Abstract
Introduction
Although peripheral blood analysis has become increasingly automated, microscopy is the only available method for the diagnosis of anisocytosis and poikilocytosis. The aims of the study were to compare RBC volume data obtained with two different analysers and by manual assessment of smears and to compare this data between dogs in various stages of heart failure secondary to degenerative mitral valvular (DMV) disease. The impact of diuretic administration on RBC morphology was also assessed.
Material and Methods
Sixty-eight dogs, 56 in different stages of DMV disease and 12 as healthy controls, were studied. Impedance and flow cytometry haematological analyses were performed for each animal. Additionally, two smears were prepared for manual analysis. RBC structure, staining, and size differences were recorded.
Results
There were no significant differences between the blood morphological parameters assessed using haematological analysers nor between dogs receiving diuretic treatment and those not treated. Based on the manual smear, significantly higher erythrocyte anisocytosis was observed in the dogs with symptomatic DMV disease than in the control group.
Conclusion
Haematological analysers based on impedance and flow cytometry provide reliable and comparable morphological results in dogs with heart failure. However, microscopic assessment of blood smears is a more reliable tool to detect erythrocyte anisocytosis.
Keywords: dog, anisocytosis, mitral valve disease, haematology
Introduction
Although peripheral blood analysis has become increasingly automatised, microscopic blood analysis remains an important tool in haematological diagnostics and is the only available method for the diagnosis of anisocytosis and poikilocytosis. Many morphological abnormalities can only be detected by the human eye. Hence, morphological blood analysis is offered by most laboratories, as it is a fast, cheap, and widely available technique. The assessment of red blood cell morphology provides valuable information on the functioning of many organs (such as the liver, spleen, and kidneys) and the causes of anaemia. Changes in erythrocyte morphology may be caused by dietary deficiencies or protein dysfunctions as well as disorders in the lipid composition of cell membranes (1, 5, 14, 20).
Mechanical factors may damage the cell membrane, leading to erythrocyte fragmentation (20). Red blood cell indicators, such as the mean corpuscular volume (MCV), mean corpuscular haemoglobin, mean cell haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), and red blood cell distribution width (RDW) provide information concerning the size and shape of erythrocytes. MCV is particularly useful in differentiation of anaemia. In dogs, the MCV ranges from 60 to 75 fL (17). An increased MCV value may suggest the presence of large erythrocytes (macrocytes), while a decreased MCV value suggests the presence of small red blood cells (microcytes). The presence of microcytes as well as macrocytes may occur with a normal MCV as this parameter describes the mean erythrocyte volume. MCH and MCHC determine the erythrocyte mass and haemoglobin concentration, respectively. Decreased values indicate hypochromasia, which is visible in a manual blood smear as central pallor within an erythrocyte. Increased MCH and MCHC values are observed in the presence of intra-erythrocyte Heinz bodies as well as in lipaemia and haemolysis. The RDW is a parameter that is routinely assessed by a haematological analyser and is a marker of anisocytosis, i.e. the concomitant occurrence of normocytes, macrocytes, and microcytes in a sample. The RDW is usually increased if there are iron, vitamin B12, or other nutritional deficiencies. It may also be greater following blood transfusions in inflammatory or renal disease or when excessive erythrocyte lysis occurs (11). In humans, RDW is an important diagnostic marker of heart disease (3, 4, 7) and correlates with patient morbidity and mortality (7). It is also considered a useful prognostic marker for predicting mortality in patients undergoing high-risk gastrointestinal surgery (15). Increased RDW has been observed in dogs with pulmonary hypertension (13). In dogs, there are breed differences in the erythrocyte volume. Greyhounds have a physiologically higher MCV compared to other breeds (16). Macrocytosis is often observed in miniature dog breeds and standard poodles (19), while microcytosis is seen in Asian dog breeds, such as the Akita or Shiba Inu (22). An increase in anisocytosis is observed in regenerative and non-regenerative anaemia caused by dyserithropoiesis (9).
Normal erythrocytes are the shape of a double-concave disc, the so-called discocyte, and are characterised by little variation in shape and size. Because of their size, the surface-to-volume ratio is greater than in spheroidal cells. This causes more effective gas exchange between cells and their surroundings and ensures high erythrocyte deformability. In effect, this allows erythrocytes to pass through narrow blood vessels and return to their correct shape. Erythrocytes adopt various shapes depending on the disease, these shapes being known as poikilocytes. They may be divided according to shape into echinocytes, acanthocytes, schistocytes, dacrocytes, codocytes, keratocytes, ovalocytes, stomatocytes, and spherocytes. Echinocytes contain numerous irregular protrusions on their cell surface. They are present in glomerulonephritis, uraemia, lymphomas, alkalosis, and following the administration of furosemide and doxorubicin. They may appear as artefacts if the collected blood volume is too low in relation to the EDTA content in the vial (5). Acanthocytes contain from two to ten characteristic projections, do not contain central pallor and are a non-specific sign of disorders in lipid metabolism. Their presence is usually associated with liver disorders, as well as DIC, angiosarcoma, and nephritis in dogs (5, 10). Schistocytes, which are fragments of erythrocytes, are characteristic of disseminated intravascular coagulation syndrome (DIC) and are observed in iron deficiency, chronic kidney failure, vasculitis, and heart valvular disease (5, 23). Dacrocytes, which are drop-shaped erythrocytes, occur in myeloproliferation and splenomegaly. They are also present in ruminants with iron deficiency (6). Codocytes, also known as target cells, have an excessive cell membrane compared to the cell haemoglobin content. They occur in association with liver damage, kidney disease, and bile duct and spleen disorders. They may be present in iron deficiency anaemia (5, 23). Keratocytes are characterised by the presence of pseudovacuoles in the periphery of the cell, which may form a cavity within the erythrocyte. They form as a result of cell membrane damage following doxorubicin administration and are also observed in iron deficiency anaemia and liver disease (6, 9). Ovalocytes are oval erythrocytes that may appear in the blood in the presence of kidney and liver diseases (6). The same shape usually describes the central pallor in stomatocytes, which may appear in increased erythropoiesis and a hereditary disease known as stomatocytosis (occurring in Alaskan dwarf Malamutes and miniature schnauzers) (6). Spherocytes, which are small cells without a central pallor, are characteristic of hereditary spherocytosis and autoimmune haemolytic anaemia (9).
Although manual smears and their analysis are frequently performed in dogs, the changes in the shape of erythrocytes in various stages of heart failure and caused by drugs such as diuretics have not been previously assessed. The primary aim of the study was to compare the morphological data on erythrocyte volume obtained with two different analysers and manual assessment of smears. The secondary aim of the study was to compare the erythrocyte volume between dogs in various stages of heart failure in the course of chronic mitral valve disease. In addition, the impact of diuretic administration on the erythrocyte morphology was assessed.
Material and Methods
The study was performed on 68 dogs (43 males and 25 females) with a mean age of 10.8 ± 2.5 years. Following the cardiac examination that consisted of learning the patient history and carrying out a physical examination, chest radiography, and transthoracic echocardiography, the dogs were assigned to study groups. Group A included healthy controls and comprised 12 dogs with a mean age of 8.2 ± 3.0 years. Two mixed-breed dogs, five Beagles, two Border collies, one Nova Scotia duck tolling retriever, one miniature schnauzer, and one Cavalier King Charles spaniel were the group’s members. Dogs with different stages of degenerative mitral valvular (DMV) disease were assigned according to the American College of Veterinary Internal Medicine (ACVIM) consensus guidelines classification for DMV disease into respective groups. Group B1 included asymptomatic dogs with no echocardiographic evidence of cardiac enlargement secondary to DMV disease as well as those with minimal cardiac remodelling not severe enough to qualify them for group B2. This group was made up of eight dogs and one bitch. Group B2 consisted of asymptomatic dogs with echocardiographic findings of heart enlargement: left atrium-to-aorta ratio of ≥1.6 and left ventricular internal diameter in diastole ≥1.7 normalised for body weight. The sex distribution was six dogs and four bitches. Overall for group B, the mean age was 10.9 ± 2.6 years and the breeds represented were mixed (six animals), dachshund (four), miniature schnauzer (three), shih-tzu (two), fox terrier, cocker spaniel, Yorkshire terrier, and Cavalier King Charles spaniel (one of each breed). Group C included dogs with past clinical signs of heart failure secondary to DMV disease and its size was 27 animals, these being 18 dogs and 9 bitches aged 11.4 ± 1.9 years. The group combined 12 mixed breed dogs, four miniature schnauzers, three Cavalier King Charles spaniels, three shih-tzu, two dachshunds, one medium poodle, one Pekingese, and one bull terrier. Group D included 10 canine patients with end-stage DMV disease with clinical signs of heart failure refractory to standard pharmacological treatment. Aged 11.8 ± 1.9 years as the mean, the animals had breed allegiances of mixed (four), Cavalier King Charles spaniel, bull terrier, Yorkshire terrier, miniature poodle, dachshund, and Pekingese (one of each). Dogs with acute heart failure were not included in this study (2, 12). Venous blood was collected from all the patients for a haematological examination. It was collected into two sample tubes with dipotassium ethylenediaminetetraacetic acid (K2EDTA) maintaining the correct anticoagulant-to-blood ratio. Next, the blood was gently mixed using a haematological mixer. Two haematological analyses on two different apparatuses were performed within 30 min of blood collection. The first analyser (Scil Vet Animal Blood Counter (ABC), Horiba ABX, Montpellier, France) exploits blood impedance and the second (LaserCyte Dx, IDEXX Laboratories, Westbrook, MN, USA) is based on flow cytometry. Two manual smears were prepared on glass slides immediately after the haematological analysis. They were dried for 12 h at room temperature in a dust-free room, and then stained using the Pappenheim method and the May–Grunwald and Giemsa stains. First, the samples were immersed in 1mL of an undiluted May– Grunwald solution for 5 min. Then, they were left in 1mL of pH 7.2 buffer for 3 min. Next, they were immersed in 1:9 diluted Giemsa solution and stained for 20 min. The dye was then rinsed off with water and the samples were left to dry at room temperature. The samples were initially examined under low magnification (10×) to determine the quality of the smear, the distribution of erythrocytes and leukocytes, the presence of cell aggregations, and free spaces between cells. The size, shape, and staining of the erythrocytes were assessed under high magnification (40×). Then, a single erythrocyte layer was examined under very high magnification (100×), where half of the cells were in contact with one another. The leukocytes were counted according to the Schilling differential cell count. The erythrocyte structure and staining and size differences were recorded. Anisocytosis and poikilocytosis were examined using a 4-grade scale proposed by John W. Harvey (10, 24) (Table 1). This scale provides a means of grading anisocytosis and poikilocytosis severity into four levels. A score of 1+ denotes finding the smallest number of cells with changed morphology and a score of 4+ signifies finding the largest number of these cells according to the stepped ranges presented in Table 1. If echinocytes were present, the smear was repeated and dried using two techniques. The first technique was immediate smear drying following its preparation. When the second technique was used, the smear was left to dry freely, which eliminated the possibility of incorrect smear preparation as a cause for echinocyte formation.
Table 1.
Results of semi-quantitative erythrocyte morphological analysis based on the number of abnormal cells in a single cell layer at 1,000 × magnification. Adapted from (24)
| 1+ | 2+ | 3+ | 4+ | |
| Anisocytosic cells | ||||
| Dog | 7–15 | 16–20 | 21–29 | > 30 |
| Polychromatic cells | ||||
| Dog | 2–7 | 8–14 | 15–29 | > 30 |
| Hypochromic cells | 1–10 | 11–50 | 51–200 | > 200 |
| Poikilocytosic cells | 3–10 | 11–50 | 51–200 | > 200 |
| Codocytes | 3–5 | 6–15 | 16–30 | > 30 |
| Spherocytes | 5–10 | 11–50 | 51–150 | > 150 |
| Echinocytes | 5–10 | 11–100 | 101–250 | > 250 |
| Other shapes* | 1–2 | 3–8 | 9–20 | > 20 |
*acanthocytes, schistocytes, keratocytes, ovalocytes, dacrocytes, drepanocytes, stomatocytes
The data underwent statistical analysis using the GraphPad Prism 5.0 package (GraphPad, San Diego, CA, USA). The Shapiro–Wilk normality test was performed to assess data normality. Two groups of related variables were assessed using the paired t-test, and any correlations between groups of variables were determined using the Pearson correlation coefficient. P ≤ 0.05 was considered statistically significant.
Results
Full blood and blood serum from the dogs were analysed. The results of complete blood counts in individual groups are presented in Table 2, and those of the semi-quantitative microscopic erythrocyte analysis are presented in Table 3.
Table 2.
Comparison of chosen haematological parameter results obtained using two different analysers Group
| Group | ||||||
|---|---|---|---|---|---|---|
| Analyser | ||||||
| A | B1 | B2 | Cc | Dc | ||
| WBC (K/μL) | Laser Cyte | 7.40 ± 2.61 | 7.31 ± 2.63 | 8.94 ± 2.59 | 8.62 ± 2.74 | 10.22 ± 2.58 |
| Vet ABC | 7.68 ± 2.41 | 7.39 ± 2.23 | 8.76 ± 2.84 | 9.12 ± 2.90 | 10.32 ± 2.87 | |
| RBC (M/μL) | Laser Cyte | 6.86 ± 0.63 | 7.30 ± 0.89 | 7.10 ± 0.90 | 6.99 ± 0.69 | 6.95 ± 1.23 |
| Vet ABC | 6.98 ± 0.59 | 7.51 ± 0.91 | 7.05 ± 0.55 | 7.14 ± 0.68 | 7.14 ± 1.27 | |
| HCT (%) | Laser Cyte | 49.04 ± 5.65 | 51.33 ± 5.11 | 50.19 ± 0.10 | 48.56 ± 4.29 | 47.9 ± 7.42 |
| Vet ABC | 49.75 ± 4.59 | 52.5 ± 623 | 48.9 ± 4.00 | 49.1 ± 4.66 | 48.6 ± 7.76 | |
| HGB (mmol/L) | Laser Cyte | 10.13 ± 0.87 | 10.77 ± 1.08 | 10.34 ± 0.72 | 10.21 ± 0.92 | 10.05 ± 1.30 |
| VetABC | 10.01 ± 1.07 | 11.39 ± 2.58 | 9.94 ± 0.77 | 10.09 ± 1.31 | 11.35 ± 3.66 | |
| MCH (fmol) | Laser Cyte | 1.47 ± 0.08 | 1.47 ± 0.11 | 1.46 ± 0.12 | 1.46 ± 0.10 | 1.46 ± 0.11 |
| Vet ABC | 1.43 ± 0.07 | 1.41 ± 0.06 | 1.41 ± 0.06 | 1.38 ± 0.06 | 1.38 ± 0.05 | |
| MCHC (mmol/L) | Laser Cyte | 20.73 ± 1.21 | 20.99 ± 1.48 | 20.71 ± 1.39 | 20.94 ± 1.20 | 21.23 ± 1.32 |
| Vet ABC | 19.91 ± 0.82 | 20.14 ± 0.32 | 20.19 ± 0.37 | 20.12 ± 0.41 | 20.17 ± 0.24 | |
| MCV (fL) | Laser Cyte | 71.36 ± 2.79 | 70.52 ± 3.72 | 70.74 ± 3.30 | 69.54 ± 4.82 | 68.62 ± 3.70 |
| Vet ABC | 71.4 ± 2.91 | 70 ± 2.62 | 69.8 ± 2.82 | 68.8 ± 2.35 | 68.2 ± 2.55 | |
| RDW (%) | Laser Cyte | 15.83 ± 0.93 | 15.59 ± 0.58 | 15.27 ± 0.29 | 15.59 ± 0.63 | 15.53 ± 0.41 |
| Vet ABC | 14.06 ± 0.72 | 13.75 ± 1.25 | 14.13 ± 0.49 | 14.54 ± 0.77 | 13.86 ± 1.20 | |
| PLT (K/μL) | Laser Cyte | 295.1 ± 73.96 | 326 ± 75.10 | 424.9 ± 95.08 | 420.64 ± 116.1 | 364.3 ± 142.24 |
| Vet ABC | 278.2 ± 68.82 | 289.7 ± 64.3 | 417.5 ± 150.2 | 387.96 ± 127.5 | 309.25 ± 86.30 | |
Presented as mean values ± standard deviation (SD)
Table 3.
Semi-quantitative analysis of erythrocyte morphology in five groups of dogs: A, B1, B2, C, and D
| Study group | Intensification of changes | Anisocytosis | Macrocytosis | Echinocytes | Codocytes | Dacrocytes | Keratocytes | Schistocytes | Acanthocytes | Ovalocytes | Stomatocytes | Crystallised haemoglobin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2/8*, ** | 2/8 | |||||||||||
| 1+ | (25%) | 2/8 | (25%) | |||||||||
| A | 2+ | (25%) | ||||||||||
| 1/8 | ||||||||||||
| n = 8 | 3+ | (12.5%) | ||||||||||
| 4+ | ||||||||||||
| 2/8 | 1/8 | 1/8 | 2/8 | |||||||||
| 1+ | (25%) | (12.5%) | (12.5%) | (25%) | ||||||||
| 2/8 | 1/8 | 1/8 | 1/8 | |||||||||
| B1 | 2+ | (25%) | (12.5%) | (12.5%) | (12.5%) | |||||||
| 1/8 | ||||||||||||
| n = 8 | 3+ | (12.5%) | ||||||||||
| 4+ | ||||||||||||
| 1+ | 3/10 | 1/10 | 1/10 | 3/10 | 1/10 | 2/10 | ||||||
| (30%) | (10%) | (10%) | (30%) | (10%) | (20%) | |||||||
| B2 | 2+ | 1(/10 10%) | ||||||||||
| n = 10 | 3+ | |||||||||||
| 4+ | ||||||||||||
| 1+ | 15/26 *, | 6/26 | 4/26 | 7/26 | 1/26 | 4/26 | 2/26 | 1/26 | 4/26 | 1/26 | 9/26 | |
| (57.7%) | (23%) | (15.4%) | (26.9%) | (3.8%) | (15.4%) | (7.7%) | (3.8%) | (15.4%) | (3.8%) | (34.6%) | ||
| 5/26 | 1/26 | 7/26 | 2/26 | 5/26 | ||||||||
| C | 2+ | (19.2%) | (3.8%) | (26.9%) | (7.7%) | (19.2%) | ||||||
| n = 26 | 3+ | 2/26 | 2/26 | |||||||||
| (7.7%) | (7.7%) | |||||||||||
| 4+ | ||||||||||||
| 3/8 **, | 1/8 | 2/8 | 1/8 | 2/8 | 2/8 | 1/8 | 2/8 | 2/8 | 3/8 | |||
| 1+ | (37.5%) | (12.5%) | (25%) | (12.5%) | (25%) | (25%) | (12.5%) | (25%) | (25%) | (37.5%) | ||
| 1/8 | 2/8 | |||||||||||
| D | 2+ | (12.5%) | (25%) | |||||||||
| n = 8 | 3+ | |||||||||||
| 4+ | ||||||||||||
There were no statistically significant differences in the morphological parameters between the studied groups (Table 2). In addition, there were no significant differences between the values of the blood morphological parameters assessed using the Laser Cyte IDEXX and the Vet ABC analyser. The MCV in dogs is much smaller than in humans, which may render changes in the RDW less evident (10). For this reason, it can be assumed that this marker has less diagnostic value in dogs. A positive correlation of the erythrocyte results obtained with both types of analysers for all the patients (r = 0.865, P < 0.0001), only healthy patients (r = 0.904, P < 0.0001), and only patients with heart failure (r = 0.841, P < 0.0001) was observed. There were no differences in the number of erythrocytes (P = 0.86) or RDW (P = 0.95) between dogs receiving furosemide and those with no diuretic treatment (P = 0.12).
Based on the manual smear, significantly higher erythrocyte anisocytosis was observed in dogs with symptomatic DMV disease (groups C and D) compared to the control group (P < 0.0001) (Table 3). In the control group, a 1+ degree of anisocytosis was observed in two out of eight specimens. In group C, 15/26 of the specimens were found to have a 1+ degree of anisocytosis, while 5/26 had a 2+ degree (Fig. 1). In group D, three out of eight dogs had 1+ anisocytosis and one dog had 2+. Macrocytosis was observed in smears from groups B2, C, and D. In group C, the presence of macrocytes was seen in 7/26 smears and was rated as 1+ in 6/26 cases and as 2+ in 1 case. No macrocytes were found in groups A (control) or B1. Echinocytes were present in all groups, their highest percentage occurring in group C, where 7/26 specimens where classified as 2+ (Fig. 2).
Figs 1–6.
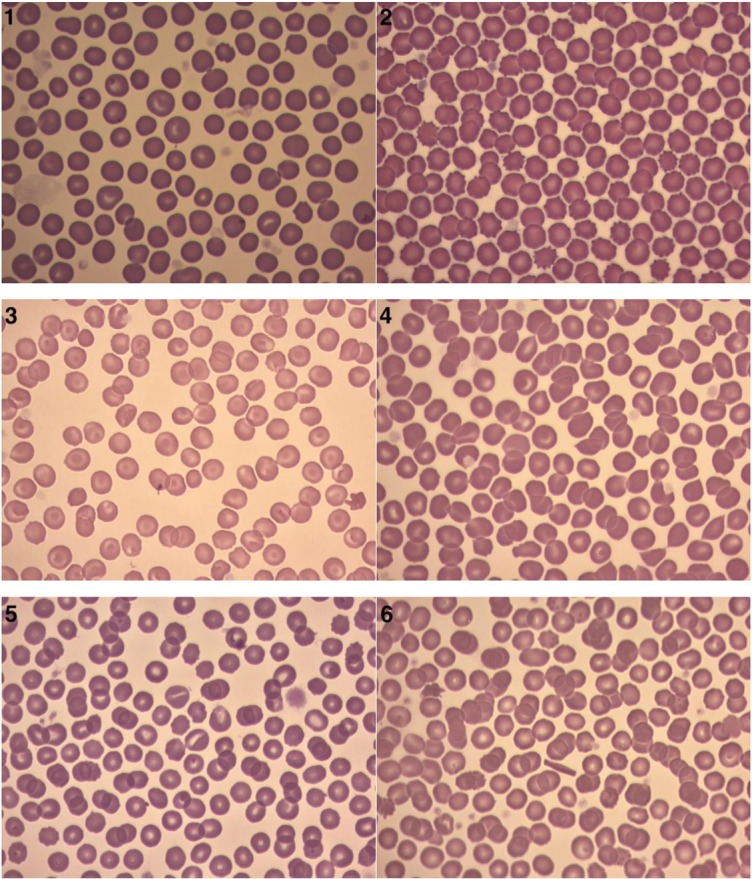
Sample blood smears representing: 1 – anisocytosis; 2 – echinocytes; 3 – codocytes; 4 – dacryocytes; 5 – stomatocytes; and 6 – crystallised haemoglobin
Codocytes were present in one out of eight dogs from the control group, and their presence was assessed as 3+. In the remaining groups B1, B2, C, and D, codocytes were observed in more than one smear (Fig. 3). Codocytes were found in 9/26 smears from group C to an extent scored as 1+. Various amounts of dacrocytes were found in groups B1, C, and D (Fig. 4). A 2+ intensity of dacrocytes was observed in 5/26 smears in group C. No dacrocytes were found in the control group or in group B1. Keratocytes were found in all groups of dogs with DMV disease (B, C, D) but not in the control group. The largest percentage content of karyocytes was observed in groups C (4/26 specimens) and D (2/8 specimens). Schistocytes, ovalocytes, and stomatocytes (Fig. 5) were only detected in groups C and D. Acanthocytes were present in one smear from group C.
A larger number of erythrocytes with crystallised haemoglobin was found in animals with a high degree of heart failure than in the dogs without heart failure. Crystallised haemoglobin was present in 17% of the specimens from group C, 22% and 20% of the specimens in groups B1 and B2, respectively, 33% of the specimens in group C, and 37% of the specimens in group D (Fig. 6).
Discussion
DMV disease predominantly affects miniature and small breed dogs over eight years of age and is found to occur more frequently in males than females, which is why males were in the majority in the present study. All the dogs were treated according to the 2019 ACVIM guidelines (12).
In the present study, two analysers using different measurement methods were used to assess blood morphology. The Scil Vet ABC uses the impedance method for analysis, which means that it counts most erythrocyte components and platelets based on their volumes and a change in current conductivity. The second analyser, the Laser Cyte flow cytometer, uses a combination of optic detection and flow fluorescence to perform measurements. This technique assesses not only cell size, but also the intracellular structure, i.e., the size and shape of the nucleus and its segmentation and granulation, which enables more accurate cell identification and improves result reliability. The results of the morphological analyses performed by the two analysers did not differ significantly.
The RDW results are interesting. RDW is a parameter that is routinely assessed by haematological analysers and is a marker of anisocytosis, i.e., the simultaneous occurrence of normocytes, macrocytes, and microcytes. In humans, the mean erythrocyte volume ranges from 80 to 96 fL and is much larger than in dogs (60–75 fL). The presence of anisocytosis in humans may be associated with larger differences in erythrocyte size than in dogs. Hence, it may be assumed that commercial analysers accurately calculate RDW and differences in cell sizes in humans.
This may explain why RDW in humans is of material importance in heart disease, while it remains a controversial parameter in dogs (3, 4, 7, 8, 13, 21). Some authors observed changes in the RDW in dogs with DMV disease without pulmonary hypertension, DMV disease with postcapillary pulmonary hypertension and DMV disease with precapillary pulmonary hypertension. They found that the RDW was increased in dogs with pre- and post-capillary pulmonary hypertension as well as severe pulmonary hypertension compared to the control groups of healthy dogs (13, 21). Due to the discrepancies concerning the diagnostic value of an automatically determined RDW, the authors chose to manually assess the blood smears, which provided additional information. While the RDW results obtained from both analysers were within the reference range for dogs, the manual microscopic analysis of the blood smears revealed the presence of erythrocyte anisocytosis. In the dogs with advanced heart failure, the degree of poikilocytosis was significantly greater. Codocytes and dacrocytes were observed in the dogs with heart failure. Moreover, schistocytes, ovalocytes, and stomatocytes were found in the manual blood smears. In addition, crystallised haemoglobin was discovered in the dogs with advanced heart failure. Echinocytes may appear following furosemide administration. However, in the present study, no association was found between poikilocytosis and the use of diuretics. The presence of echinocytes in all studied groups is most likely associated with the presence of the EDTA anticoagulant, which may cause cell contraction in vivo (10).
In healthy animals, mainly mature and well-formed erythrocytes called normocytes enter the bloodstream. However, in certain pathological conditions, bone marrow may release cells with abnormal morphology. The presence of poikilocytosis and anisocytosis in dogs with heart failure may therefore become a helpful diagnostic marker. The assessment of the manual smear can be performed quickly and cost-effectively, which should also prompt the routine performance of this examination when heart failure is suspected. It is important to note that a complete blood count with the use of haematological analysers and microscopic evaluation has its limitations. For proper evaluation of blood smears it is necessary to prepare them within an hour of collection. The result of smear evaluation is influenced by four components: the quality of the smear, staining, the quality of the microscope, and the experience of the examiner. In the case of haematological analysers, the result is affected by haemolysis and lipaemia, which distorts the results for haemoglobin, MCH, and MCHC. Platelet aggregates affect proper analysis of the platelet count, falsely increasing the mean corpuscular volume of erythrocytes. An accurate MCV determination is crucial for RDW measurement, as it is calculated from MCV. For this reason, regardless of the method used for automatic measurement of complete blood count, verification of the result should be based on manual evaluation of the blood smear.
In conclusion, haematological blood analysers based on impedance and flow cytometry provide reliable and comparable results of blood morphology in dogs with heart failure. The red blood cell distribution width measured automatically did not differ between the dogs with mitral valve regurgitation and healthy controls, while erythrocyte poikilocytosis increased with the severity of heart failure. Hence, the microscopic assessment of blood smears is a more reliable tool to detect erythrocyte anisocytosis.
Footnotes
Conflict of Interest
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This research was supported by statutory funding for research and development from the Polish Ministry of Science and Higher Education assigned to the Faculty of Veterinary Medicine, Wroclaw University of Environmental and Life Sciences.
Animal Rights Statement: None required.
References
- 1.An X.U., Mohandas N.. Disorders of red cell membrane. Br J Haematol. 2008;141:367–375. doi: 10.1111/j.1365-2141.2008.07091.x. [DOI] [PubMed] [Google Scholar]
- 2.Atkins C., Bonagura J., Ettinger S., Fox P., Gordon S., Häggström J., Hamlin R., Keene B., Luis-Fuentes V., Stepien R.. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009;23:1142–1150. doi: 10.1111/j.1939-1676.2009.0392.x. [DOI] [PubMed] [Google Scholar]
- 3.Aung N., Ling H.Z., Cheng A.S., Aggarwal S., Flint J., Mendonca M., Rashid M., Kang S., Weissert S., Coats C.J., Richards T., Thomas M., Woldman S., Okonko D.O.. Expansion of the red cell distribution width and evolving iron deficiency as predictors of poor outcome in chronic heart failure. Int J Cardiol. 2013;168:1997–2002. doi: 10.1016/j.ijcard.2012.12.091. [DOI] [PubMed] [Google Scholar]
- 4.Campora C., Freeman K.P., Lewis F.I., Gibson G., Sacchini F., Sanchez-Vazquez M.J., Rainer C., Kawanishi D.T., Chandraratna P.A., Bauersachs R.M., Reid C.L., Rahimtoola S.H., Meiselman H.J.. Changes in blood rheology in patients with stable angina pectoris as a result of coronary artery disease. Circulation. 1987;76:15–20. doi: 10.1161/01.cir.76.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Daleke D.L.. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–242. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Degórski A., Winnicka A. Atlas hematologiczny psów i kotów. Galaktyka; Łódź: 2013. pp. 24–68. [Google Scholar]
- 7.Felker G.M., Allen L.A., Pocock S.J., Shaw L.K., McMurray J.J., Pfeffer M.A., Swedberg K., Wang D., Yusuf S., Michelson E.L., Granger C.B.. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50:40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 8.Guglielmini C., Poser H., Pria A.D., Drigo M., Mazzotta E., Berlanda M., Luciani A.. Red blood cell distribution width in dogs with chronic degenerative valvular disease. J Am Vet Med Assoc. 2013;243:858–862. doi: 10.2460/javma.243.6.858. [DOI] [PubMed] [Google Scholar]
- 9.Harvey J.W. Atlas of Veterinary Hematology: Blood and Bone Marrow of Domestic Animals. W.B Saunders; Philadelphia: 2001. [Google Scholar]
- 10.Harvey J.W. Veterinary hematology: a diagnostic guide and color atlas. Elsevier/Saunders; St Louis: 2012. [Google Scholar]
- 11.Hellhammer K., Zeus T., Verde P.E., Veulemanns V., Kahlstadt L., Wolff G., Erkens R., Westenfeld R., Navarese E.P., Merx M.W., Rassaf T., Kelm M.. Red cell distribution width in anemic patients undergoing transcatheter aortic valve implantation. World J Cardiol. 2016;8:220–230. doi: 10.4330/wjc.v8.i2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keene B.W., Atkins C.E., Bonagura J.D., Fox P.R., Häggström J., Fuentes V.L., Oyama M.A., Rush J.E., Stepien R., Uechi M.. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2019;33:1127–1140. doi: 10.1111/jvim.15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzotta E., Guglielmini C., Menciotti G., Contiero B., Baron Toaldo M., Berlanda M., Poser H.. Red blood cell distribution width, hematology, and serum biochemistry in dogs with echocardiographically estimated precapillary and postcapillary pulmonary arterial hypertension. Am Vet Med Assoc. 2013;243:858–862. doi: 10.1111/jvim.14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasini E.M, Lutz H.U., Mann M., Thomas A.W.. Red blood cell (RBC) membrane proteomics – Part I: proteomics and RBC physiology. J Proteomics. 2010;73:403–420. doi: 10.1016/j.jprot.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Pluta M., Klocek T., Krzych Ł.J.. Diagnostic accuracy of red blood cell distribution width in predicting in-hospital mortality in patients undergoing high-risk gastrointestinal surgery. Anaesthesiol Intensive Ther. 2018;50:277–282. doi: 10.5603/AIT.a2018.0037. [DOI] [PubMed] [Google Scholar]
- 16.Porter J.A. Jr, Canaday W.R. Jr. Hematologic values in mongrel and greyhound dogs being screened for research use. J Am Vet Med Assoc. 1971;159:1603–1606. [PubMed] [Google Scholar]
- 17.Rebar A.H. Hemogram Interpretation for Dogs and Cats. Gloyd Group Inc; Wilmington: 1998. [Google Scholar]
- 18.Savarese A., Probo M., Locatelli C., Gazzonis A.L., Zanzani S.A., Traini G., Vitiello T., Brambilla P.G.. Iron status in dogs with myxomatous mitral valve disease. Pol J Vet Sci. 2018;21:507–515. doi: 10.24425/122625. [DOI] [PubMed] [Google Scholar]
- 19.Schalm O.W.. Erythrocyte macrocytosis in miniature and toy poodles. Canine Pract. 1976;3:55–57. [Google Scholar]
- 20.Spychalska J.. Red blood cell membranopathies – pathogenesis, clinical presentation, and diagnosis. Hematologia. 2012;3:81–119. [Google Scholar]
- 21.Swann J.W., Sudunagunta S., Covey H.L., English K., Hendricks A., Connolly D.J.. Evaluation of red cell distribution width in dogs with pulmonary hypertension. J Vet Cardiol. 2014;16:227–235. doi: 10.1016/j.jvc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Tanabe Y.. Phylogenetic studies of dogs with emphasis on Japanese and Asian breeds. Proc Japan Acad. 2006;82:375–387. doi: 10.2183/pjab.82.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thrall M.A. Thrall M.A., Baker D.C., Campbell T.W. Veterinary Hematology and Clinical Chemistry. Blackwell Publishing; Ames: 2006. Erythrocyte morphology; pp. 69–82. edited by. [Google Scholar]
- 24.Weiss D.J.. Uniform evaluation and semiquantitative reporting of hematologic data in veterinary laboratories. Vet Clin Pathol. 1984;13:27–31. doi: 10.1111/j.1939-165x.1984.tb00836.x. [DOI] [PubMed] [Google Scholar]


