Abstract
Background:
Nonalcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease (CLD). NAFLD is also related to obesity and metabolic syndromes, which are common in Saudi Arabia. However, it is yet unclear what proportion of CLD cases is because of NAFLD in Saudi Arabia.
Objective:
To investigate the prevalence and clinical characteristics of NAFLD among patients with CLD in Saudi Arabia.
Materials and Methods:
This retrospective study included all patients with any CLD who had successfully undergone transient elastography (FibroScan) examination at King Abdulaziz University Hospital, Jeddah, Saudi Arabia, between April 2015 and April 2018. These CLD patients were then grouped as NAFLD and non-NAFLD patients. Serum hemoglobin, alanine aminotransferase, gamma-glutamyl transferase, albumin and bilirubin levels; platelet count and international normalized ratio within 1 month after the FibroScan examination were assessed. For NAFLD patients, glycated hemoglobin levels and abdominal ultrasound examination results were also assessed. Statistical analysis was carried out using Student’s t-test and linear regression.
Results:
The prevalence of NAFLD among CLD patients was 22.5% (111 of 494 CLD patients), and it was the third most common CLD after chronic hepatitis B and C. Compared with non-NAFLD patients, NAFLD patients had significantly higher mean age (53.65 ± 12.7 vs. 48.07 ± 14.6 years; P < 0.001), mean serum alanine aminotransferase level (61.84 vs. 50.23 IU/L; P < 0.001) and mean controlled attenuation parameter (297.83 vs. 238.41; P < 0.001). NAFLD patients also had a higher rate of ultrasound-detected features of cirrhosis (16.2% vs. 3.7%, P < 0.001), but there was no significant difference in fibrosis severity. In addition, their mean glycated hemoglobin level (6.85) was elevated (range: 5–13). Age and platelet count were significantly correlated with presence of cirrhosis.
Conclusion:
NAFLD is the third most common CLD in Western Saudi Arabia, and it is associated with older age and metabolic syndromes, with one-third of the patients having advanced fibrosis or cirrhosis.
Keywords: Chronic liver disease, diabetes, nonalcoholic fatty liver disease, obesity, Saudi Arabia
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is now a leading cause of chronic liver disease (CLD).[1] In fact, it is the most common cause of abnormal liver enzyme levels in both Eastern and Western countries and has a global prevalence of 25%, with Middle East having the highest prevalence.[1,2,3] NAFLD is a spectrum of disease that varies from fatty liver changes on abdominal imaging with normal serum transaminases to nonalcoholic steatohepatitis, which is associated with elevated serum transaminase levels and advanced liver cirrhosis in extreme cases.[4,5] NAFLD is related to metabolic syndromes, obesity and diabetes mellitus (DM).[6,7] Obesity is the main risk factor for metabolic syndrome; in the Middle East, more than one-third of the population is obese.[8,9] Similarly, the prevalence of DM is progressively increasing to alarming figures in the Saudi population.[10,11]
In the general population of Saudi Arabia, the prevalence of NAFLD has been reported as 16.6%.[12] In one study, 8% of liver donors were found to have steatosis, and 24.9% were either obese or had DM, resulting in donor rejection.[13] Moreover, the prevalence of NAFLD among Saudi diabetic patients was reported to be as high as 55%.[14] However, to the best of the authors’ knowledge, in Saudi Arabia, it is not yet known what proportion of CLDs are because of NAFLD. Therefore, this study was conducted at King Abdulaziz University Hospital (KAUH), the largest academic medical center in the Western region of Saudi Arabia, with the aim of investigating the prevalence and clinical characteristics of NAFLD among patients with CLD.
MATERIALS AND METHODS
Study design
This study was a retrospective chart review of all patients with CLDs (including NAFLD) who had undergone transient elastography (FibroScan) examination at KAUH between April 2015 and April 2018. The study was conducted after obtaining an ethical approval from the Ethical Committee of the Faculty of Medicine at King Abdulaziz University (Ref no. 358-14) on January 11, 2015.
Study population
Patients who had CLD and underwent an assessment for liver fibrosis using transient elastography (FibroScan) during the study period were screened. Only patients with a successful examination, defined as 10 successful readings with an interquartile range of ≤30% and at least a 70% success rate, were included in this study. The exclusion criterion was patients with incomplete or unavailable laboratory results within 1 month from the time of the FibroScan examination. The patients were divided into two groups: NAFLD and non-NAFLD (i.e.,, patients with all other CLDs). KAUH is a tertiary academic medical center that receives referral from different parts of the Western region of Saudi Arabia, and thus this study cohort is expected to be reflective of the pattern of CLD in the region.
Definitions
NAFLD was diagnosed based on fatty changes on abdominal imaging studies, elevated or normal serum alanine aminotransferase (ALT) levels and the absence of alcohol intake and other causes of liver disease. Viral hepatitis, hepatitis B virus (HBV) and hepatitis C virus (HCV) were diagnosed according to the presence of serological markers for HBV or HCV, or both, and positive viral DNA for HBV and RNA for HCV, according to the TaqMan polymerase chain reaction method. Autoimmune hepatitis (AIH) was diagnosed based on the original and simplified criteria for diagnosing AIH.[15,16] Primary biliary cholangitis was diagnosed according to the patient’s clinical presentation and a positive antimitochondrial antibody result. Overlap syndromes were diagnosed based on the presence of overlapping features of two autoimmune liver diseases. Drug-induced chronic liver injury was diagnosed based on persistent elevation of liver enzymes at 3 months after stopping the causative drug and evidence of liver fibrosis on the FibroScan examination.
Data collection
Patients’ data were collected from both the gastroenterology/hepatology unit database and the hospital’s information system. For all patients, demographic data, including age, sex and nationality, and data regarding the history of DM, hypertension (HTN) and hyperlipidemia were collected. Further, the body mass index (BMI) of all NAFLD patients was available, as it is routinely measured before FibroScan examination at KAUH, and was included for analysis.
Regarding laboratory data, the authors obtained the complete blood count results for the hemoglobin, platelets count, serum ALT, serum gamma-glutamyl transferase, serum albumin, serum bilirubin and international normalized ratio within 1 month of the FibroScan examination. For NAFLD patients, the glycated hemoglobin (HbA1c) level was obtained within 6 weeks of the FibroScan examination and the lipid profile was checked for fasting triglycerides levels within 1 month of the FibroScan examination.
Results of the abdominal ultrasound examination were obtained for all patients. Cirrhosis on an ultrasonogram was defined as the presence of evidence of portal HTN collaterals, splenomegaly or both. Transient elastography was performed using the 2005 FibroScan (Echosens, Paris, France). All examinations were conducted by an expert FibroScan technician who performs all cases in KAUH. All results were revised and verified by one of the two senior hepatologists in the unit.
Statistical analysis
Descriptive analysis was used to obtain the frequencies, means and standard deviations. Student’s t-test was used to compare the means between the NAFLD and non-NAFLD groups. Linear regression analysis was used for different variables to predict factors that were associated with cirrhosis in each group separately. A backward linear regression analysis was carried out to predict persistent factors associated with radiological evidence of cirrhosis. All statistical analyses were conducted using SPSS version 22 (IBM Corp., Armonk, NY, USA). P ≤ 0.005 was considered statistically significant.
RESULTS
After excluding 15 patients based on the exclusion criterion, 494 CLD patients were included in this study. Of these, 22.5% (111) had NAFLD, making it the third most common CLD after chronic hepatitis B (CHB) and chronic hepatitis C (CHC) [Table 1]. The age of NAFLD patients ranged from 22 to 86 years, and the mean age was significantly higher in the NAFLD group than in the non-NAFLD group (53.65 ± 12.7 vs. 48.07 ± 14.6 years; P < 0.001). In addition, there were significantly higher numbers of patients with DM, HTN and hyperlipidemia in the NAFLD group than in the non-NAFLD group [Table 2].
Table 1.
Distribution of patients according to the cause of chronic liver disease (n = 494)
| Diagnosis | Number of patients (%) |
|---|---|
| NAFLD | 111 (22.5) |
| CHB | 166 (33.6) |
| CHC | 153 (31) |
| AIH | 30 (6.1) |
| Methotrexate | 19 (3.8) |
| Chronic cholestasis of unknown etiology | 1 (0.2) |
| Overlap syndrome | 4 (0.8) |
| PBC | 3 (0.6) |
| CHB + CHC | 6 (1.2) |
| AIH due to DILI | 1 (0.2) |
NAFLD – Nonalcoholic fatty liver disease; CHB – Chronic hepatitis B; CHC – Chronic hepatitis C; PBC – Primary biliary cholangitis; AIH – Autoimmune hepatitis; DILI – Drug-induced liver injury
Table 2.
Demographic and clinical data for NAFLD and non-NAFLD patients
| Variable | Number of NAFLD patients (%) | Number of Non-NAFLD patients (%) | P |
|---|---|---|---|
| Nationality | |||
| Saudi | 74 | 225 | 0.081 |
| Non-Saudi | 37 | 158 | |
| Sex | |||
| Male | 51 | 172 | 0.465 |
| Female | 60 | 211 | |
| Age years | 53.65±12.7 | 48.07±14.6 | <0.001 |
| DM | 54 (48.6) | 77 (20.1) | <0.001 |
| HTN | 46 (41.4) | 65 (17) | <0.001 |
| Fatty liver on ultrasound | 64 (57.7) | 16 (4.1) | <0.001 |
| Hyperlipidemia | 29 (26.1) | 27 (7) | <0.001 |
| Cirrhosis on ultrasound | 18 (16.2) | 14 (3.7) | <0.001 |
DM – Diabetes mellitus; HTN – Hypertension; NAFLD – Nonalcoholic fatty liver disease
The NAFLD group had a significantly higher mean serum ALT level and a higher controlled attenuation parameter (CAP) than the non-NAFLD group [Table 3]. Moreover, CAP showed AUROC of 0.744 in the detection of steatosis in NAFLD [Figure 1]. The NAFLD group tended to have more severe fibrosis than the non-NAFLD group, but this difference was not statistically significant (P = 0.31) [Table 4]. The serum ALT level was significantly lower in 18 patients who had evidence of cirrhosis on abdominal ultrasound examination than in those without such evidence (40.4 ± 19 IU/L vs. 71 ± 47.5 IU/L, respectively; P < 0.001). In the NAFLD group, patients with mild fibrosis (F1) had significantly higher serum triglyceride level (mean 2.45 ± 1.2) than those with cirrhosis (F4) (mean 1.38 ± 0.68; P = 0.012). However, there was no difference in the serum triglyceride level between the intermediate stages of fibrosis. Finally, the HbA1c level was significantly lower in patients without fibrosis (F0) than in those with cirrhosis (F4) (mean 6.1 ± 1.4 vs. 7.2 ± 2.5, respectively; P = 0.026). Table 4 shows the number and percentage of patients with F0 and F4.
Table 3.
Comparison of body mass index and laboratory results between NAFLD and non-NAFLD patients
| Variable (normal range) | Category | Mean±SD | P |
|---|---|---|---|
| BMI (18.5-24.9) | Non-NAFLD | 29.52±15.516 | 0.42 |
| NAFLD | 30.62±8.682 | ||
| Hg (12-15 g/dL) | Non-NAFLD | 12.79±2.633 | 0.032 |
| NAFLD | 13.33±2.149 | ||
| Platelets (150-400 K/uL) | Non-NAFLD | 255.67±122.668 | 0.866 |
| NAFLD | 257.65±102.321 | ||
| Albumin (35-40 g/L) | Non-NAFLD | 34.40±6.178 | 0.065 |
| NAFLD | 35.60±5.886 | ||
| ALT (30-65 U/L) | Non-NAFLD | 50.23±73.671 | 0.041 |
| NAFLD | 61.84±43.498 | ||
| GGT (5-85 U/L) | Non-NAFLD | 80.91±233.541 | 0.747 |
| NAFLD | 85.97±100.361 | ||
| Bilirubin (1-17 umol/L) | Non-NAFLD | 16.45±34.509 | 0.438 |
| NAFLD | 14.15±24.539 | ||
| INR (1.1-1.4 s) | Non-NAFLD | 1.10±0.268 | 0.308 |
| NAFLD | 1.05±0.413 | ||
| CAP | Non-NAFLD | 238.41±66.865 | <0.001 |
| NAFLD | 297.83±60.765 | ||
| Stiffness score kpa | Non-NAFLD | 10.35±11.329 | 0.224 |
| NAFLD | 12.09±13.704 |
Hg – Hemoglobin; ALT – Alanine aminotransferase; INR – International normalize ratio; CAP – Controlled attenuation parameter; NAFLD – Nonalcoholic fatty liver disease; BMI – Body mass index; GGT – Gamma-glutamyltransferase
Figure 1.
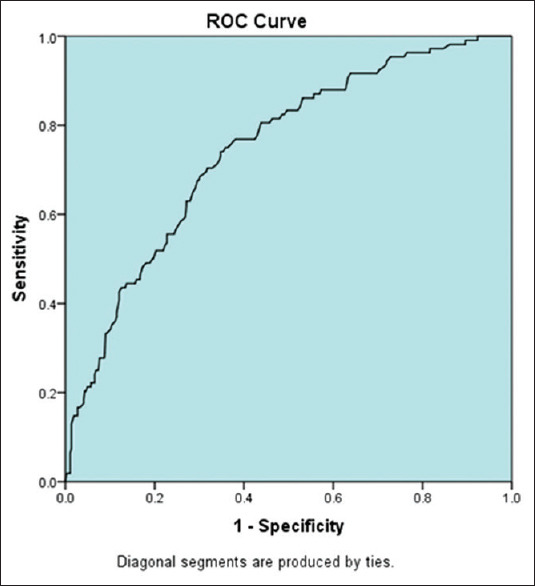
Areas under receiver operating characteristic curve for controlled attenuation parameter in detecting steatosis in nonalcoholic fatty liver disease patients
Table 4.
Level of fibrosis (F score) on FibroScan
| Fibrosis score | Number of patients (%) | Total | |
|---|---|---|---|
| Non-NAFLD | NAFLD | ||
| F0 | 160 (41.8) | 34 (30.6) | 194 |
| F1 | 73 (19.1) | 31 (27.9) | 104 |
| F2 | 37 (9.7) | 10 (9) | 47 |
| F3 | 27 (7) | 10 (9) | 37 |
| F4 | 86 (22.5) | 26 (23.4) | 112 |
| Total | 383 | 111 | 494 |
P=0.314 nonsignificant. NAFLD – Nonalcoholic fatty liver disease
In the linear regression analysis, age and platelet count showed a significant relationship with the presence of cirrhosis on the abdominal ultrasonogram both for NAFLD and non-NAFLD patients [Table 5]. Furthermore, in the backward analysis for linear regression, both platelet count and hemoglobin maintained a significant association with the ultrasound findings of cirrhosis for NAFLD patients. On the other hand, for non-NALFD CLD patients, age, sex, platelets count, albumin and ALT were significantly correlated with cirrhosis [Table 6].
Table 5.
Multiple regression analysis for factors associated with the presence of cirrhosis on ultrasound for NAFLD and non- NAFLD patients
| Coefficients | |||||
|---|---|---|---|---|---|
| Model | Unstandardized coefficients | Standardized coefficients | t | Significant | |
| B | SE | β | |||
| Non-NAFLD patients | |||||
| Constant | 1.382 | 0.227 | 6.083 | <0.001 | |
| Stiffness score | 0.000 | 0.002 | −0.011 | −0.110 | 0.912 |
| INR | −0.008 | 0.063 | −0.010 | −0.124 | 0.901 |
| Bilirubin | 0.000 | 0.001 | −0.046 | −0.430 | 0.668 |
| GGT | 0.000 | 0.000 | −0.658 | −4.359 | <0.001 |
| ALT | 0.001 | 0.001 | 0.309 | 1.843 | 0.068 |
| Albumin | 0.007 | 0.003 | 0.184 | 2.189 | 0.030 |
| Platelets | 0.000 | 0.000 | 0.266 | 3.046 | 0.003 |
| Hg | 0.001 | 0.005 | 0.018 | 0.222 | 0.825 |
| BMI | 0.002 | 0.002 | 0.058 | 0.718 | 0.474 |
| AGE | 0.004 | 0.001 | 0.301 | 3.749 | <0.001 |
| Sex | −0.030 | 0.033 | −0.071 | −0.909 | 0.365 |
| NAFLD patients | |||||
| Constant | −1.009 | 0.836 | −1.207 | 0.235 | |
| Stiffness Score | 0.003 | 0.004 | 0.110 | 0.713 | 0.480 |
| INR | 0.174 | 0.157 | 0.196 | 1.104 | 0.277 |
| Bilirubin | −0.003 | 0.002 | −0.239 | −1.608 | 0.116 |
| GGT | −0.002 | 0.001 | −0.247 | −1.895 | 0.066 |
| ALT | 0.002 | 0.002 | 0.191 | 1.145 | 0.260 |
| Albumin | 0.005 | 0.011 | 0.072 | 0.416 | 0.680 |
| Platelets | 0.002 | 0.001 | 0.599 | 3.822 | 0.001 |
| Hg | 0.084 | 0.038 | 0.462 | 2.203 | 0.034 |
| BMI | 0.002 | 0.006 | 0.042 | 0.322 | 0.749 |
| Age | 0.010 | 0.005 | 0.344 | 2.040 | 0.049 |
| Sex | 0.112 | 0.134 | 0.132 | 0.833 | 0.411 |
Dependent variable: Cirrhosis on imaging study. NAFLD – Nonalcoholic fatty liver disease; INR – International normalized ratio; Hg – Hemoglobin; ALT – Alanine aminotransferase; BMI – Body mass index; GGT – Gamma-glutamyl transferase; SE – Standard error
Table 6.
Backward multiple regression analysis for factors associated with evidence of cirrhosis on abdominal imaging
| Model | Unstandardized coefficients | Standardized coefficients | t | Significant | |
|---|---|---|---|---|---|
| B | SE | β | |||
| Non-NAFLD patients | |||||
| Constant | −1.545 | 0.728 | −2.122 | 0.055 | |
| Sex | 0.564 | 0.238 | 0.515 | 2.368 | 0.036 |
| Age | 0.019 | 0.006 | 0.518 | 3.305 | 0.006 |
| ALT | −0.002 | 0.001 | −0.651 | −3.627 | 0.003 |
| Albumin | 0.023 | 0.009 | 0.382 | 2.462 | 0.030 |
| Platelets | 0.002 | 0.000 | 0.600 | 4.249 | 0.001 |
| NAFLD patients | |||||
| Bilirubin | 0.008 | 0.002 | 1.288 | 4.698 | 0.001 |
| Constant | 0.177 | 0.312 | 0.566 | 0.575 | |
| Platelets | 0.002 | 0.000 | 0.482 | 3.871 | 0.000 |
| Hg | 0.081 | 0.024 | 0.415 | 3.328 | 0.002 |
Hg – Hemoglobin; NAFLD – Nonalcoholic fatty liver disease; ALT – Alanine aminotransferase. Dependent factor is cirrhosis on imaging
DISCUSSION
The current study found that NAFLD is the third most common cause of CLDs in the Western region of Saudi Arabia. To the best of the authors’ knowledge, this is the first study to investigate the prevalence of NAFLD among CLD patients in Saudi Arabia. The higher prevalence of CHB and CHC than NAFLD can be explained by the high prevalence of chronic viral hepatitis in Saudi Arabia.[15,16] However, the direct-acting antiviral therapy for CHC and control of CHB by the neonatal vaccination program are effective measures that are likely to drastically reduce the presence of viral hepatitis in Saudi Arabia.[17,18] In contrast, the growing epidemic of metabolic syndromes is likely to increase the incidence of NAFLD in Saudi Arabia, and its estimated prevalence in the general population by 2030 is 48%,[12,19] which is similar to the estimated NAFLD pattern across other Western and Eastern countries.[1,2,3,20]
The current study had a higher percentage of females with CLDs (i.e.,, overall and in both groups) than males. This is in contrast to previous findings on liver disease, where it was shown that in the reproductive age, liver disease are likely to affect females lesser than males, possibly due to the protective effect of sex hormones.[21,22] However, it should be noted that the mean age of patients in this study was >45 years, and as the protective effect of estrogen against liver disease is expected to be lost after menopause,[21] this may have contributed to the current study finding.
The current study found that there was a significant association between NAFLD and DM, HTN and hyperlipidemia as well as patients in this group were significantly older than those in the non-NAFLD group. This result adds to current evidence regarding the association of NAFLD with metabolic syndromes in Saudi Arabia.[8,12,14,23]
The current study result did not find a significant difference in the BMI between the NAFLD and non-NAFLD patients. This is inconsistent with the previous studies that have shown an association of NAFLD with high BMI.[12,20] This difference might be because of differences in the studied population. In the current study, one-fifth of the non-NAFLD patients had evidence of metabolic syndrome, 20% had DM and 17% had HTN, whereas previous studies have assessed the prevalence of NAFLD in healthy general population.
When first described, NAFLD was considered a benign disease, but more recent evidence suggest that it has progressed into an advanced stage.[5,6,7] In our cohort, one-sixth of the patients with NAFLD had evidence of portal HTN on abdominal ultrasonography, which was four-folds higher than that of non-NAFLD patients. This finding indicates that NAFLD patients at risk of disease progression should be carefully monitored.[4,5,18] The higher serum ALT level and CAP in the NAFLD than in the non-NAFLD patients may reflect the nature of NAFLD progression in patients from Saudi Arabia. A previous study on noninvasive assessment of NAFLD from our center had shown a similar association between elevated serum ALT and advanced fibrosis.[24] On the other hand, both national and international guidelines on NAFLD diagnosis and management have shown the association of elevated serum ALT in NASH and liver disease progression to advanced fibrosis.[4,5,20]
The pathogenesis of NAFLD is related to pathological triglyceride deposition in the liver due to insulin resistance, resulting in exudative stress and liver damage.[25,26] In the present study, NAFLD patients with early-stage fibrosis had higher serum triglyceride levels than those without fibrosis, likely representing early stages of liver damage in NAFLD. With liver disease advancement, patients with cirrhosis had lower triglyceride levels than those with early-stage fibrosis. Triglyceride deposition in the liver is an early-stage NAFLD pathogenesis.[20] Older age is an important predictor for the progression of liver disease to cirrhosis.[27,28] In our cohort, older age was a predictor for cirrhosis in NAFLD and non-NAFLD patients. Similarly, a significant association was found between platelet count and cirrhosis. Thrombocytopenia is an important predictor for liver cirrhosis and it is an outcome of several factors that include splenic sequestration and reduced production from the bone marrow. The platelet count is expected to progressively diminish with the advancement of cirrhosis.[29]
Limitations and recommendations
The retrospective nature of the study is a limitation because it did not allow a proper inclusion of patients, as patients had to be excluded because of incomplete or unavailable laboratory results. Nonetheless, given that this study was conducted in a tertiary referral academic center, the included sample is likely to be representative of the region.
More studies are needed from different regions in Saudi Arabia and at a national level to understand the overall prevalence. In addition, studies should also be conducted to determine the genetic risk factors associated with NAFLD in Saudi Arabia. Finally, the authors recommend additional studies around the optimal management of NAFLD and associated metabolic syndromes to delay or stop the progression of liver disease Saudi Arabia.
CONCLUSION
This study found that NAFLD is the third most common CLD in the Western region of Saudi Arabia. In addition, NAFLD is associated with metabolic syndromes, DM and HTN, with about one-third of the patients having advanced stage fibrosis or cirrhosis.
Ethical consideration
Ethical approval of this study was obtained from the Ethical Committee of the Faculty of Medicine of King Abdulaziz University (Ref 358-14), Jeddah, Saudi Arabia, on January 11, 2015. Need for informed consent was waived because this was a retrospective study.
Peer review
This article was peer reviewed by three independent and anonymous reviewers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal S, Duseja AK. Non-alcoholic fatty liver disease: East versus West. J Clin Exp Hepatol. 2012;2:122–34. doi: 10.1016/S0973-6883(12)60101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the World. Clin Liver Dis. 2016;20:205–14. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the study of liver diseases. Hepatol. 2018;67:328–57. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO).EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Byrne CD, Targher G. NAFLD: A multisystem disease. J Hepatol. 2015;6(Suppl 1):S47–64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Streba LA, Vere CC, Rogoveanu I, Streba CT. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: An open question. WJG. 2015;21:4103–10. doi: 10.3748/wjg.v21.i14.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Memish ZA, El Bcheraoui C, Tuffaha M, Robinson M, Daoud F, Jaber S, et al. Obesity and associated factors – Kingdom of Saudi Arabia, 2013. Prev Chronic Dis. 2014;11:E174. doi: 10.5888/pcd11.140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Othaimeen AI, Al-Nozha M, Osman AK. Obesity: An emerging problem in Saudi Arabia Analysis of data from the national nutrition survey. East Mediterr Health J. 2007;13:441–8. [PubMed] [Google Scholar]
- 10.Al-Rubeaan K, Al-Manaa HA, Khoja TA, Ahmad NA, Al-Sharqawi AH, Siddiqui K, et al. Epidemiology of abnormal glucose metabolism in a country facing its epidemic: SAUDI-DM study. J Diabetes. 2015;7:622–32. doi: 10.1111/1753-0407.12224. [DOI] [PubMed] [Google Scholar]
- 11.Al-Rubeaan K, Al-Manaa H, Khoja T, Ahmad N, Al-Sharqawi A, Siddiqui K, et al. The Saudi abnormal glucose metabolism and diabetes impact study (Saudi-DM) Ann Saudi Med. 2014;34:465–75. doi: 10.5144/0256-4947.2014.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Hamoudi W, El-Sabbah M, Ali S, Altuwaijri M, Bedewi M, Adam M, et al. Epidemiological, clinical, and biochemical characteristics of Saudi patients with nonalcoholic fatty liver disease: A hospital-based study. Ann Saudi Med. 2012;32:288–92. doi: 10.5144/0256-4947.2012.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Hamoudi W, Abaalkhail F, Bendahmash A, Allam N, Hegab B, Elsheikh Y, et al. The impact of metabolic syndrome and prevalent liver disease on living donor liver transplantation: A pressing need to expand the pool. Hepatol Int. 2016;10:347–54. doi: 10.1007/s12072-015-9664-7. [DOI] [PubMed] [Google Scholar]
- 14.Akbar DH, Kawther AH. Nonalcoholic fatty liver disease in Saudi type 2 diabetic subjects attending a medical outpatient clinic: Prevalence and general characteristics. Diabetes Care. 2003;26:3351–2. doi: 10.2337/diacare.26.12.3351-a. [DOI] [PubMed] [Google Scholar]
- 15.Abdo AA, Sanai FM, Al-Faleh FZ. Epidemiology of viral hepatitis in Saudi Arabia: Are we off the hook? Saudi J Gastroenterol. 2012;18:349–57. doi: 10.4103/1319-3767.103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdo AA, Sanai FM. Viral hepatitis in Saudi Arabia. An unfinished story. Saudi Med J. 2015;36:785–6. doi: 10.15537/smj.2015.7.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al Ghamdi SS, Fallatah HI, Fetyani DM, Al-Mughales JA, Gelaidan AT. Long-term efficacy of the hepatitis B vaccine in a high-risk group. J Med Virol. 2013;85:1518–22. doi: 10.1002/jmv.23658. [DOI] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Alswat K, Aljumah AA, Sanai FM, Abaalkhail F, Alghamdi M, Al Hamoudi WK, et al. Nonalcoholic fatty liver disease burden – Saudi Arabia and United Arab Emirates, 2017-2030. Saudi J Gastroenterol. 2018;24:211–9. doi: 10.4103/sjg.SJG_122_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alswat KA, Fallatah HI, Al-Judaibi B, Elsiesy HA, Al-Hamoudi WK, Qutub AN, et al. Position statement on the diagnosis and management of non-alcoholic fatty liver disease. Saudi Med J. 2019;40:531–40. doi: 10.15537/smj.2019.6.23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu I. Impact of oestrogens on the progression of liver disease. Liver Int. 2003;23:63–9. doi: 10.1034/j.1600-0676.2003.00811.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Lazo M, Koteish A, Kao WH, Shih MH, Bonekamp S, et al. Oral contraceptive pill use is associated with reduced odds of nonalcoholic fatty liver disease in menstruating women: Results from NHANES III. J Gastroenterol. 2013;48:1151–9. doi: 10.1007/s00535-012-0715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alsabaani AA, Mahfouz AA, Awadalla NJ, Musa MJ, Al Humayed SM. Non-alcoholic fatty liver disease among type-2 diabetes mellitus patients in Abha City, South Western Saudi Arabia. Int J Environ Res Public Health. 2018;15:2521. doi: 10.3390/ijerph15112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallatah HI, Akbar HO, Fallatah AM. Fibroscan compared to FIB-4, APRI, and AST/ALT ratio for assessment of liver fibrosis in Saudi patients with nonalcoholic fatty liver disease. Hepat Mon. 2016;16:e38346. doi: 10.5812/hepatmon.38346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day CP. From fat to inflammation. Gastroenterol. 2006;130:207–10. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatol. 2010;52:1836–46. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 27.Sajja KC, Mohan DP, Rockey DC. Age and ethnicity in cirrhosis. J Investig Med. 2014;62:920–6. doi: 10.1097/JIM.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol. 2015;31:184–91. doi: 10.1097/MOG.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore AH. Thrombocytopenia in cirrhosis: A review of pathophysiology and management options. Clin Liver Dis (Hoboken) 2019;14:183–6. doi: 10.1002/cld.860. [DOI] [PMC free article] [PubMed] [Google Scholar]


