Abstract
Background
Preservatives have to be added in food, pharmaceuticals and cosmetics products to maintain their shelf life. However, the existing chemical based preservatives have been associated with severe side effects that compel the researchers to find better safe preservatives based on natural products. G-6-P synthase is an important enzyme for bacterial and fungal cell wall synthesis and offers as a potential target to find better G-6-P synthase inhibitors based antimicrobial compounds. Naringenin, a flavanone, has been reported for a wide range of pharmacological activities including antimicrobial activity, which makes it a potential candidate to be explored as novel G-6-P synthase inhibitor.
Results
The synthesis of naringenin derivatives with potent G-6-P synthase inhibitor having remarkable antioxidant, antimicrobial and preservative efficacy was performed. Among the synthesized compounds, the compound 1 possessed good antioxidant activity (IC50 value, 6.864 ± 0.020 µM) as compared to standard ascorbic acid (IC50 value, 8.110 ± 0.069 µM). The antimicrobial activity of synthesized compounds revealed compound 1 as the most potent compound (pMIC 1.79, 1.79, 1.49, 1.49, 1.49 and 1.49 μM/mL for P. mirabilis, P. aeruginosa, S. aureus, E. coli, C. albicans and A. niger respectively) as compared to standard drugs taken. The compound 2 showed comparable activity against P. mirabilis (pMIC 1.14 μM/mL), C. albicans (pMIC 1.14 μM/mL) while the compound 3 also showed comparable activity against C. albicans (pMIC 1.16 μM/mL) as well A. niger (pMIC 1.46 μM/mL), likewise the compound 4 showed comparable activity against P. mirabilis (pMIC 1.18 μM/mL) as compared to the standard drugs streptomycin (pMIC 1.06, 1.36, 1.06 and 1.96 μM/mL for P. mirabilis, P. aeruginosa, S. aureus and E. coli respectively), ciprofloxacin (pMIC 1.12, 1.42, 1.12 and 1.42 μM/mL for P. mirabilis, P. aeruginosa, S. aureus and E. coli respectively), ampicillin (pMIC 1.14, 0.84, 0.84 and 1.74 μM/mL for P. mirabilis, P. aeruginosa, S. aureus and E. coli respectively) and fluconazole (pMIC 1.08 and 1.38 μM/mL for C. albicans and A. niger respectively). The molecular docking with the target G-6-P synthase pdb id 1moq resulted with an better dock score for compound 1 (− 7.42) as compared to standard antimicrobial drugs, ciprofloxacin (− 5.185), ampicillin (− 5.065) and fluconazole (− 5.129) that supported the wet lab results. The preservative efficacy test for compound 1 in White Lotion USP showed the log CFU/mL value within the prescribed limit and results were comparable to standard sodium benzoate, ethyl paraben and propyl paraben as per USP standard protocol.
Conclusions
The synthesized naringenin derivatives exhibited significant G-6-P synthase inhibitory potential with good selectivity towards the selected target G-6-P synthase. Compound 1, bearing nitro group showed good antioxidant, antimicrobial and preservative efficacy compared with the standard drugs taken. The mechanistic insight about the compounds within the active site was completed by molecular docking that supported the results for novel synthesized G-6-P synthase inhibitors.
Keywords: G-6-P synthase, Naringenin derivatives, DPPH, Preservative efficacy
Introduction
The use of packaged foods containing various additive’s viz. artificial sweeteners, colorants, stabilizers, preservatives etc. has greatly increased in recent years. As per recent data available it is estimated that 75% of the contemporary diet is packaged food and on an average every person consumes 3.6 to 4.5 kg of food additives per year [1].
Among other additives the preservative such as sodium benzoate, ethyl paraben, propyl paraben, butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), etc. plays a vital role to maintain the shelf life of various food, pharmaceuticals and cosmetic products [2–4]. However, the existing chemical preservatives have been associated with serious side effects viz. estrogenic effect, breast cancer, malignant melanoma, contact eczema, endocrine disruption, etc. [5–12]. Hence, there is an urgent need for the discovery of novel and safer preservatives for use in food, pharmaceuticals and cosmetic products.
G-6-P synthase is a complex enzyme involved in the formation of UDP-N-acetyl glucosamine and catalyzes the initial step in hexosamine biosynthesis. One of these catalyzed products, N-acetyl glucosamine, is an important part of the peptidoglycan layer of bacterial and fungal cell wall. Hence, G-6-P synthase may act as potential target for discovery of novel antimicrobial compounds which could be evaluated for their preservative efficacy to find better and safe preservatives [13, 14].
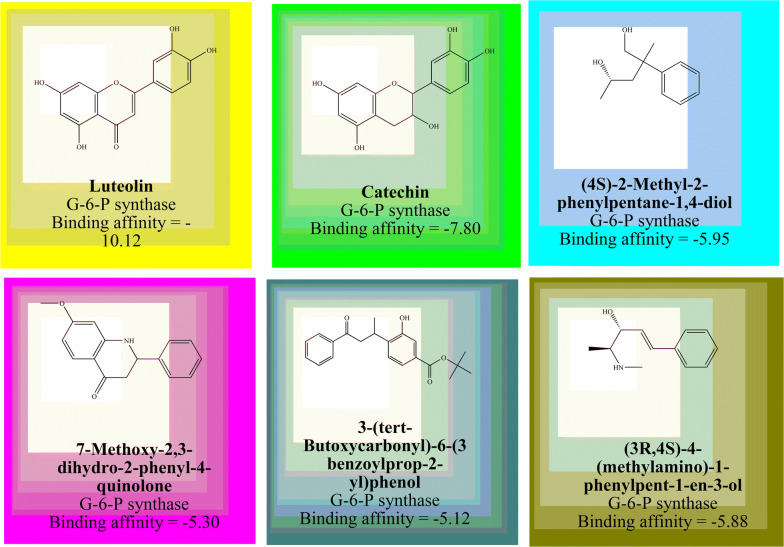
The complex 3-D crystal structure of G-6-P synthase can be utilized for molecular docking to explore the structural requirements for the pharmacophore complex. Flavonoids such as luteolin, catechin, (4S)-2-Methyl-2-phenylpentane-1,4-diol, 7-Methoxy-2,3-dihydro-2-phenyl-4 quinolone, 3-(tert-Butoxycarbonyl)-6-(3 benzoylprop-2-yl)phenol and (3R,4S)-4-(methylamino)-1-phenylpent-1-en-3-ol also have been explored for G-6-P synthase inhibition [15–18]. Some flavonoids along with their G-6-P synthase inhibitory dock score have been shown in Fig. 1.
Fig. 1.
G-6-P synthase inhibitory profile of flavonoids and their derivatives cited in the recent literature
Naringenin is a naturally occurring bioflavonoid present in various fruits, vegetables and honey which is used as a dietary supplement due to its low toxicity [19–21]. Naringenin has been reported for its diverse pharmacological profile including its antibacterial property as shown in Fig. 2 [22–41].
Fig. 2.
Pharmacological potential of Naringenin
Further, naringenin could be utilized as a potential candidate for evaluation of its G-6-P synthase inhibitory response. Hence, it was planned to synthesize and investigate the naringenin derivatives for their antioxidant, antimicrobial, preservative efficacy and in silico evaluation for G-6-P synthase inhibition.
Results and discussion
Chemistry
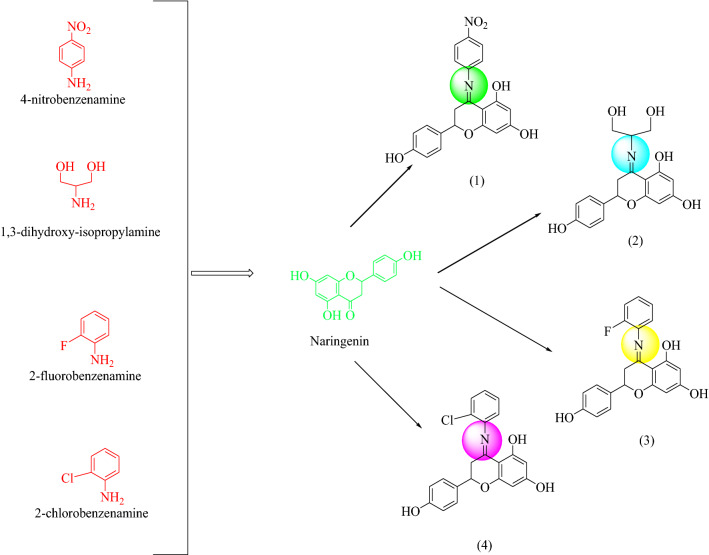
Naringenin derivatives were synthesized according to Kriza et al. 2011 with slight modifications as shown in Scheme 1 [42]. The chemical structures of all the synthesized compounds were confirmed by FTIR, 1H NMR, 13C NMR, mass spectroscopy and elemental analysis which were in agreement with the structures.
Scheme 1.
Synthetic route for the synthesis of naringenin derivatives
For the synthesis of naringenin derivatives substituted aniline (0.01 mol) was taken in a round bottom flask and concentrated hydrochloric acid was added drop wise with continuous stirring. Equimolar concentration of naringenin (0.01 mol) was dissolved in ethanol (50 mL) and was refluxed for 80–100 h at 80 °C on heating mantle. All the compounds in series were synthesized according to the standard procedure outlined in Scheme 1. Completion of reaction was confirmed by TLC under UV lamp and FTIR spectra.
Formation of compound 1, 2, 3 and 4 was confirmed by peaks of IR, NMR, mass spectroscopy. In positive chemical ionization most of the naringenin derivatives showed (M++1), M+ (molecular ion peak), (M++2) and in negative chemical ionization mode showed (M+1), (M+2), M+. The elemental analysis established the synthesis of naringenin derivatives where the percentage of C, H and N in the synthesized compounds was observed within defined limits. The reaction mixture was concentrated, after that precipitates formed were filtered off and dried. Crude products were recrystallized by alcohol which yielded the final compounds 1–4.
Antioxidant activity
DPPH radical scavenging activity
All the synthesized compounds were evaluated for antioxidant profile by using DPPH radical scavenging assay method (Table 1). The compound 1 was observed as the most potent antioxidant compound (IC50 6.864 ± 0.020 µM) as compared to standard L-ascorbic acid (IC50 8.110 ± 0.069 µM). However, compounds 3 and 4 showed moderate antioxidant activity (IC50 7.170 ± 0.028 µM and 7.801 ± 0.077 µM, respectively) as compared to standard. The electron withdrawing strongly deactivating nitro group in compound 1 may be responsible for better antioxidant activity. The presence of weakly deactivating electron withdrawing chloro and fluoro groups present in compound 3 and 4 have moderate antioxidant activity. IC50 value of synthesized naringenin derivatives has been shown in Fig. 3.
Table 1.
Antioxidant IC50 values of synthesized compounds
| S. no. | Compound(s) | IC50 (µM)a |
|---|---|---|
| 1. | Compound 1 | 6.864 ± 0.020 |
| 2. | Compound 2 | 26.210 ± 0.151 |
| 3. | Compound 3 | 7.170 ± 0.028 |
| 4. | Compound 4 | 7.801 ± 0.077 |
| 5. | Naringenin | 13.765 ± 0.408 |
| 6. | Standard (l-ascorbic acid) | 8.110 ± 0.069 |
aValues are expressed as mean ± SEM, n = 3
Fig. 3.
IC50 value of different synthesized compunds with respect to standard l-ascarbic acid
Antimicrobial activity
Minimum inhibitory concentration
The antimicrobial activity of synthesized compounds revealed compound 1 as the most potent compound (pMIC 1.79, 1.79, 1.49, 1.49, 1.49 and 1.49 μM/mL for P. mirabilis, P. aeruginosa, S. aureus, E. coli, C. albicans and A. niger respectively) as compared to standard drugs taken. The compound 2 showed comparable activity against P. mirabilis (pMIC 1.14 μM/mL), C. albicans (pMIC 1.14 μM/mL) while the compound 3 also showed comparable activity against C. albicans (pMIC 1.16 μM/mL) as well A. niger (pMIC 1.46 μM/mL), likewise the compound 4 showed comparable activity against P. mirabilis (pMIC 1.18 μM/mL) as compared to the standard drugs streptomycin (pMIC 1.06, 1.36, 1.06 and 1.96 μM/mL for P. mirabilis, P. aeruginosa, S. aureus and E. coli, respectively), ciprofloxacin (pMIC 1.12, 1.42, 1.12 and 1.42 μM/mL for P. mirabilis, P. aeruginosa, S. aureus and E. coli, respectively), ampicillin (pMIC 1.14, 0.84, 0.84 and 1.74 μM/mL for P. mirabilis, P. aeruginosa, S. aureus and E. coli, respectively) and fluconazole (pMIC 1.08 and 1.38 μM/mL for C. albicans and A. niger, respectively). In general, the results of MIC studies (Table 2) revealed that the synthesized compounds have better anti bacterial and anti fungal potential as compared to standard drugs streptomycin, ciprofloxacin, ampicillin and fluconazole. The graphically representation of the pMIC values of test and standard compounds have been shown in Fig. 4.
Table 2.
pMIC values (μM/mL) of synthesized naringenin derivatives against different standard microbial strains
| Compound(s) | PMIC values in μM/mL | |||||
|---|---|---|---|---|---|---|
| P. mirabilis | P. aeruginosa | S. aureus | E. coli | C. albicans | A. niger | |
| Compound 1 | 1.79 | 1.79 | 1.49 | 1.49 | 1.49 | 1.49 |
| Compound 2 | 1.14 | 1.14 | 0.83 | 1.14 | 1.14 | 0.83 |
| Compound 3 | 0.86 | 1.16 | 0.86 | 0.86 | 1.16 | 1.46 |
| Compound 4 | 1.18 | 0.88 | 0.88 | 1.18 | 0.88 | 1.18 |
| Naringenin | < 0.73 | < 0.73 | < 0.73 | < 0.73 | < 0.73 | < 0.73 |
| Streptomycin | 1.06 | 1.36 | 1.06 | 1.96 | – | – |
| Ciprofloxacin | 1.12 | 1.42 | 1.12 | 1.42 | – | – |
| Ampicillin | 1.14 | 0.84 | 0.84 | 1.74 | – | – |
| Fluconazole | – | – | – | – | 1.08 | 1.38 |
Fig. 4.
Antimicrobial activity (pMIC in µM/mL) of synthesized naringenin derivatives against different microorganisms
Preservative efficacy study
The most active antimicrobial compound 1 was selected for the evaluation of its preservative efficacy. The results of preservative efficacy testing performed in triplicate and were reported as mean values in Table 3.
Table 3.
Log CFU/mL values of the selected compound 1
| Compound(s) | E. coli | P. aeruginosa | S. aureus | C. albicans | A. niger | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CFU/mL after days | 14 days | 28 days | 14 days | 28 days | 14 days | 28 days | 14 days | 28 days | 14 days | 28 days |
| Compound 1 | 3.190 ± 0.008 | 3.496 ± 0.12 | 3.306 ± 0.16 | 3.406 ± 0.016 | 3.486 ± 0.012 | 3.486 ± 0.012 | 3.200 ± 0.081 | 3.313 ± 0.016 | 3.306 ± 0.016 | 3.463 ± 0.020 |
| Sodium Benzoate | 3.213 ± 0.012 | 3.323 ± 0.24 | 3.282 ± 016 | 3.210 ± 0.037 | 3.863 ± 0.044 | 3.166 ± 0.047 | 3.076 ± 0.088 | 2.800 ± 0.081 | 3.166 ± 0.012 | 3.320 ± 0.014 |
| Propyl Paraben | 3.280 ± 0.57 | 3.246 ± 0.36 | 3.310 ± 0.016 | 3.306 ± 0.016 | 3.883 ± 0.023 | 3.516 ± 0.012 | 3.940 ± 0.028 | 3.530 ± 0.016 | 3.113 ± 0.065 | 3.403 ± 0.012 |
| Ethyl Paraben | 3.336 ± 0.020 | 3.090 ± 0.148 | 3.246 ± 0.36 | 3.340 ± 0.014 | 3.166 ± 0.047 | 3.210 ± 0.008 | 3.520 ± 0.014 | 3.200 ± 0.018 | 3.043 ± 0.041 | 3.300 ± 0.081 |
# Initial microbial count in inoculums 1 × 105–1 × 106
Compound 1 showed the values of log CFU/mL reduction within the prescribed limit and the results were comparable to that of the standard preservatives sodium benzoate, propyl paraben and methyl paraben. The preservative efficacy of compound 1 in White lotion USP and degree of microbial log reduction has been represented in Fig. 5.
Fig. 5.
Preservative efficacy of compound 1 in White lotion USP and degree of microbial log reduction
Structure activity relationship (SAR) studies
Design strategy of naringenin derivative for G-6-P inhibition and antioxidant activity has been represented in Fig. 6. The structure activity relationship of the synthesized naringenin derivatives with their antioxidant activity results were summarized as:
Substitution of naringenin with aliphatic amines produced biological activity but aromatic substitution showed greater activity than aliphatic i.e. compound 2 showed the lowest activity as compared to other.
Substitution with aromatic amine at para position increased the activity with increase in electronegativity i.e. compound 1 was more active than compound 3 and 4.
Replacement of para position with nitro group produced the highest activity i.e. compound 1 was most active in the series.
Exchange at para position produced more activity as compared to ortho position substitution.
Fig. 6.
Design strategy of Naringenin derivatives for G-6-P synthase inhibition and antioxidant activity
Molecular docking study
Molecular docking studies were carried out to identify the binding affinities and interaction between the inhibitors and pdb id 1moq of G-6-P synthase protein by using Glide software (Schrodinger Inc. U.S.A. Maestro version 11). Dock score and binding of compound 1, 2, 3 and 4 with G-6-P synthase have been shown in Table 4 and Fig. 7. After, docking results of compound 1 with G-6-P synthase protein suggested the formation of the hydrogen bond between NO2 and Thr 402. Additionally, the molecule has been stabilized by residues such as Ser 347, Thr 352, Ser 303, Gln 348, Ala 602, Asn 600 and Asp 354. The binding orientation of compound 2 within the catalytic site of G-6-P synthase exhibited backbone hydrogen bonding with Glu 488. The molecule is stabilized by residues such as Asp 354, Lys 603, Glu 488, Lys 487 and Ala 400. The compound 3 showed interaction with Arg 599. The molecule was enclosed by residues such as Val 399, Thr 302, Lys 487 and Leu 484. In compound 4 hydrogen bonding was shown by Thr 606 and ligand was entrapped by the residue sequence of Val 399, Lys 487, Cys 300 and Ser 328. Docking results of G-6-P synthase showed that the synthetic compounds have comparable docking score as compared to the standard drugs taken. All the ligands showed variable degrees of hydrogen bond interaction, hydrophobic interactions, electrostatic interactions, ionic interactions and π–π stacking with the various amino acid residues in the binding pockets of G-6-P synthase.
Table 4.
G-6-P synthase inhibition showed by synthesized naringenin derivatives
| S. no. | Compound(s) | Structure of G-6-P synthase inhibitors | Dock score |
|---|---|---|---|
| 1. | Compound 1 |
|
− 7.42 |
| 2. | Compound 2 |
|
− 4.29 |
| 3. | Compound 3 |
|
− 3.30 |
| 4. | Compound 4 |
|
− 4.02 |
| 5. | Naringenin |
|
− 6.36 |
| 6. | Standard | Streptomycin | − 5.795 |
| Ciprofloxacin | − 5.185 | ||
| Ampicillin | − 5.065 | ||
| Fluconazole | − 5.129 |
Fig. 7.
Binding of compounds 1, 2, 3 and 4 with G-6-P synthase
ADME study
The evaluation of different ADME parameters has been represented in Table 5. It was observed that all the synthesized compounds fulfilled the standard Rule of Five [43]. All the synthesized compounds qualified the conditions for various descriptors like LogP, HBA, HBD and MW. All these parameters were in suitable range for drug-like characteristics. In addition, according to Veber et al., 2002 for better bioavailability rotatable bonds should be ≤ 10 as the rotatable bonds in ligand impart elasticity [44]. The values of QPlogBB should be > 1.0 CNS active compounds and value < 1.0 CNS inactive compounds. QPPCaco cell permeability should be in a range from 4–70 [45–47]. In the present study, all the synthesized compounds exhibited a suitable drug-like profile.
Table 5.
ADMET profile of various newly synthesized naringenin derivatives
| Compound(s) | Mol. Wt. | No. of rotatable bond | DonorHB | AcceptHB | QPlogPo/w | QPlogBB | QPPMDCK | QPPCaco |
|---|---|---|---|---|---|---|---|---|
| Compound 1 | 392.10 | 5 | 5 | 4 | 2.084 | 0.081 | 0.053 | 1.877 |
| Compound 2 | 345.12 | 3 | 3 | 3 | 2.490 | 0.138 | 11.251 | 2.773 |
| Compound 3 | 365.11 | 4 | 2 | 4 | 4.29 | 2.445 | 0.282 | 10.982 |
| Compound 4 | 381.08 | 3 | 4 | 2 | 1.278 | 3.355 | 0.162 | 20.169 |
Conclusion
In conclusion, the above mentioned wet and dry laboratory studies highlight the underlying mechanism of G-6-P synthase inhibition. The rational development of inhibitors and specificity of naringenin derivatives to be discovered as the novel preservatives. Moreover, the synthesized compounds were also found as wonderful antioxidants towards DPPH with remarkable potential as compared to the reference compounds.
Experimental
Materials and methods
All the chemicals required for experiments were of analytical grade and were purchased from Loba Chemie (Mumbai, India), SRL (Mumbai, India), and Sigma Aldrich (Germany). Nutrient agar, nutrient broth, sabouraud dextrose agar and sabouraud dextrose broth required for antimicrobial and preservative efficacy were obtained from Hi-media Laboratories. Streptomycin, ciprofloxacin, ampicillin and fluconazole were obtained as gift sample from Belco Pharma, Bahadurgarh, India. Microbial strains S. aureus MTCC 3160, P. aeruginosa MTCC 1934, E. coli MTCC 45, C. albicans MTCC 183 and A. niger MTCC 282 strains were purchased from MTCC, Chandigarh, India. Chemical reactions were monitored by TLC on silica gel plates in iodine and UV chambers. Sonar melting point apparatus in open capillary tube was used for the recording of melting points. 1H NMR and 13C NMR spectra were confirmed in DMSO and deuterated CDCl3 on Bruker Avance II 400 NMR spectrometer at a frequency of 400 MHz downfield to tetramethyl silane standard. FTIR spectra were recorded on Perkin Elmer FTIR spectrophotometer with the help of KBr pellets technique. Waters Micromass Q-ToF Micro instrument was used for Mass spectrum recording.
General procedure for the synthesis of naringenin derivatives
Substituted aniline (0.01 mol) was taken in a round bottom flask, concentrated hydrochloric acid was added drop wise with continuous stirring. Equimolar concentration of naringenin (0.01 mol) was dissolved in ethanol (50 mL) and was re fluxed for 80-100 h on heating mantle. All the compounds in the series were synthesized according to the standard procedures as outlined in Scheme 1. Completion of reaction was monitored by TLC. Reaction mixture was concentrated and the precipitates formed were filtered off and dried. The crude product was recrystallized using alcohol which yielded the final compounds 1-4.
Spectral data
2-(4-hydroxyphenyl)-4-(4-nitrophenylimino) chroman-5, 7-diol
Rf TLC mobile phase: Chloroform: Acetone (8:5) = 0.63; Yield = 55%; M.P. = 190–192 °C; M.Wt. = 317.29; IR (KBr pellets) cm−1: 1081 (–C–O–C), 1156 (–C–C–), 1305 (–NO2), 1599 (–C=C–), 1632 (–C=N–), 2921 (–C–H–), 3479 (–OH–); 1H NMR (400 MHz, DMSO-d6) δ = 11.94 (s, 1H), 11.10 (s, 1H), 8.17 (d, J = 8.5 Hz, 2H), 8.04 (s, 1H), 7.29 (d, J = 9.3 Hz, 2H), 7.28 (d, J = 7.5 Hz, 2H), 6.80 (d, J = 7.3 Hz, 2H), 6.28 (s, 1H), 6.27 (s, 1H), 5.28 (t, J = 9.0 Hz, 1H), 3.15 (d, J = 7.3 Hz, 1H), 3.00 (d, J = 7.3 Hz, 1H); 13C NMR (400 MHz, CDCL3) δ = 166.11, 165.34, 163.98, 161.90, 153.71, 152.59, 146.42, 133.96, 131.14, 126.43, 125.72, 124.08, 123.64, 117.42, 103.05, 97.89, 95.36, 77.13, 38.79, 27.19, 22.70; MS ES + (ToF): m/z 392.10 [M++2]; CHNS: Calc (C12H16N2O2): C, 64.28; H, 4.11; N, 7.14; O, 24.47; Found C, 64.25; H, 4.14; N, 7.17; O, 24.44.
4-(1,3-dihydroxypropan-2-ylimino)-2-(4-hydroxyphenyl)chroman-5,7-diol
Rf TLC mobile phase: Chloroform: Acetone (8:5) = 0.66; Yield = 50%; M.P. = 173–175 °C; M.Wt. = 345.32; IR (KBr pellets) cm−1: 1074 (–C–O–C–), 1251 (–C–C–), 1513 (–C=C–), 1631 (–C=N–), 2831 (–C–H–), 3295 (–OH–); 1H NMR (400 MHz, DMSO-d6) δ = 11.60 (s, 1H), 11.10 (s, 1H), 8.04 (s, 1H), 7.28 (d, J = 7.2 Hz, 2H), 6.80 (d, J = 7.3 Hz, 2H), 6.28 (s, 1H), 6.24 (s, 1H), 5.25–5.24 (m, 1H), 3.95 (d, J = 8.1 Hz, 2H), 3.64 (q, J = 9.0 Hz, 2H), 3.50 (q, J = 9.6 Hz, 2H), 3.47–3.45 (m, 1H), 3.13 (d, J = 8.6 Hz, 1H), 2.86 (d, J = 8.6 Hz, 1H); 13C NMR (400 MHz, CDCL3) δ = 164.81, 162.47, 161.87, 161.58, 158.28, 130.84, 128.42, 115.98, 107.15, 103.33, 96.95, 78.20, 72.30, 63.75, 37.92, 27.60, 22.32, 14.16; MS ES + (ToF): m/z 345.12 [M++2]; CHNS: Calc (C18H19NO6): C, 62.60; H, 5.55; N, 4.06; O, 27.80; Found C, 62.63; H, 5.52; N, 4.09; O, 27.82.
4-(2-fluorophenylimino)-2-(4-hydroxyphenyl)chroman-5,7-diol
Rf TLC mobile phase: Chloroform: Acetone (8:5) = 0.64; Yield = 23%; M.P. = 165-167 °C; M.Wt. = 365.35; IR (KBr pellets) cm−1: 753 (–F–), 1082 (–C–O–C), 1241 (–C–C–), 1612 (–C=C–), 1632 (–C=N–), 2833 (–C–H–), 3350 (–OH–); 1H NMR (400 MHz, DMSO-d6) δ = 11.78 (s, 1H), 11.10 (s, 1H), 8.04 (s, 1H), 7.47 (d, J = 8.8 Hz, 1H), 7.31 (dt, J = 15.7, 8.4 Hz, 2H), 7.28–7.26 (m, 3H), 6.80 (d, J = 7.4 Hz, 2H), 6.31 (s, 1H), 6.28 (s, 1H), 5.33 (t, J = 8.5 Hz, 1H), 3.04 (d, J = 7.7 Hz, 1H), 2.92 (d, J = 8.5 Hz, 1H); 13C NMR (400 MHz, CDCL3) δ = 165.92, 165.91, 165.24, 163.73, 161.86, 132.64, 132.61, 126.96, 126.94, 126.50, 126.48, 125.25, 114.89, 114.86, 102.91, 97.83, 95.53, 72.64, 39.18, 20.46; MS ES+ (ToF): m/z 365.11 [M++2]; CHNS: Calc (C21H16FNO4): C, 69.04; H, 4.41; F, 5.20; N, 3.83; O, 17.52; Found C, 69.01; H, 4.44; F, 5.23; N, 3.84; O, 17.55.
4-(2-chlorophenylimino)-2-(4-hydroxyphenyl)chroman-5,7-diol
Rf TLC mobile phase: Chloroform: Acetone (8:5) = 0.66; Yield = 60%; M.P. = 155-157 °C; M.Wt. = 381.81; IR (KBr pellets) cm−1: 754 (–Cl–Str), 1062 (–C–O–), 1155 (–C–C–), 1602 (–C=C–) 1633 (–C=N–), 2834 (–C–H–), 3284 (–OH–); 1H NMR (400 MHz, DMSO-d6) δ = 11.78 (s, 1H), 11.10 (s, 1H), 8.04 (s, 1H), 7.55 (d, J = 6.9 Hz, 1H), 7.39 (t, J = 8.0 Hz, 1H), 7.28 (d, J = 8.0 Hz, 2H), 7.26 (d, J = 8.3 Hz, 1H), 7.17 (d, J = 7.6 Hz, 1H), 6.80 (d, J = 7.5 Hz, 2H), 6.19 (s, 1H), 6.17 (s, 1H), 5.34 (t, J = 8.9 Hz, 1H), 3.04 (d, J = 8.7 Hz, 1H), 2.94 (d, J = 9.1 Hz, 1H); 13C NMR (400 MHz, CDCL3) δ = 165.10, 163.08, 161.26, 159.81, 143.28, 139.86, 129.24, 128.98, 128.45, 128.28, 127.73, 127.42, 126.85, 124.29, 107.38, 102.08, 95.02, 76.72, 38.77, 17.39, 14.71; MS ES+ (ToF): m/z 381.08 [M++2]; CHNS: Calc (C21H16ClNO4): C, 66.06; H, 4.22; Cl, 9.29; N, 3.67; O, 16.76; Found C, C, 66.09; H, 4.20; Cl, 9.26; N, 3.69; O, 16.72.
Antioxidant activity
DPPH radical scavenging assay
Antioxidant activity of the synthesized compounds was determined by DPPH (2, 2-diphenyl-1-pycrilhydrazil hydrate) radical scavenging method. Briefly, 0.1 mM solution of DPPH in methyl alcohol was prepared and 1 mL of this solution was added to 3 mL of sample or standard with a concentration of 12.5, 25, 50, 75 and 100 μg/mL. Discolorations were measured at 517 nm after incubation for 30 min at 30 °C in the dark. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. The IC50 values of given samples were calculated by using formula:
Here, Ac was the absorbance of the control and As was the absorbance of the sample [48, 49].
Antimicrobial activity
Minimum inhibitory concentration (MIC)
The antimicrobial activity of the synthesized compounds were performed against S. aureus MTCC 3160, P. aeruginosa MTCC 1934, E. coli MTCC 45, P. mirabilis MTCC 3310, C. albicans MTCC 183 and A. niger MTCC 282 by using the tube dilution method [50]. Dilutions of test and standard compounds were prepared in double strength nutrient broth I.P. (bacteria) or sabouraud dextrose broth I.P. (fungi) [51, 52]. The slants of E. coli, P. aeruginosa, P. mirabilis and S. aureus were incubated at the 30-35 °C for 24 h. The slants of C. albicans were incubated at 20–25 °C for 48 h whereas; the slants of A. niger were incubated at 20–25 °C for 5 days. After the incubation period sterilized 0.9% NaCl solution was used to harvest the bacterial and fungal cultures from agar slant through proper shaking and then the suspensions of microorganisms were diluted with the sterile 0.9% NaCl solution to CFU count was adjusted by adjusting the density of microorganism suspension to that of 0.5 McFarland standards by adding distilled water. The number of CFU was determined by dilution pour-plate method [53]. A serial dilution of 50 µg/mL, 25 µg/mL, 12.5 µg/mL, 6.25 µg/mL, 3.12 µg/mL and 1.62 µg/mL was used for determination of MIC. The samples tubes were incubated at 37 °C for 24 h (bacteria), at 25 °C for 7 days (A. niger), and at 37 °C for 48 h (C. albicans) and the results were recorded in pMIC.
Preservative effectiveness
White lotion USP was utilized as the medium for the testing of preservative effectiveness.
Ingredients: Zinc sulfate 40 gm, sulfurated potash 40 gm and purified water q.s. to 1000 mL.
Firstly, zinc sulphate and sulfurated potash were dissolved in 450 mL of water separately and filtered. Then, sulfurated potash solution was added to zinc sulfate with stirring. At last, the required amount of water was added and mixed thoroughly and sterilized. For preservative efficacy testing, the White lotion USP was prepared using the equimolar amount of compounds 1-4 as novel preservatives by replacing sodium benzoate, methyl paraben and propyl paraben from the formula [54].
Challenge microorganism
Staphylococcus aureus MTCC 3160, P. aeruginosa MTCC 1934, E. coli MTCC 45, C. albicans MTCC 183 and A. niger MTCC 282 were used as common contaminants in the study as prescribed in USP for preservative efficacy testing in the pharmaceutical preparations.
Preparation of ioculums
The slants of E. coli, P. aeruginosa and S. aureus were incubated at the 30–35 °C for 24 h. The slants of C. albicans were incubated at 20–25 °C for 48 h whereas; the slants of A. niger were incubated at 20–25 °C for 5 days [55].
Test procedure
White lotions USP was added in final containers and were used in challenge test. The preparation was inoculated with 0.5–1% volume of microbial inoculum having a concentration of 1 × 105–1 × 106 CFU/mL [56]. Inoculated samples were mixed thoroughly to ensure homogeneous microorganism distribution and incubated. The CFU/mL of the product was determined at an interval of 0 days, 7 days, 14 days, 21 days, and 28 days in agar plates. Log CFU/mL of white lotion USP was calculated as not less than 2.0 log reductions from initial count at 14 days of incubation and no increase in CFU from 14 days count at 28 days in case of bacteria and no increase from the initial calculated count at 14 and 28 days [57].
In silico molecular docking studies
The Schrodinger, Inc. (New York, USA) software Maestro 11 was used for the computational calculations and docking studies. Laboratory for Enzyme Inhibition Studies, Department of Pharmaceutical Sciences, M.D. University, Rohtak, INDIA was used for the computational work. The receptor-grid files were generated by grid-receptor generation program Glide [58]. Grid-based ligand docking utilized the hierarchical sequence of filters to produce possible conformations of the ligand in the active-site region of the protein receptor. At this stage, crude score values and geometric filters were prepared out unlikely binding modes. The next filter phase involves a grid-based force field evaluation and refinement of docking experiments including torsional and rigid-body movements of the ligand [59]. The remained docking evaluations were subjected to a Monte Carlo procedure to minimize the energy score. A conjugate gradient minimization protocol was used in all calculations [60].
The energy differences were calculated using the equation:
Protein preparation
The X-ray protein structure co-ordinates of pdb id 1moq were downloaded from Protein Data Bank from www.rcbs.org [61] and were prepared with the help of the Schrödinger protein preparation wizard ‘Prepwiz’ [62, 63]. PDB id 1moq (resolution 1.57 A°) was selected on the basis of the lowest resolution and availability. All the waters molecules except metals co-ordinated and present between the ligand and protein were removed. The energy-restrained structure of the protein G-6-P synthase was constructed with the help of OPLS-2005 force field.
Ligand Preparation
The three-dimensional structural library was prepared using the Chemdraw software and proceeded for energy minimization using the LigPrep tool for the correction of coordinates, ionization, stereochemistry and tautomeric structure to gain the appropriate conformation through the addition or removal of hydrogen bonds. The partial charges were computed according to the OPLS-2005 force field (32 stereo isomers, tautomers and ionization) at biological pH and used for molecular docking studies.
Acknowledgements
The authors are highly thankful to the Head, Department of Pharmaceutical Sciences, M.D. University, Rohtak for providing essential facilities to accomplish this research study. The authors are also thankful to Dr. Vinod Devaraji Application Scientist Schrödinger LLC for his support to carry out the computational work.
Abbreviations
- ADMET
Absorption, distribution, metabolism, excretion & toxicity
- G-6-P Synthase
Glucosamine-6-phosphate synthase
- CYP P450
Cytochromes P450
- OATP1B1
Solute carrier organic anion transporter family member 1B1
- DHEAS
Dehydroepiandrosterone
- PPAR
Peroxisome proliferator-activated receptors
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- UDP-N-acetyl glucosamine
Uridine diphosphate N-acetylglucosamine
- FTIR
Fourier-transform infrared spectroscopy
- 1H NMR
Proton nuclear magnetic resonance
- 13C NMR
Carbon 13 nuclear magnetic resonance
- UV
Ultra violet
- TLC
Thin layer chromatography
- IC50
Inhibitory concentration
- MIC
Minimum inhibitory concentrations
- CFU
Colony forming unit
- HBA
Hydrogen bond acceptor
- HBD
Hydrogen bond donor
- MW
Molecular weight
- MTCC
Microbial type culture collection
- DMSO
Dimethyl sulfoxide
- BOD
Biological oxygen demand
- USP
United States Pharmacopoeia
- PDB ID
Protein Data Bank Identification
- OPLS
Optimized potential for liquid simulations
- Q.S.
Quantity sufficient
Authors’ contributions
The authors AL, SS and AK have designed, synthesized and carried out the work in equal contribution. All authors read and approved the final manuscript.
Funding
No funding received for this research work from outside sources.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zengin N, Yuzbasıoglu D, Unal F, Yilmaz S, Aksoy H. The evaluation of the genotoxicity of two food preservatives: sodium benzoate and potassium benzoate. Food Chem Toxicol. 2011;49(4):763–769. doi: 10.1016/j.fct.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 2.Reddy MV, Aruna G, Parameswari SA, Banu BH, Jayachandra PR. Estimated daily intake and exposure of sodium benzoate and potassium sorbate through food products in school children of Tirupati, India. Int J Pharm and Pharmaceut Sci. 2015;7(7):129–133. [Google Scholar]
- 3.Denyer SP, King RO (1988) Microbial quality assurance in pharmaceuticals, cosmetics and toiletries. Ed. Bloomfield SF, Baird R, Leak RE and Leech R. Chichester: Ellis Horwood 156–170
- 4.Pawar HA, Shenoy AV, Narawade PD, Soni PY, Shanbhag PP, Rajal VA. Preservatives from nature: a review. Int J Pharm Phytopharmacol Res. 2011;1(2):78–88. [Google Scholar]
- 5.The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers (2002) The determination of certain formaldehyde releasers in Cosmetic products, 1–9
- 6.Gue L. What’s Iinside? That counts a survey of toxic ingredients in our cosmetics. Vancouver: David Suzuki Foundation; 2010. pp. 1–26. [Google Scholar]
- 7.Sedlewicz LB (2011) Current trends in cosmetic preservation. Schulke inc
- 8.David Suzuki Foundation (2010) What’s inside? The “dirty dozen” ingredients investigated in the david Suzuki foundation survey of chemicals in cosmetics 1-19
- 9.Rastogi SC, Jensen GH, Petersen MR, Worsoe IM, Christoffersen C (1999) Preservatives in skin creams. Analytical Chemical Control of Chemical Substances and Chemical Preparations. National Environmental Research Institute. Denmark. NERI Technical Report No. 297:1–67
- 10.Darbre PD, Harvey PW. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxi. 2008;28(5):561–578. doi: 10.1002/jat.1358. [DOI] [PubMed] [Google Scholar]
- 11.Tavares RS, Martins FC, Oliveira PJ, Ramalho-Santos J, Peixoto FP. Parabens in male infertility-is there a mitochondrial connection. Reprod Toxicol. 2009;27(1):1–7. doi: 10.1016/j.reprotox.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Lundov MD, Moesby L, Zachariae C, Johansen JD. Contamination versus preservation of cosmetics: a review on legislation, usage, infections, and contact allergy. Cont Derm. 2009;60(2):70–80. doi: 10.1111/j.1600-0536.2008.01501.x. [DOI] [PubMed] [Google Scholar]
- 13.Satyendra RV, Vishnumurthy KA, Vagdevi HM, Rajesh KP, Manjunatha H, Shruthi A. In vitro antimicrobial and molecular docking of dichloro substituted benzoxazole derivatives. Med Chem Res. 2012;21(12):4193–4199. doi: 10.1016/j.ejmech.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Bearne SL, Blouin C. Inhibition of Escherichia coli glucosamine-6-phosphate synthase by reactive intermediate analogues, the role of the 2-amino function in catalysis. J Biol Chem. 2002;75(1):135–140. doi: 10.1074/jbc.275.1.135. [DOI] [PubMed] [Google Scholar]
- 15.Krishna PKV, Harish BGV, Kumar SSR, Kumar GK. Antibacterial activity of leaf extract of Delonix elata and molecular docking studies of luteolin. J Biochem Tech. 2012;3(5):S193–S197. [Google Scholar]
- 16.Fikrika H, Ambarsari L, Sumaryada T (2016) Molecular docking studies of catechin and its derivatives as anti-bacterial inhibitor for glucosamine-6-phosphate synthase, IOP Conf. Series. Earth and Environmental Science 31:012009
- 17.Deepa M, Devi PR, Alam Md A. In silico antimicrobial activity of active phyto compounds from the leaf extract of Vitex negundo linn. against glucosamine-6-phasphate synthase. World J Pharm Pharmaceut Sci. 2016;5(1):1144–1156. [Google Scholar]
- 18.Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bugianesi R, Catasta G, Spigno P, D’Uva A, Maiani G. Naringenin from cooked tomato paste is bioavailable in men. J Nutr. 2002;132:3349–3352. doi: 10.1093/jn/132.11.3349. [DOI] [PubMed] [Google Scholar]
- 20.Xu XH, Ma CM, Han YZ, Li Y, Liu C, Duan ZH, Wang HL, Liu DQ, Liu RH. Protective effect of naringenin on glutamate-induced neurotoxicity in cultured hippocampal cells. Arch Biol Sci Belgrade. 2015;67(2):639–646. [Google Scholar]
- 21.Tomas-Barberan FA, Clifford MN. Flavanones, chalcones and dihydrochalcones- nature, occurence and dietary burden. J Sci Food and Agric. 2000;80:1073–1080. [Google Scholar]
- 22.Kumar S, Tiku BA. Biochemical and molecular mechanisms of radio protective effects of naringenin, a phytochemical from citrus fruits. J Agric Food Chem. 2016;64(8):1676–1685. doi: 10.1021/acs.jafc.5b05067. [DOI] [PubMed] [Google Scholar]
- 23.Bear WL, Teel RW. Effects of citrus flavonoids on the mutagenicity of heterocyclic amines and on cytochrome P450 1A2 activity. Anticancer Res. 2000;20:3609–3614. [PubMed] [Google Scholar]
- 24.Ueng YF, Chang YL, Oda Y, Park SS, Liao JF, Lin MF. In vitro and in vivo effects of naringin on cytochrome P450-dependent monooxygenase in mouse liver. Life Sci. 1999;65:2591–2602. doi: 10.1016/s0024-3205(99)00528-7. [DOI] [PubMed] [Google Scholar]
- 25.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Harbi MS. Hepatoprotective effect and antioxidant capacity of naringenin on arsenic induced liver injury in rats. Int J Pharm Pharmaceut Sci. 2016;8(4):103–108. [Google Scholar]
- 27.Goldwasser J, Cohen PY, Yang E, Balaguer P, Yarmush ML, Nahmias Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: role of PPAR alpha, PPAR gamma and LXR alpha. PLoS ONE. 2010;5(8):e12399. doi: 10.1371/journal.pone.0012399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casas M, Prat G, Robledo P, Barbanoj M, Kulisevsky J, Jane F. Scopolamine prevents tolerance to the effects of caffeine on rotational behavior in 6-hydroxydopamine-denervated rats. Eur J Pharmacol. 1999;366(1):1–11. doi: 10.1016/s0014-2999(98)00911-x. [DOI] [PubMed] [Google Scholar]
- 29.Papiez MA. Influence of naringenin on the activity of enzymes participating in steroidogenesis in male rats. Annales Academiae Medicae Bialostocensis. 2004;49:120–122. [PubMed] [Google Scholar]
- 30.Wang X, Wolkoff AW, Morris ME. Flavonoids as a novel class of human organic anion-transporting polypeptide OATP1B1 (OATP-C) modulators. Drug Metab Dispos. 2005;33:1666–1672. doi: 10.1124/dmd.105.005926. [DOI] [PubMed] [Google Scholar]
- 31.Celiz G, Daz M, Audisio MC. Antibacterial activity of naringin derivatives against pathogenic strains. J Appl Microbiol. 2011;1(3):731–738. doi: 10.1111/j.1365-2672.2011.05070.x. [DOI] [PubMed] [Google Scholar]
- 32.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 33.Burda S, Oleszek W. Antioxidant and antiradical activity of flavonoids. J Agric Food Chem. 2001;49:2774–2779. doi: 10.1021/jf001413m. [DOI] [PubMed] [Google Scholar]
- 34.Kanno S, Tomizawa A, Hiura T, Osanai Y, Shouji A, Ujibe M, Ohtake T, Kimura K, Ishikawa M. Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice. Biol Pharm Bull. 2005;28:527–530. doi: 10.1248/bpb.28.527. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Jiang ZF, Pan Q, Song CY, Zhang WH. Anti-cancer effect of naringenin chalcone is mediated via the induction of autophagy, apoptosis and activation of PI3K/Akt signalling pathway. Bangladesh J Pharmacol. 2016;11:684–690. [Google Scholar]
- 36.Goldwasser J, Cohen PY, Lin W, Kitsberg D, Balaguer P, Polyak SJ. Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism. J Hepatol. 2011;55:963–971. doi: 10.1016/j.jhep.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du G, Jin L, Han X, Song Z, Zhang H, Liang W. Naringenin: a potential immune modulator for inhibiting lung fibrosis and metastasis. Cancer Res. 2009;69:3205–3321. doi: 10.1158/0008-5472.CAN-08-3393. [DOI] [PubMed] [Google Scholar]
- 38.Cui W, Zhang J, Wang Q, Gao K, Zhang W, Yang J. A novel synthesis of naringenin and related flavanones. J Chem Res. 2014;38:686–689. [Google Scholar]
- 39.Wang HK, Yeh CH, Iwamoto T, Satsu H, Shimizu M, Totsuka M. Dietary flavonoid naringenin induces regulatory T cells via an aryl hydrocarbon receptor mediated pathway. J Agric Food Chem. 2012;60(9):2171–2178. doi: 10.1021/jf204625y. [DOI] [PubMed] [Google Scholar]
- 40.Yilma AN, Singh SR, Morici L, Dennis VA. Flavonoid naringenin: a potential immunomodulator for Chlamydia trachomatis inflammation. Mediat Inflamm. 2013;2013:102457. doi: 10.1155/2013/102457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Wang JF, Jing D, Wei JY, Wang YN, Dai XH, Wang X, Luo MJ, Tan W, Deng XM, Niu XD. Inhibition of α-toxin production by sub inhibitory concentrations of naringenin controls Staphylococcus aureus pneumonia. Fitoterapia. 2013;86:92–99. doi: 10.1016/j.fitote.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Kriza A, Ignat I, Stanica N, Draghici C. Synthesis and characterization of Cu(II), Co(II) and Ni(II) complexes with Schiff bases derived from isatin. Rev Chim. 2011;62:696–701. [Google Scholar]
- 43.Hopkins AL, Groom CR. The drug gable genome. Nat Rev Drug Discov. 2002;1:727–733. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 44.Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 45.Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick HE, Groove R. MDCK (Madin Darby Canine Kidney) cells: a tool for membrane permeability screening. J Pharm Sci. 1999;88(1):28–33. doi: 10.1021/js9803205. [DOI] [PubMed] [Google Scholar]
- 46.Kulkarni A, Han Y, Hopfinger AJ. Predicting Caco-2 cell permeation coefficients of organic molecules using membrane-interaction QSAR analysis. J Chem Inf Comput Sci. 2002;42(2):331–342. doi: 10.1021/ci010108d. [DOI] [PubMed] [Google Scholar]
- 47.Teague SJ, Davis AM, Leeson PD, Opera TA. The design of lead like combinatorial libraries. Chem Int Ed Eng. 1999;38:3743–3748. doi: 10.1002/(SICI)1521-3773(19991216)38:24<3743::AID-ANIE3743>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 48.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. [Google Scholar]
- 49.Mohamed SK, Ahmed AAA, Yagi SM, Alla AEWHA. Antioxidant and antibacterial activities of total polyphenols isolated from pigmented sorghum (Sorghum bicolor) Lines. J Genet Eng Biotechn. 2009;7(1):51–58. [Google Scholar]
- 50.Cappucino JG, Sherman N. Microbiology - A laboratory manual. Boston: Addison Wesley; 1999. p. 263. [Google Scholar]
- 51.Indian Pharmacopoeia Vol-I (2007) The controller of publications, New Delhi 37
- 52.Kowser MM, Fatema N. Determination of MIC and MBC of selected azithromycin capsule commercially available in Bangladesh. The ORION Med J. 2009;32(1):619–620. [Google Scholar]
- 53.Andrews JM. Determination of minimum inhibitory concentration. J Antimicrob Chem. 2001;48(S1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 54.Narang R, Narasimhan B, Judge V, Ohlan S, Ohlan R. Evaluaton of preservatve effectiveness in an official antacid preparaton. Acta Pharmaceutica Sciencia. 2009;51:225–229. [Google Scholar]
- 55.Indian Pharmacopoeia (2010) Indian Pharmacopoeia Commission, Ghaziabad, India 27-28
- 56.Dafale NA, Semwal UP, Agarwal PK, Sharma P, Singh GN. Valuation of preservative effectiveness in antacid, cough syrup and ophthalmic solution by microbial challenge test. Int J Pharm. 2014;1(3):193–199. [Google Scholar]
- 57.The United States Pharmacopoeia . Antmicrobial effectiveness testing. Rockville: United States Pharmacopoeial Conventon Inc.; 2004. pp. 2148–2150. [Google Scholar]
- 58.Glide, version 6.6 (2015) Schrodinger, LLC, New York, America
- 59.Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 60.Godschalk F, Genheden S, Soderhjelm P, Ryde U. Comparison of MM/GBSA calculations based on explicit and implicit solvent simulations. Phy Chem. 2013;15:7731–7739. doi: 10.1039/c3cp00116d. [DOI] [PubMed] [Google Scholar]
- 61.Teplyakov A, Obmolova G, Badet-Denisot MA, Badet B, Polikarpov I. Involvement of the C terminus in intramolecular nitrogen channeling in glucosamine 6-phosphate synthase: evidence from a 1.6 Å crystal structure of the isomerase domain. Structure. 1998;6:1047–1055. doi: 10.1016/s0969-2126(98)00105-1. [DOI] [PubMed] [Google Scholar]
- 62.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE. Glide: a new approach for rapid, accurate docking and scoring. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 63.Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL. Glide: a new approach for rapid, accurate docking and scoring. Enrichment factors in database screening. J Med Chem. 2004;47:1750–1759. doi: 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.