Abstract
Background
The objectives of this study were to determine the objective effective response rate, survival, and safety of radiotherapy combined with gefitinib in patients with locally advanced non-small cell lung cancer (NSCLC) who were unfit for surgery or concurrent chemoradiotherapy.
Methods
The patients with the locally advanced NSCLC who were unfit to receive surgery or concurrent chemoradiotherapy, received thoracic intensity-modulated radiotherapy (IMRT) combined with gefitinib 250 mg daily.
Results
29 patients were enrolled between July 2014 and March 2017. 28 patients was in the analysis. Of the 28 patients, 21 (75.0%) experienced a partial response, 5 (17.9%) had stable disease, and 2 (7.1%) experienced progression of disease. The objective response rate was 75.0%, and the disease control rate was 92.9%. The median follow-up time was 51 months. The disease progression showed in 25 (89.3%) patients, including local progression in 19 (67.9%) and distant metastasis in 16 (57.1%). The median overall survival and progression-free survival time (PFS) were 26 and 11 months, respectively. The 3-, 4-, 5-year survival rates were 39.0, 30.1 and 30.1%, respectively. The 3-, 4-, 5-year PFS rates were 14.3, 9.5 and 9.5%. Two patients developed grade 3 acute adverse events. Seven patients developed grade 2 acute irradiation pneumonitis, and there was no grade 3 acute irradiation pneumonitis.
Conclusions
For patients with locally advanced NSCLC who are not eligible for surgery or concurrent chemoradiotherapy, IMRT combined with gefitinib can improve the objective effective rate and is generally well-tolerated.
Keywords: Non-small cell lung cancer, Radiotherapy, Molecular targeted therapy, Gefitinib
Background
Lung cancer is a malignant tumor with one of the highest morbidity and mortality rates in the world, and non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers [1]. Approximately 30% of NSCLC are at a locally advanced stage at the time of diagnosis. Concurrent chemoradiotherapy (CRT) plus Durvalumab is the standard treatment for unresectable locally advanced NSCLC; however, not all patients with unresectable stage III NSCLC are able to tolerate concurrent CRT. Previous clinical trials have shown that among patients treated with concurrent CRT, the incidence of grade 3 and more irradiation pneumonitis and esophagitis ranged from 0 to 18% and 3.4–32%, respectively [2–11]. The PACIFC study [12], durvalumb after chemoradiotherapy in stage III NSCLC, showed that a total of 30.5% of the patients in the durvalumab and 26.1% of those in the placebo group had grade 3 and more adverse events.
Among patients who are unable to tolerate concurrent CRT, the conventional treatment regimens are sequential chemotherapy and radiotherapy, or radiotherapy only, and the median overall survival (OS) is 11–16 months [2–9].
Coupled with further development of lung cancer genomics, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) provide an effective treatment for patients with the advanced lung adenocarcinoma. There is currently a lot of interest in targeted therapy, which has a higher efficiency and a lower toxicity.
Several trials have shown that EGFR inhibitors have a radiotherapy sensitization effect when combined with radiotherapy. The Cancer and Leukemia Group B (CALGB30106) trial [13], which evaluated the feasibility of poor-risk patients receiving radiotherapy and gefitinib (Group A) and those with a more favorable risk profile receiving concurrent CRT and gefitinib (Group B), showed that Group A had a longer MST than Group B. In RTOG 0972, a phase II clinical trial of induction chemotherapy followed by thoracic radiotherapy (TRT) and erlotinib in 75 poor-risk patients with stage III NSCLC, showed the median OS and progression-free survival time (PFS) were 17 and 11 months, respectively, and the 1-year overall survival (OS) was 75% [14].
These trials demonstrate that radiotherapy combined with targeted therapy may be a potentially beneficial treatment for patients with locally advanced NSCLC who are unable to tolerate concurrent CRT. We conducted a phase II trial to evaluate the effectiveness of radiotherapy combined with gefitinib in patients with locally advanced NSCLC who were unfit for surgery or concurrent CRT. The primary objective of this study was to determine the objective response rate. The secondary objectives included assessing the OS, the median OS, PFS, and the safety of radiotherapy combined with gefitinib.
Patients and methods
Patient selection
The inclusion criteria were: (1) histologically or cytologically confirmed unresectable stage III NSCLC; (2) no thoracic radiotherapy history; (3) tumor objectively measured; (4) age ≥ 18 years; (5) Karnofsky Performance Status ≥70; (6) predicted survival ≥3 months; (7) without severe disease of the vital organs; (8) provision of written informed consent.
The exclusion criteria were: (1) neurological disease; (2) history of recent treatment for another malignant tumor; (3) previous participation in clinical trials of other new drugs; and (4) previous targeted therapy.
The unresectable stage IIIA and IIIB NSCLC mainly refers to the following image or lymph node pathological evidence: 1. The more ipsilateral mediastinal lymph nodes merging into a great lump or multi-region metastasis (IIIA: T1–3 N2 or IIIB: T4N2); 2. The contralateral hilar,mediastinal lymph nodes; the ipsilateral /contralateral scalene, supraclavicular lymph node metastasis (IIIB: T1- N3); 3. Lesions invades into the heart, the aorta and esophagus (IIIB: T4N0–1).
The patients of NSCLC, unfitting for concurrent chemoradiotherapy, refer to the following situations: the patients refuse the concurrent chemoradiotherapy; the patients have so much basic disease that they can’t toletate the concurrent CRT.
Treatment
Patients were treated with a combination of radiotherapy and gefitinib:
Radiotherapy: Photon beams were applied with an intensity of 6-MV. The gross tumor volume (GTV) was defined as the volume of the primary disease as well as any involved regional lymph nodes (the short axis of at least 1 cm on computed tomography scan). The clinical target volume (CTV) included the primary tumor plus a margin of 6–8 mm, elective nodal regions including ipsilateral hilar, paratracheal, and subcarinal nodal regions were also included in the CTV. The planning target volume (PTV) included the CTV plus a margin of 5 mm in 3 dimensions. The prescription dose was 95% PTV 60–66 Gy/2 Gy/30–33 f.
Gefitinib: An oral dose of 250 mg was administered daily, starting on Day 1. If the treatment was effective, it was continued after radiotherapy had been completed.
Response and toxicity criteria
The Response Evaluation Criteria in Solid Tumors (RECIST) guideline version 1.0 was used to evaluate the short-term effectiveness. The National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 was used to evaluate the toxicity.
Study design
The study was a single-arm, phase II open-label clinical trial.
Calculation of sample size
In the JCOG0301 trial [15], the objective effective rate of concurrent CRT was 51.5%. We expected that the objective effective rate of TRT with gefitinib would be 68%. According to the sample size calculations, at least 27 patients would be required. The target sample size was increased by 10% to 30 patient to allow for participant withdrawals.
Statistical analysis
The statistical analysis was performed using SPSS version 20.0 (IBM Corp, Armonk, NY, USA). Kaplan-Meier plots were used for survival analysis. Log-rank tests were used to assess the significance of associations in the univariate analysis. Cox regression was using to assess factors associated with survival in the multivariate analysis. Results with p < 0.05 were considered statistically significant.
Results
Patient characteristics
From July 2014 to March 2017, 29 patients were enrolled, of which 28 were included in the analysis. One patient refused to continue radiotherapy because of the fever, so the patient was excluded from the analysis. The median age of the participants was 63 years. Of the participants, 7 (25.0%) and 21 (75.0%) were diagnosed with stages III A and III B NSCLC, respectively. Seventeen participants (60.7%) had previously received platinum-based induction chemotherapy (Table 1).
Table 1.
Patients’ characteristics
| Characteristics | N(%) |
|---|---|
| Sex | |
| Male | 24 (85.7) |
| Female | 4 (14.3) |
| Age | |
| Median | 63 |
| Range | 42–77 |
| Smoking index | |
| 0 | 7 (25.0) |
| 1–400 | 1 (3.6) |
| ≥ 400 | 20 (71.4) |
| KPS status | |
| 70 | 2 (7.1) |
| 80 | 16 (57.1) |
| 90 | 9 (32.1) |
| 100 | 1 (3.6) |
| Weight loss within 6 months before the treatment | |
| 0 | 19 (67.9) |
| <5% | 4 (14.3) |
| ≥ 5% | 5 (17.9) |
| T stage | |
| T1 | 1 (3.6) |
| T2 | 10 (35.7) |
| T3 | 9 (32.1) |
| T4 | 8 (28.6) |
| N stage | |
| N1 | 3 (10.7) |
| N2 | 9 (32.1) |
| N3 | 16 (57.1) |
| Clinical stage | |
| IIIA | 7 (25.0) |
| IIIB | 21 (75.0) |
| Histological type | |
| • Squamous cell carcinoma | 15 (53.6) |
| Adenocarcinoma | 12 (42.9) |
| Neuroendocrine carcinoma | 1 (3.6) |
| Inducing chemotherapy before TRT | |
| Yes | 17 (60.7) |
| No | 11 (39.3) |
Treatment regimens
Of the 28 participants, 21 received intensity-modulated radiotherapy and 7 received volumetric-modulated arc therapy. Twenty eight patients completed TRT with a dose of 54–60Gy.Twenty three patients accepted TRT with 60Gy. Three patients completed TRT with 58Gy. Because the CTV of the 3 patients is too large and the V20 of double lungs is beyond 28%, the total dose reduced to 58Gy. Two patients accepted TRT with 54Gy, because they suffered from grade 2 radiation pneumonia.
Response to treatment and toxicity
Of the 28 patients, 21 (75.0%) experienced a partial response (PR), 5 (17.9%) had stable disease (SD), and 2 (7.1%) experienced progression of disease (PD). None of the patients experienced a complete response (CR). The objective response rate (CR + PR) was 75.0%, and the disease control rate (CR + PR + SD) was 92.9%. Twenty-five participants (89.3%) experienced a relapse: 19 (67.9%) experienced a local relapse, and 16 (57.1%) experienced a distant relapse, including 10 participants (35.7%) who experienced both a local and a distant relapse.
Overall, the toxicity results demonstrated the feasibility of this approach (Table 2). One patient (3.6%) experienced Grade 3 liver dysfunction, esophagitis, and diarrhea, respectively. Seven participants (25.0%) experienced grade 2 pneumonitis and there was no grade 3 acute irradiation pneumonitis.
Table 2.
Acute adverse events
| Toxicity | Toxicity Grade | |||
|---|---|---|---|---|
| 1 N(%) |
2 N(%) |
3 N(%) |
4 N(%) |
|
| Skin | ||||
| Rash | 9 (32.1) | 0 | 0 | 0 |
| Radiation dermatitis | 18 (64.3) | 0 | 0 | 0 |
| Hematologic toxicity | ||||
| Anemia | 9 (32.1) | 0 | 0 | 0 |
| Leukopenia | 9 (32.1) | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 |
| Gastrointestinal toxicity | ||||
| Nausea | 7 (25.0) | 3 (10.7) | 0 | 0 |
| Vomiting | 1 (3.6) | 1 (3.6) | 0 | 0 |
| Diarrhea | 1 (3.6) | 0 | 1 (3.6) | 0 |
| Esophagitis | 9 (32.1) | 9 (32.1) | 1 (3.6) | 0 |
| Hypohepatia | 8 (28.6) | 0 | 1 (3.6) | |
| Renal function | 6 (21.4) | 0 | 0 | 0 |
| Pneumonitis | 2 (7.1) | 7 (25.0) | 0 | 0 |
Survival
Follow-up was censored on 15 December 2019. The 7median follow-up time was 51 months. The median PFS was 11 months, and the MST was 26 months. The 3-, 4- and 5-year survival rates were 39.0, 30.1 and 30.1%, respectively. The 3-, 4- and 5-year PFS rates were 14.3, 9.5 and 9.5%, respectively. (Fig. 1).
Fig. 1.
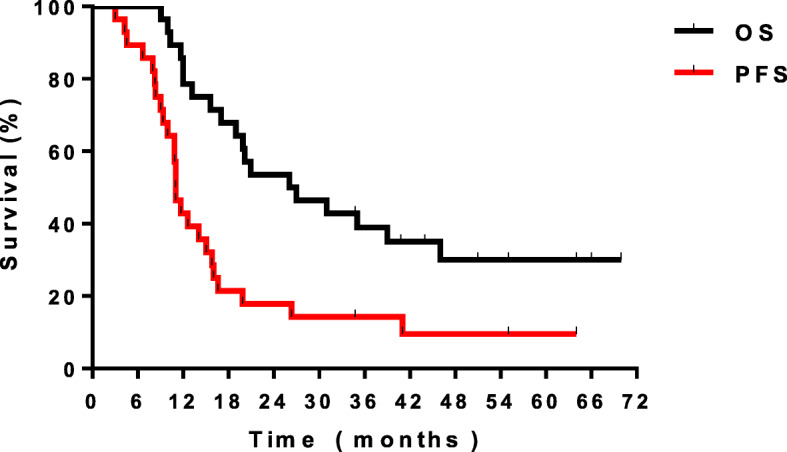
The O S and P FS curves of the w hole group
Univariate analysis showed that only disease stage was associated with OS (p = 0.043, 95%CI 11.998–40.109), and with PFS (p = 0.000, 95%CI 9.899–12.114).
The median OS of participants with stages III A and III B disease were 12 and 35 months, respectively. Participants with stage III B disease had a significantly better OS and PFS than patients with stage III A disease.
Epidermal growth factor receptor mutation
Molecular data were available for 13 participants. The EGFR-activating mutation was detected in 6 of the 13 participants (46.2%). The 6 participants with the EGFR-activating mutation had a significantly better the median OS (39 months) than the 20 participants with the EGFR wild-type or non-adenocarcinoma (20 months) without significant difference.
Discussion
In this trial, the PR rate and the objective effective response rate were both 75.0%, and the disease control rate was 92.9%, and no participants experienced a CR. In previous clinical trials of concurrent CRT the CR rate was 9–28%, the PR rate was 28–81%, and the objective effective response rate was 43–85% [2–11, 15]. Among participants in previous studies who received radiotherapy only, or sequential chemotherapy and radiotherapy, the CR rate was 1.3–30%, the PR rate was 30–65%, and the objective effective response rate was 39–66%. Although none of the participants in our study experienced a CR, the PR rate and the objective effective response rate were comparable to those of previous studies.
The reason for the absence of any participants with a CR in this study may be attributable to: the high proportion of participants with stage III B disease (75%); high PTV (64.3% had a PTV volume > 450 ml); the relatively small proportion of participants with adenocarcinoma (42.9%). The relatively high PR rate and objective effective response rate compared to previous studies may be attributable to the higher rates of completion of radiotherapy (82.1%) and targeted therapy (85.7%).
In the CALB30106 trial [13] the high-risk group (radiotherapy and gefitinib) had a median OS of 19.0 months and median PFS of 13.4 months; while the low-risk group (concurrent CRT and gefitinib) had a median OS of 13 months and a median PFS of 9.2 months. The survival time of high-risk patients who received sequential CRT with gefitinib is promising. Spanish researchers [16] studied 90 patients with locally advanced NSCLC. The median OS was 11.4 months among those who received radiotherapy only, and 8.9 months among those who received radiotherapy with erlotinib, and the median PFS was 15.3 months and 12.9 months, respectively. Wang et al. reported that EGFR-TKI concurrent with thoracic radiotherapy in treating stage IIIB/IV NSCLC had local control rate of 96% for thoracic tumor and 1-year PFS rate of 42% [17]. Zheng et al. reported that the 1-year PFS rate of 57.1%, the median PFS 13 months and the median time to progression of irradiated lesion 20.5 months in TIK combined with radiotherapy as first line treatment for patients with stage IV NSCLC harboring EGFR active mutations [18]. In the RTOG 9410 trial [4], the median OS was 17 months among participants who received 60 Gy of CRT, and 15.6 months among those who received 70 Gy of CRT. Compared with radiotherapy only and concurrent CRT, the survival of participants who received radiotherapy combined with targeted therapy is comparable to that of the participants in our study.
In our study, the OS and PFS of participants with stage III B disease was higher than that of participants with stage III A disease. Possible reasons for this paradoxical result include: (1) participants with stage III A disease had more risk factors than those with III B disease including older age(≥65 year; III A 5/7 (71.4%);III B 8/21 (38.1%);), greater weight loss(6 months before radiotherapy: weight loss≥5%;III A 3/7 (42.9%);III B 2/21 (9.5%);), more comorbidities (including high blood pressure, diabetes, heart disease and other disease; III A 6/7 (85.7%);III B16/21 (76.2%);), less healthy lifestyles (smoking index ≥400;III A 6/7 (85.7%);III B 14/21 (66.7%);), and larger primary tumors (maximum diameter before radiotherapy≥4 cm;III A 5/7 (71.4%);III B 10/21 (47.6%);); (2) participants with stage III B disease were more likely to have adenocarcinoma and EGFR mutations than those with stage III A disease (adenocarcinoma; III A 2/7 (28.6%);III B 10/21 (47.6%);) (EGFR mutations; III A 1/7 (14.3%);III B 5/21 (23.8%);); (3) participants with stage III B disease received more induction chemotherapy (III A 3/7 (42.9%);III B 14/21 (66.7%);); (4) participants with stage III A disease suffered more acute toxicities (Grade 3 esophagitis; III A 1/7 (14.3%);III B 0/21 (0);) (Grade 3 diarrhea; III A 1/7 (14.3%);III B 0/21 (0);).
In our study, participants with the EGFR-activating mutation had a better the median OS than those with the EGFR wild-type and those with non-adeno carcinomatous tumors. This result suggests that there is an association between the EGFR mutation state and response to targeted therapy. For NSCLC, previous studies have shown that approximately 80% of patients with squamous cell carcinoma and 65% of patients with adenocarcinomas have overexpression of EGFR protein, and the overexpression state is an important factor leading to radiation resistance [19, 20]. Meta-analysis conducted in 2002 suggested that a high EGFR expression could be related to the prognosis of NSCLC [21]. However, the CALGB 30106 trial [13] did not find an association between the presence of EGFR mutations and the prognosis of NSCLC. Although the relationship between EFGR-mutation and prognosis in advanced lung adenocarcinoma was relatively clear, the heterogeneity was relatively large among participants with locally advanced lung adenocarcinoma who were able to receive radiotherapy and chemotherapy. For locally advanced lung cancer, there are no prospective studies that have assessed whether individuals with EGFR-activating mutations could benefit from targeted therapy as the first-line treatment. Our study suggests that radiotherapy combined with gefitinib could improve the survival of patients with locally advanced EGFR-activating mutation lung adenocarcinoma.
In this study, the majority of acute adverse events (92.9%) were grades 1 and 2; only 7.1% of participants experienced a grade 3 acute adverse event, and no participants experienced a grade 4 acute adverse event. These results indicate that radiotherapy combined with gefitinib was well tolerated.
Irradiation pneumonia is an important adverse event among patients treated with TRT. In our study, the incidence of grade 2 acute irradiation pneumonitis was 25.0%, but there were no cases of more than grade 3 acute irradiation pneumonitis. Compared with concurrent CRT (which has a reported rate of grade 3 acute irradiation pneumonitis of 0–18%) [2–11], targeted therapy combined with radiotherapy did not significantly increase the incidence of related adverse events. Although irradiation pneumonia did not cause any treatment-related deaths among participants in our study, the pulmonary toxicity associated with EGFR-TKIs is a cause for concern. Zheng et al. reported that the most common grade 3 adverse events were radiation pneumonitis (20%) and rash (10%) in TIK combined with radiotherapy as first line treatment for patients with stage IV NSCLC harboring EGFR active mutations [18]. A study [22] of the relationship between radiotherapy combined with erlotinib and acute irradiation pneumonitis among 24 patients with NSCLC, suggested that 9 patients (37.5%) experienced greater than grade 2 acute irradiation pneumonitis. Other studies have shown that erlotinib can damage the lung stroma [23, 24], and erlotinib combined with TRT may increase the occurrence of acute irradiation pneumonitis. Some small sample studies [25, 26] also reported there was a higher incidence of acute irradiation pneumonitis among patients treated with erlotinib combined with TRT.
This trial had several limitations. First, some patients received induction chemotherapy, but the induction chemotherapy regimens and chemotherapy cycles were not standardized. Second, this study was a single-arm, phase II clinical trial with no control group. Third, the sample was small.
Conclusions
For patients with locally advanced NSCLC who couldn’t receive surgery or concurrent chemoradiotherapy, TRT combined with gefitinib could improve the objective effective rate and be tolerated well.
Acknowledgments
We acknowledge all the investigators, patients and their families. We would like to thank Editage for English language editing.
Abbreviations
- CR
Complete response
- CRT
Chemoradiotherapy;
- CTCAE
Common terminology criteria for adverse events
- CTV
Clinical target volume
- EGFR
Epidermal growth factor receptor
- IMRT
Intensity-modulated radiotherapy
- MST
Median survival time
- NCI
National cancer institute
- NSCLC
non-small cell lung cancer
- OS
Overall survival
- PD
Progressive disease
- PFS
Progression-free survival
- PR
Partial response
- PTV
Planning target volume
- RECIST
Response evaluation criteria in solid tumors
- SD
Stable disease
- TKI
Tyrosine kinase inhibitor
- TRT
Thoracic radiotherapy
Authors’ contributions
Dr. Jun Liang had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Acquisition, analysis, or interpretation of data: Xu Yang, Wenqing Wang, Lei Deng, Tao Zhang, Nan Bi, Xiaozhen Wang, Dongfu Chen, Zongmei Zhou. Drafting of the manuscript: Zhixue Fu. Supervision: Luhua Wang, Jun Liang. The author (s) read and approved the final manuscript.
Funding
This study was funded by a grant from the Chinese Geriatric Oncology Society (CGOS-02-2014-1-1-01500).
Availability of data and materials
The data and materials of this study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This trial had approved by Ethics Committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences. Approval No. 14–091/881.
Consent for publication
Not applicable.
Competing interests
The authors have declared no conflicts of interest.
Footnotes
This study was previously oral presented as the 60th annual meeting of American Society for therapeutic Radiology and Oncology, San Antonio, 21-24 October 2018.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhixue Fu, Email: 1263042468@qq.com.
Xu Yang, Email: yx1016534625@163.com.
Wenqing Wang, Email: wwq4350524@163.com.
Lei Deng, Email: dengleipumc@163.com.
Tao Zhang, Email: zhangt10@126.com.
Nan Bi, binan_email@163.com.
Xiaozhen Wang, Email: wangxiaozhen2008@yahoo.com.
Dongfu Chen, Email: cdf1825@sina.com.
Zongmei Zhou, Email: zhouzongmei2013@163.com.
Luhua Wang, Email: wlhwq@yahoo.com.
Jun Liang, Email: liang23400@163.com.
References
- 1.American Cancer Society . Cancer facts and figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 2.Schaake-Koning C, van den Bogaert W, Dalesio O, Festen J, Hoogenhot J, van Houtte P, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med. 1992;326:524–530. doi: 10.1056/NEJM199202203260805. [DOI] [PubMed] [Google Scholar]
- 3.Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 4.Curran WJ, Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradia- tion for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, et al. Concurrent versus sequential chemorad- iotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004;46:87–98. doi: 10.1016/j.lungcan.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Huber RM, FlentjeM SM, PÖllinger B, Gosse H, Willner J, et al. Simultaneouschemoradiotherapy compared with radiotherapy alone after induction chemotherapy in inoperable stage IIIA or IIIB non small-cell lung cancer: study CTRT99/97by the bronchial carcinoma therapy group. J Clin Oncol. 2006;24:4397–4404. doi: 10.1200/JCO.2005.05.4163. [DOI] [PubMed] [Google Scholar]
- 7.Clamon G, Herndon J, Cooper R, Chang AY, Rosenman J, Green MR. Radiosensitization with carboplatin for patients with unresectable stage III non-small-cell lung cancer: a phase III trial of the Cancer and leukemia group B and the eastern cooperative oncology group. J Clin Oncol. 1999;17:4–11. doi: 10.1200/JCO.1999.17.1.4. [DOI] [PubMed] [Google Scholar]
- 8.Belderbos J, Uitterhoeve L, van Zandwijk N, Belderbos H, Rodrigus P, van de Vaart P, et al. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972-22973) Eur J Cancer. 2007;43:114–121. doi: 10.1016/j.ejca.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Groen HJ, van der Leest AH, Fokkema E, Timmer PR, Nossent GD, Smit WJ, et al. Continuously infused carboplatin used as radiosensitizer in locally unresectable non-small-cell lung cancer: A multicenter phase III study. Ann Oncol. 2004;15:427–432. doi: 10.1093/annonc/mdh100. [DOI] [PubMed] [Google Scholar]
- 10.Fournel P, Robinet G, Thomas P, Souquet PJ, Léna H, Vergnenégre A, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-saint-Etienne d'Oncologie Thoracique-Groupe Francais de PneumoCancerologie NPC 95-01 study. J Clin Oncol. 2005;23:5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 11.Gervais R, Ducolone A, Lechevalier T, Legroumellec A, Lemarie E, Quoix E, et al. Conventional radiation (RT) with daily carboplatin (Cb) compared to RT alone after induction chemotherapy (ICT) [vinorelbine (Vr)-cisplatine (P)]: final results of a randomized phase III trial in stage III unresectable non small cell lung (NSCLC) cancer. Study CRG/BMS/NPC/96 of the French lung cancer study group FNCLCC and IFCT. J Clin Oncol. 2005;23:7016. doi: 10.1200/jco.2005.23.16_suppl.7016. [DOI] [Google Scholar]
- 12.Ready N, Jänne P, Bogart J, DiPetrillo T, Garst J, Graziano S, et al. Chemoradiotherapy (CRT) and geftinib (G) in stage III non-small cell lung cancer (NSCLC): a CALGB stratifed phase II trial. J Clin Oncol. 2006;24(Suppl 1):7046. doi: 10.1200/jco.2006.24.18_suppl.7046. [DOI] [Google Scholar]
- 13.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with Durvalumab after Chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 14.Lilenbaum R, Samuels M, Wang X, Kong FM, Jamme PA, Masters G, et al. A phase II study of induction chemotherapy followed by thoracic radiotherapy and erlotinib in poor-risk stage III non-small-cell lung cancer: results of CALGB 30605 (Alliance)/RTOG 0972 (NRG)[J] J Thorac Oncol. 2015;10:143–147. doi: 10.1097/JTO.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atagi S, Kawahara M, Yokoyama A, Okamoto H, Yamamoto N, Ohe Y, et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small cell lung cancer: a randomized controlled, phase 3 trial by the Japan clinical oncology group (JCOG 0301) Lancet Oncol. 2012;13:671–678. doi: 10.1016/S1470-2045(12)70139-0. [DOI] [PubMed] [Google Scholar]
- 16.Martínez E, Martínez M, Rico M, Hemández B, Casas F, Viñolas N, et al. Feasibility, tolerability, and effcacy of the concurrent addition of erlotinib to thoracic radiotherapy in locally advanced unresectable non-small cell lung cancer: a phase II trial. Oncol Targets Ther. 2016;9:1057–1066. doi: 10.2147/OTT.S89755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Xia TY, Wang YJ, Li HQ, Li P, Wang JD, et al. Prospective study of epidermal growth factor receptor tyrosine kinase inhibitors concurrent with individualized radiotherapy for patients with locally advanced or metastatic non-small cell lung cancer. Int J Radiat Oncol. 2011;81:e59–e65. doi: 10.1016/j.ijrobp.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Zheng LP, Wang Y, Xu Z, Yang Q, Zhu G, Liao XY, et al. Concurrent EGFR-TKI and thoracic radiotherapy as first line treatment for stage IV non-small-cell lung cancer harboring EGFR active mutations. Oncologist. 2019;24:1031–e612. doi: 10.1634/theoncologist.2019-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 20.Dacic S, Flanagan M, Cieply K, Ramalingam S, Luketich J, Belani C, et al. Significance of EGFR protein expression and gene amplification in non-small cell lung carcinoma. Am J Clin Pathol. 2006;125:860–865. doi: 10.1309/H5UW6CPCWWC92241. [DOI] [PubMed] [Google Scholar]
- 21.Meert AP, Martin B, Delmotte P, Berghmans T, Lafitte JJ, Mascaux C, et al. The role of EGFR expression on patient survival in lung cancer: a review with meta-analysis. Eur Respir J. 2002;9:75–81. doi: 10.1183/09031936.02.00296502. [DOI] [PubMed] [Google Scholar]
- 22.Zhuang H, Yuan Z. Radiation pneumonitis in patients with non-small cell lung cancer treated with erlotinib concurrent with thoracic radiotherapy. J Thorac Oncol. 2014;9:882–885. doi: 10.1097/JTO.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 23.Ren S, Li Y, Li W, Zhao Z, Jin C, Zhang D. Fatal asymmetric interstitial lung disease after erlotinib for lung cancer. Respiration. 2012;84:431–435. doi: 10.1159/000339508. [DOI] [PubMed] [Google Scholar]
- 24.Tsubata Y, Hamada A, Sutani A, Isobe T. Erlotinib-induced acute interstitial lung disease associated with extreme elevation of the plasma concentration in an elderly non-small-cell lung cancer patient. J Cancer Res Ther. 2012;8:154–156. doi: 10.4103/0973-1482.95201. [DOI] [PubMed] [Google Scholar]
- 25.Nanda A, Dias-Santagata DC, Stubbs H, O’Hara CJ, Zaner KS, Lynch TJ, et al. Unusual tumor response and toxicity from radiation and concurrent erlotinib for non-small-cell lung cancer. Clin Lung Cancer. 2008;9:285–287. doi: 10.3816/CLC.2008.n.044. [DOI] [PubMed] [Google Scholar]
- 26.Chang CC, Chi KH, Kao SJ, Hsu PS, Tsang YW, Chang HJ, et al. Upfront geftinib/erlotinib treatment followed by concomitant radiotherapy for advanced lung cancer: a mono-institutional experience. Lung Cancer. 2011;73:189–194. doi: 10.1016/j.lungcan.2010.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials of this study are available from the corresponding author on reasonable request.


