodate the increased need for critical resources and intensive care unit beds. In addition, we demonstrated favorable outcomes with a single 400 mg dose of tocilizumab, while majority of prior studies used higher doses with multiple infusions (i.e. 800 mg tocilizumab with a repeat dose in 12 h) [23,24]. Hence, our results may be instructive to evaluate low–dose tocilizumab in large prospective studies because similar outcomes with fewer side effects might be achieved with low–dose tocilizumab. In conclusion, our findings suggest that use of tocilizumab in selected patients with severe COVID–19 may provide clinical benefit. These findings require validation from ongoing clinical trials of tocilizumab in COVID–19 patients.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
Funding
This study was not funded by any source.
Acknowledgements
This study was not funded by any source. We acknowledge all health–care workers involved in management of patients.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100418.
Appendix. Supplementary materials
References
- 1.Bedford J., Enria D., Giesecke J. COVID–19: towards controlling of a pandemic. Lancet. 2020;395(10229):1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss P., Murdoch D.R... Clinical course and mortality risk of severe COVID–19. Lancet. 2020;395(10229):1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Pesenti A., Cecconi M. Critical Care Utilization for the COVID–19 Outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 5.Kandel N., Chungong S., Omaar A., Xing J. Health security capacities in the context of COVID–19 outbreak: an analysis of international health regulations annual report data from 182 countries. Lancet. 2020;395(10229):1047–1053. doi: 10.1016/S0140-6736(20)30553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P., McAuley D.F., Brown M. COVID–19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033. doi: 10.1016/S0140-6736(20)30628-0. –4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conti P., Ronconi G., Caraffa A. Induction of pro–inflammatory cytokines (IL–1 and IL–6) and lung inflammation by Coronavirus–19 (COVI–19 or SARS–CoV–2): anti–inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2) doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Casals M., Brito-Zerón P., López-Guillermo A. Adult haemophagocytic syndrome. Lancet. 2020;383(9927):1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 9.Radbel J., Narayanan N., Bhatt P.J. Use of Tocilizumab for COVID-19-induced cytokine release syndrome: a cautionary case report. Chest. 2020 doi: 10.1016/j.chest.2020.04.024. published online on April 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID–19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134–020–05991–x. published online on March 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID–19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. published online on March 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X., Zhao B., Qu Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically Ill COVID-19 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa449. published online on April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le R.Q., Li L., Yuan W. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell–induced severe or life–threatening cytokine release syndrome. Oncologist. 2018;23(8):943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020 doi: 10.1073/pnas.2005615117. published online on May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capra R., Rossi N.D., Mattioli F. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020 doi: 10.1016/j.ejim.2020.05.009. published online on May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. published online on May 22. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31022-9. published online on April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao B., Wang Y., Wen D. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid–19 in critically ill patients in the Seattle region – Case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. published online on March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019–nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. published online on April 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toniati P., Piva S., Cattalini M. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102568. published online on May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colaneri M., Bogliolo L., Valsecchi P. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020 doi: 10.3390/microorganisms8050695. published online on May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. published online on May 22. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Lorenzo G.D., Trolio RD. Coronavirus disease (COVID-19) in Italy: analysis of risk factors and proposed remedial measures coronavirus disease (COVID-19) in Italy: analysis of risk factors and proposed remedial measures. Front Med (Lausanne) 2020 doi: 10.3389/fmed.2020.00140. published online on April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khafaie M.A., Rahim F. Cross-country comparison of case fatality rates of COVID-19/SARS-COV-2. Osong Public Health Res Perspect. 2020;11(2):74–80. doi: 10.24171/j.phrp.2020.11.2.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.