Abstract
Anthracyclines are archetypal representatives of the tetracyclic type II polyketide natural products that are widely used in cancer chemotherapy. Although the synthesis of this class of compounds has been a subject of several investigations, all known approaches are based on annulations, relying on the union of properly prefunctionalized building blocks. Herein, we describe a conceptually different approach using a polynuclear arene as a starting template, ideally requiring only functional decorations to reach the desired target molecule. Specifically, tetracene was converted to (±)-idarubicinone, the aglycone of the FDA approved anthracycline idarubicin, through the judicious orchestration of Co- and Ru-catalyzed arene oxidation and arenophile-mediated dearomative hydroboration. Such a global functionalization strategy, the combination of site-selective arene and dearomative functionalization, provided the key anthracycline framework in five operations and enabled rapid and controlled access to (±)-idarubicinone.
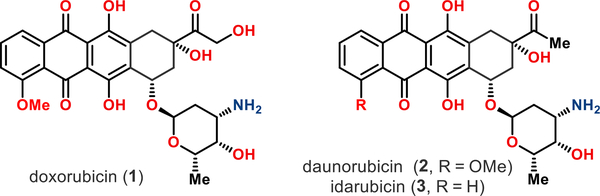
The Streptomyces-produced type II polyketides doxorubicin (l)1 and daunorubicin (2)2 are among the most effective and most often used chemotherapeutics owing to their broad-spectrum of anticancer activity (Figure 1).3 For example, doxorubicin (l) is used for the treatment of breast and bladder cancers, childhood solid tumors, soft tissue sarcomas, and aggressive lymphomas.4 Similarly, daunorubicin (2) is primarily used as an antileukemic drug for multiple myeloma, acute myeloid leukemia, acute lymphocytic leukemia, and Kaposi’s sarcoma.5 Although extremely effective, anthracyclines threaten patients with cumulative dose-dependent cardiotoxicity, severely limiting their long-term application as well as their use in patients with pre-existing cardiovascular risk.6 Therefore, significant research efforts have been devoted to the identification of derivatives with improved pharmacological properties.7 The successful result of one such medicinal chemistry campaign is idarubicin (3),8 an FDA approved anticancer agent with superior therapeutic efficacy and reduced cardiotoxicity relative to daunorubicin (2).9
Figure 1.
Structures of doxorubicin (l), daunorubicin (2), and idarubicin (3).
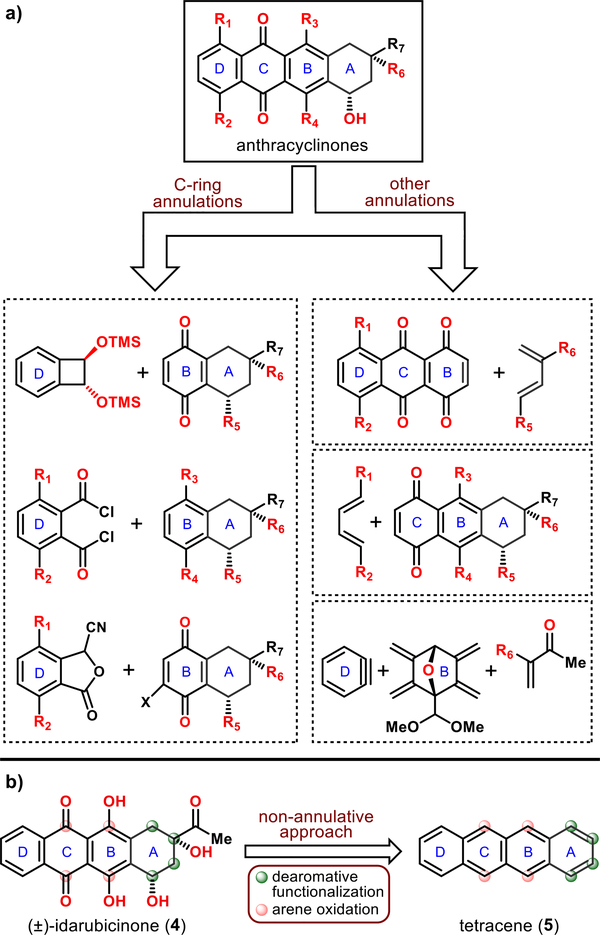
The need for tailored analogs has made anthracyclines the subject of rigorous investigation within the synthetic community.10 Thus, many innovative pathways to the aglycon anthracyclines (anthracyclinones) have been established, all of which rely upon annulation to forge one of the rings (see Figure 2a). The most commonly employed unifying disconnection is C-ring annulation, achieved through cycloadditions, cationic cyclizations, or anionic processes (Figure 2a, left inset).11 Moreover, cycloadditions were also explored to forge other rings of the tetracyclic core of these molecules (Figure 2a, right insets).12 Herein, we report a conceptually different, nonannulative approach to anthracyclinones, starting from a simple aromatic hydrocarbon via a global functionalization strategy (Figure 2b). Specifically, (±)-idarubicinone (4) was synthesized from tetracene (5), an ideal aromatic precursor containing the essential tetracyclic framework, through a manifold of arene functionalizations and a site-selective dearomative elaboration.
Figure 2.
(a) Selected annulation-based strategies to anthracyclinones. (b) This work: synthesis of (±)-idarubicinone (4) from tetracene (5) using a nonannulative approach.
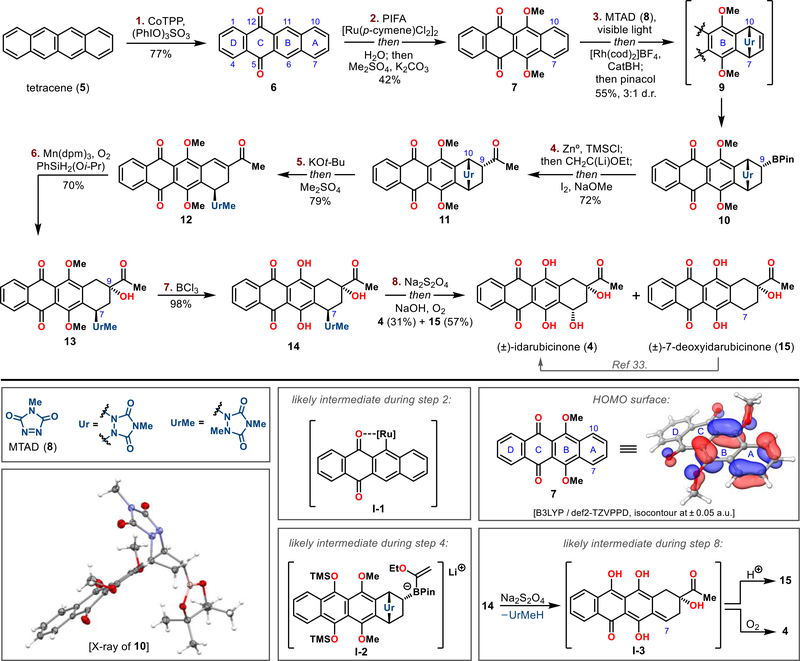
Following this global functionalization strategy, we commenced our studies by exploring functionalization reactions of tetracene (5), which would establish the proper oxidation states of the internal rings B and C within idarubicinone (4) (Figure 3). Thus, inspired by a similar transformation reported on anthracene, we achieved the first oxidation of 5 with catalytic amounts of cobalt(II) tetraphenylporphyrin (CoTPP, 5 mol %) and phenyliodine(III) sulfate as an oxidant,13 delivering 5,12-tetracenequinone (6) in 77% yield. Although this transformation proceeded readily, the second oxidation to the corresponding 6,11-dihydroxy-5,12-tetracenequinone derivative 7 proved more challenging. Several oxidants known for direct arene oxidation, such as CAN, Frémy’s salt, hypervalent iodine reagents, or oxidizing metal complexes,14 were found to be unsuitable for this transformation. This setback was not surprising, as this type of peri-oxidation remains a largely unsolved synthetic challenge owing to the high oxidation potential of quinones. Therefore, we decided to evaluate C–H activation, anticipating that the quinone carbonyl groups would serve as weakly coordinating directing groups for the peri-(C-6) and (C-11) positions15. After examining several carbonyl-directed hydroxylation protocols, we developed a one-pot procedure involving a modification of Ru-catalyzed sp2 C–H oxygenation pioneered by Ackermann ([Ru(cymene)-Cl2]2 and PIFA),16 followed by sequential one-pot hydrolysis and methylation to give desired product 7. Control experiments revealed that this functionalization likely proceeds through the peri-selective formation of ruthenacycle intermediate I-1, delivering phenol derivative, which underwent further oxidation to the hydroquinone stage in the presence of excess PIFA.17
Figure 3.
Synthesis of (±)-idarubicinone (4) from tetracene (5). Reagents and conditions: 1. CoTPP (5 mol %), (PhIO)3SO3, CH2Cl2, 25 °C, 2 h, 77%; 2. [Ru(p-cymene)Cl2]2 (2.5 mol %), PIFA, DCE, 100 °C, 12 h; then H2O, 100 °C, 12 h; then Me2SO4, K2CO3, (CH3)2CO, 74 °C, 24 h, 42%; 3. MTAD (8), CH2Cl2, −50 °C, 36 h; then [Rh(cod)2]BF4 (10 mol %), dppb (10 mol %), HBcat, THF, −30 °C, 12 h; then pinacol, −78 to 25 °C, 12 h, 55% (3:1 dr); 4. Zn0, TMSCl, THF, ultrasonication, 40 °C, 30 min; then then CH2C(Li)OEt, −78 to −25 °C, 30 min; then I2, −78 to −25 °C, 30 min; then NaOMe, −78 to 25 °C, 4 h; then 1 M HCl, 25 °C, 2 h, 72%; 5. KOt-Bu, THF, −78 °C, 20 min; then Me2SO4, −78 to 0 °C, 1.5 h, 79%; 6. Mn(dpm)3 (10 mol %), PhSiH2(Oi-Pr), O2, i-PrOH/DCE 1:1, 0 to 25 °C, 2 h, 70%; 7. BCl3, CH2Cl2, −78 °C, 1 h, 98%; 8. Na2S2O4, H2O/THF/MeOH 1:1:1, −20 °C, 20 min; then NaOH, −20 °C, 60 s; then O2, −20 °C, 5 min, 31% of 4 and 57% of 15.
With arene oxidation completed, which set the required oxidation state of the B and C rings, we turned our attention to the dearomative functionalization of the terminal ring A. We have recently reported a series of dearomatization strategies that employ visible-light-promoted para-cycloaddition between arenes and the arenophile N-methyl-1,2,4-triazoline-3,5-dione (MTAD, 8) and subsequent in situ manipulation of the resulting cycloadducts.18 With polynuclear arenes, we consistently observed highly site-selective cycloadditions onto the terminal rings. Because tetracenequinone derivative 7 contains two such regions, rings A and D, amenable to cycloaddition with MTAD, another level of complexity to this process was introduced. However, based on previous studies, we know that the relevant mechanistic feature of this process is a photoinduced charge- and electron-transfer from the arene to the arenophile;19 therefore, the HOMO of the arene should dictate the site-selectivity in polynuclear aromatic settings. Accordingly, computational studies (at the B3LYP/def2-TZVPPD level of theory) of 7 predicted a strong bias for the A ring, which has profoundly larger HOMO orbital coefficients, (see Figure 3, bottom inset for the corresponding HOMO surface). Indeed, this prediction correlated well with experiment, as we observed exclusive cycloaddition onto the A-ring to provide intermediate 9. With this site-selective dearomatization, we explored several strategies to introduce the remaining two carbon atoms needed to complete the idarubicinone framework. We found that the arenophile-based cycloaddition in combination with in situ Rh-catalyzed alkene hydroboration (7 → [9] → 10) installed the boron moiety as a suitable handle for the introduction of the requisite acetyl group. Several hydroboration procedures were evaluated, but ultimately the cationic rhodium complex [Rh(cod)2BF4] with 1,4-bis(diphenylphosphino)butane (dppb) and catecholborane provided the best outcome (for optimization details, see Table S1 in Supporting Information).20 Although catecholborane was essential for the hydroboration step, the inherent instability of the resulting alkyl catechol boronic ester required immediate transesterification of catechol to pinacol to enable product isolation in higher yields.21 Importantly, following this protocol, we were able to prepare multigram quantities of boronic ester 10 in a single pass in 55% yield and an endo/exo 3:1 dr (see Figure 3, bottom inset for an X-ray diffraction structure of 10).
Elaboration of organoborate 10 to the full skeleton of idarubicinone required installation of a two-carbon fragment through a seemingly straightforward B-alkyl Suzuki coupling reaction. However, since several standard Pd- and Ni-catalyzed reaction conditions failed,22 we decided to explore the C–C bond forming strategies involving the rich chemistry of boron 1,2-metalate rearrangements. Particularly, we were keen to explore Zweifel olefination with lithiated ethoxyvinyl ether,23 which would provide rapid access to the C-9 acetyl group. Nevertheless, a major pitfall of this design was the presence of the quinone and its general incompatibility with organolithium reagents. Indeed, prospecting experiments involving boronic ester 10 and 1-ethoxyvinyllithium resulted in the addition of organolithium species to quinone, delivering a mixture of products without any traces of the desired olefinated product. To address this chemoselectivity issue, we developed a one-pot process that involved in situ masking of the quinone. Thus, a tetrahydrofuran (THF) solution of boronic ester 10 was sonicated with Zn powder in the presence of trimethylsilyl chloride (TMSCl), resulting in the formation of a fully protected bis-hydroquinone.24 This intermediate was exposed to a freshly prepared 1-lithioethyl vinyl ether to form the boronate complex I-2, which was immediately subjected to Zweifel olefination by addition of iodine and base.25 Concurrently with olefination, the excess iodine also oxidized the labile silylated hydroquinone back to the quinone, and workup of the reaction mixture with an aqueous HCl solution hydrolyzed the newly introduced vinyl ether to the corresponding methyl ketone 11. Remarkably, this one-pot operation involved several distinct transformations and was performed on a multigram scale in 72% yield.26
Although the arenophile-mediated dearomative hydroboration and subsequent Zweifel olefination introduced the desired methyl ketone, this sequence also installed a bridging urazole moiety, which had to be strategically transmuted to reveal the fully decorated A ring of idarubicinone (4). This task was partially accomplished by treatment of ketone 11 with base followed by Me2SO4, initiating β-elimination of urazole at position C-10 with subsequent methylation of the urazole hydrazyl nitrogen, furnishing α,β-unsaturated ketone 12 in 79% yield. The N-alkylation of the urazole motif proved necessary to prevent undesired side reactions during subsequent manipulations (for details, see Table S2 in Supporting Information). Finally, subjecting olefin 12 to Mukaiyama hydration conditions27 selectively introduced the tertiary alcohol at position C-9, as α-ketol product 13 was obtained in 70% yield as a single diastereoisomer. Notably, the use of recently reported silane, PhSiH2(Oi-Pr),28 was beneficial for high conversions of this hydrogen-atom transfer process.
This hydration achieved the proper oxidation state of the A-ring, and the only difference between intermediate 13 and idarubicinone (4) at this stage resided in two hydroquinone protecting groups and the urazole moiety instead of a hydroxy group at the C-7 position. Although deprotection of methyl ethers to hydroquinone proceeded without any difficulties using BCl3 (13 → 14, 98% yield), the removal of the urazole proved to be an arduous task. Eventually, the inspiration for the direct urazole-to-hydroxy exchange was found in the Moore hypothesis for the biological mode of reactivity known as bioreductive alkylation.29 Thus, it was proposed that anthracyclines undergo in vivo quinone reduction and subsequent C-7 amino sugar elimination, producing a reactive species in the form of a phenylogous quinone methide. Moreover, this concept was demonstrated in solution with several anthracyclines, which formed the corresponding semiquinone intermediates upon subjection to specific reducing agents.30 The direct translation of these findings to our system, for example the addition of sodium dithionite to precursor 14, did not eliminate the urazole; however, after the addition of base (NaOH) we observed elimination and exclusive formation of 7-deoxyidarubicinone (15) under anaerobic conditions. This result was in accordance with the literature, since deoxygenated anthracyclinones were commonly observed upon reduction of anthracyclines.31 Mechanistically, the reduction of quinone 14 to hydroquinone, followed by base-induced elimination of the urazole, likely formed the semiquinone methide I-3, which after protonation gave the deaminated product 15. However, we noticed that in the presence of oxygen, this reactive intermediate underwent competitive oxidation,32 delivering idarubicinone (I-3 → 4). Accordingly, short exposure of 14 to an aqueous solution of sodium dithionite and NaOH, followed by rapid saturation of reaction mixture with oxygen, provided (±)-idarubicinone (4) and (±)-7-deoxyidarubicinone (15) in 31% and 57% yield. Although extensive optimization of this protocol did not result in a higher ratio of desired anthracyclinone 4 to 15 (for details, see Table S3 in Supporting Information), this deoxygenated side-product could be readily converted to aglycone 4 in one or two steps using known protocols.33
In summary, we have described a functionalization-based approach to (±)-idarubicinone (4) from tetracene (5). The salient feature of this strategy is a judicious orchestration of two arene functionalizations and dearomatization, introducing the functionality of the A, B, and C rings of the anthracyclinone skeleton. Specifically, Co- and Ru-catalyzed arene oxidations, site-selective arenophile-mediated dearomative hydroboration, and subsequent Zweifel olefination provided the fully decorated anthracyclinone framework. Moreover, adjustment of the A ring, including a formally redox neutral urazole-to-hydroxy exchange delivered (±)-idarubicinone (4) in 8 operations and 2% overall yield from tetracene (5).
Importantly, by employing a simple polynuclear hydrocarbon aromatic starting material, the described work also presents a notable departure from previously reported syntheses of anthracyclinones in which annulations were critical to the overall synthetic design. In fact, polynuclear arenes are not commonly considered in synthetic planning for construction of stereochemically complex scaffolds. However, through the development and application of new methods, the present study provides a compelling case in which tetracene serves as an ideal template for imprinting of desired functionality. Thus, the range of available polynuclear arenes, as well as numerous functionalization opportunities, can be combined to render this global functionalization approach an appealing and complementary entry for the preparation of other type II polyketide-like compounds.
Supplementary Material
ACKNOWLEDGMENTS
Financial support for this work was provided by the University of Illinois, the NIH/National Institute of General Medical Sciences (R01 GM122891), and the donors of the American Chemical Society Petroleum Research Fund (PRF#57175-DNI1). D.S. is an Alfred P. Sloan Fellow. M.O. thanks the Honjo International Scholarship Foundation. Solvias AG is acknowledged for a generous gift of chiral ligands. We thank Prof. S. E. Denmark (University of Illinois) for critical proofreading of this paper. We also thank Dr. D. Olson and Dr. L. Zhu for nuclear magnetic resonance spectroscopic assistance, Dr. D. L. Gray and Dr. T. Woods for X-ray crystallographic analysis assistance, and F. Sun for mass spectrometric assistance.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b05370.
Experimental procedures and spectral data for all new compounds (PDF)
X-ray crystallographic data for C19H24BN3O4 (CIF)
X-ray crystallographic data for C29H30BN3O8 (CIF)
REFERENCES
- (1).Arcamone F; Cassinelli G; Fantini G; Grein A; Orezzi P; Pol C; Spalla C Adriamycin 14-Hydroxydaunomycin, a New Antitumor Antibiotic From S. peucetius var. caesius. Biotechnol. Bioeng 1969, 11, 1101–1110. [DOI] [PubMed] [Google Scholar]
- (2).DiMarco A; Gaetani M; Orezzi P; Scarpinato B; Silvestrini R; Soldati M; Dasdia T; Valentini L Daunomycin, a New Antibiotic of the Rhodomycin Group. Nature 1964, 201, 706–707. [DOI] [PubMed] [Google Scholar]
- (3).Arcamone F Doxorubicin, Anticancer Antibiotics; Academic Press: New York, 1984. [Google Scholar]; (b) Arcamone F Antitumor Anthracyclines: Recent Developments. Med. Res. Rev 1984, 4, 153–188. [DOI] [PubMed] [Google Scholar]
- (4).Hortobagyi GN Anthracyclines in the Treatment of Cancer. Drugs 1997, 54, 1–7. [DOI] [PubMed] [Google Scholar]
- (5).(a) Anthracycline Antibiotics in Cancer Therapy In Developments in Oncology, 10th ed.; Muggia FM, Young CW, Carter SK., Eds.; The Hague: Martinus Nijhoff Publishers; 1982. [Google Scholar]; (b) See also ref 5. [Google Scholar]
- (6).(a) Cummings J; Anderson L; Willmott N; Smyth JF The Molecular Pharmacology of Doxorubicin in vivo. Eur. J. Cancer Clin. Oncol 1991, 27, 532–535. [DOI] [PubMed] [Google Scholar]; (b) Zucchi R; Danesi R Cardiac Toxicity of Antineoplastic Anthracyclines. Curr. Med. Chem.: Anti-Cancer Agents 2003, 3, 151–171. [DOI] [PubMed] [Google Scholar]
- (7).For example, see:Binaschi M; Bigioni M; Cipollone A; Rossi C; Goso C; Maggi CA; Capranico G; Animati F Anthracyclines: Selected New Developments. Curr. Med. Chem.: Anti-Cancer Agents 2001, 1, 113–130.
- (8).For syntheses of idaroubicinone, see: Di Marco A; Casazza AM; Giuliani F; Pratesi G; Arcamone F; Bernadi L; Franchi G; Giradino P; Patelli B; Penco S Synthesis and Antitumor Activity of 4-Demethoxyadriamycin and 4-Demethoxy-4’-epiadriamycin. Cancer Treat. Rep 1978, 62, 375–380.Swenton JS; Freskos JN; Morrow GW; Sercel AD A Convergent Synthesis of (+)-4-Demethoxydaunomycinone and (+)-Daunomycinone. Tetrahedron 1984, 40, 4625–4632.Gupta CR; Harland PA; Stoodley RJ An Efficient Enantiocontrolled Synthesis of (+)-4-Demethox-ydaunomycinone. Tetrahedron 1984, 40, 4657–4667.Tanno N; Terashima S Asymmetric Synthesis of Optically Active Anthracyclinone Intermediate and 4-Demethoxyanthracyclinones by the Use of a Novel Chiral Reducing Agent. Chem. Pharm. Bull 1983, 31, 821–836.Tamura Y; Sasho M; Akai S; Kishimoto H; Sekihachi J; Kita Y A Highly Convergent Strategy for the Synthesis of 4-Demethoxydaunomycinone and Daunomycinone: A Novel Synthesis of C4-Acetoxylated Homophthalic Anhydrides. Tetrahedron Lett. 1986, 27, 195–198.Krohn K; Rieger H; Broser E; Schiess P; Chen S; Strubin T Konvergente Synthese enantiomerenreiner Daunomycinon-Derivate. Liebigs. Ann. Chem 1988, 1988, 943–948.Carr K; Greener NA; Mullah KB; Somerville FM; Sutherland JK A Synthesis of (±)-Demethoxydaunomycinone. J. Chem. Soc., Perkin Trans 1 1992, 1975–1980.Achmatowicz O; Szechner B New Chiral Pool Approach to Anthracyclinones. The Stereoselective Synthesis of Idarubicinone. J. Org. Chem 2003, 68, 2398–2404.Rho YS; Park J; Kim G; Kim H; Sin H; Suh PW; Yoo DJ Facile Total Syntheses of Idarubicinone-7-β-D-glucuronide: Convenient Preparations of AB-Ring Synthon Using Some Carboxylic Acid Derivatives. Synth. Commun 2004, 34, 1703–1722.Gupta RC; Jackson DA; Stoodley RJ; Williams DJ Studies Related to Anthracyclines. Part 2. Synthesis of (±)-4-Demethoxydaunomycinone. J. Chem. Soc., Perkin Trans 1 1985, 525–533.Cambie RC; Larsen DS; Rickard CEF; Rutledge PS; Woodgate PD Experiments Directed Towards the Synthesis of Anthracyclinones. X. A Synthesis of (±)-4-Demethoxydaunomycinone. Aust. J. Chem 1986, 39, 487–502.Carr K; Sutherland JK A Simple Synthesis of Demethoxydaunomycinone. J. Chem. Soc., Chem. Commun 1987, 567–568.Krohn K; Tolkiehn K Stereoselektive Synthese Des 4-Desmethoxydaunomycinons. Tetrahedron Lett. 1978, 19, 4023–4026.Garland RB; Palmer JR; Schulz JA; Sollman PB; Pappo R A New Synthetic Route to 4-Demethoxydaunomycinone. Tetrahedron Lett. 1978, 19, 3669–3672.Kende AA; Curran DP; Tsay Y; Mills JE The Isobenzofuran Route to Anthracyclinones. Tetrahedron Lett. 1977, 18, 3537–3540.Broadhurst MJ; Hassall CH; Thomas GJ Anthracyclines. Part 3. The Total Synthesis of 4-Demethoxydaunomycin. J. Chem. Soc., Perkin Trans 1 1982, 2249–2255.Broadhurst MJ; Hassall CH; Thomas GJ Total Synthesis of 4-Demethoxydaunomycin. J. Chem. Soc. Chem. Commun 1982, 158–160.Allen JG; Hentemann MF; Danishefsky SJ A Powerful O-Quinone Dimethide Strategy for Intermolecular Diels–Alder Cycloadditions. J. Am. Chem. Soc 2000, 122, 571–575.Kimura Y; Suzuki M; Matsumoto T; Abe R; Terashima S A Simple and Efficient Synthesis of Optically Active (+)-4-Demethoxydaunomycinone. Bull. Chem. Soc. Jpn. 1986, 59, 415–421.
- (9).(a) Hollingshead LM; Faulds D Drugs 1991, 42, 690. [DOI] [PubMed] [Google Scholar]; (b) Cersosimo RJ Idarubicin: an Anthracycline Antineoplastic Agent. Clin. Pharm 1992, 11, 152–167. [PubMed] [Google Scholar]
- (10).For selected reviews on synthesis of anthracyclines, see:Krohn K Building Blocks for the Total Synthesis of Anthracyclinones. Prog. Chem. Org. Nat. Prod 1989, 55, 37–88.Krohn K Synthesis of Anthracyclinones by Electrophilic and Nucleophilic Addition to Anthraquinones. Tetrahedron 1990, 46, 219–318.Thomas GJ Synthesis of Anthracyclines Related to Daunomycin In Recent Progress in the Chemical Synthesis of Antibiotics; Lukacs G, Ohno M; Eds.; Springer-Verlag, 1990; pp 467–496.Wheeler DMS; Wheeler MM Stereoselective Syntheses of Doxorubicin and Related Compounds In Studies in Natural Products Chemistry; Rahman A, Ed.; Elsevier: Amsterdam, 1994; Vol. 14, pp 3–46.Achmatowicz O; Szechner B Synthesis of Enantiomerically Pure Anthracyclinones. Top. Curr. Chem 2007, 282, 143–186.
- (11).For selected examples, see:Wong CM; Popien D; Schwenk R; Raa Te. Synthetic Studies of Hydronaphthacenic Antibiotics. I. The Synthesis of 4-Demethoxy-7-O-methyl Daunomycinone. Can. J. Chem 1971, 49, 2712–2718.Ishizumi K; Ohashi N; Tanno N Stereospecific Total Synthesis of 9-Aminoanthracyclines: (+)-9-Amino-9-deoxydaunomycin and Related Compounds. J. Org. Chem 1987, 52, 4477–4485. Ref 8b.Hauser FM; Prasanna S Total Syntheses of (±)-Duanomycinone. Regiospecific Preparations of (±)-7,9-Dideoxydaunomycinone and 6,11-Dihydroxy-4-methoxy-7,8,9,10-tetrahydronaphthacene-5,9,12-trione. J. Am. Chem. Soc 1981, 103, 6378–6386.
- (12).For selected examples, see:Kelly TR; Ananthasubramanian L; Borah K; Gillard JW; Goerner RN; King PF; Lyding JM; Tsang W-C; Vaya J An Efficient, Regiospecific Synthesis of (±)-Daunomycinone. Tetrahedron 1984, 40, 4569–4577. Ref 8s.(c) Krohn K; Tolkiehn K Synthetische Anthracyclinone, XIV. Synthese neuer Derivate des Daunomycinons und des β-Rhodomycinons. Chem. Ber 1980, 113, 2976–2993.Dienes Z; Antonsson T; Vogel P Enantioselective Synthesis of (R)-(–)-2-Acetyl-2,5,12-trihydro-1,2,3,4-tetrahydro-6,11-naphthacenequinone via Diastereoselective Diels-Alder Cycloaddition. Tetrahedron Lett. 1993, 34, 1013–1016.
- (13).(a) Geraskin IM; Pavlova O; Neu HM; Yusubov MS; Nemykin VN; Zhdankin VV Comparative Reactivity of Hypervalent Iodine Oxidants in Metalloporphyrin-Catalyzed Oxygenation of Hydrocarbons: Iodosylbenzene Sulfate and 2-Iodylbenzoic Acid Ester as Safe and Convenient Alternatives to Iodosylbenzene. Adv. Synth. Catal 2009, 351, 733.–. [Google Scholar]; (b) Yusubov MS; Nemykin VN; Zhdankin VV Transition Metal-Mediated Oxidations Utilizing Monomeric Iodosyl-and Iodylarene Species. Tetrahedron 2010, 66, 5745–5752. [Google Scholar]
- (14).For selected comprehensive reviews on direct oxidation of arenes, see:Dudfield PJ Synthesis of Quinones In Comprehensive Organic Synthesis; Ley SV, Ed.; Pergamon Press: Oxford, 1991; Vol. 7, pp 345–356.Naruta Y; Maruyama K Recent Advances in the Synthesis of Quinonoid Compounds In The Chemistry of Quinonoid Compounds; Patai S, Rappoport Z, Eds.; John Wiley: New York, 1988; Vol. 2, pp 241–402.
- (15).For selected reviews on this topic, see:Gensch T; Hopkinson MN; Glorius F; Wencel-Delord J Mild Metal-Catalyzed C–H Activation: Examples and Concepts. Chem. Soc. Rev 2016, 45, 2900–2936.Huang Z; Lim HN; Mo F; Young MC; Dong G Transition Metal-Catalyzed Ketone-Directed or Mediated C–H Functionalization. Chem. Soc. Rev 2015, 44, 7764–7786.(c) De Sarkar S; Liu W; Kozhushkov S; Ackermann L Weakly Coordinating Directing Groups for Ruthenium(II)-Catalyzed C–H Activation. Adv. Synth. Catal 2014, 356, 1461–1479.(d) Engle KM; Mei T-S; Wasa M; Yu J-Q Weak Coordination as a Powerful Means for Developing Broadly Useful C–H Functionalization Reactions. Acc. Chem. Res 2012, 45, 788–802.
- (16).(a) Thirunavukkarasu VS; Ackermann L Ruthenium-Catalyzed C-H Bond Oxygenations with Weakly Coordinating Ketones. Org. Lett 2012, 14, 6206–6209. For selected examples of Ru-catalyzed C–H hydroxylation, see: [DOI] [PubMed] [Google Scholar]; (b) Yang F; Ackermann L Ruthenium-Catalyzed C–H Oxygenation on Aryl Weinreb Amides. Org. Lett 2013, 15, 718–720. [DOI] [PubMed] [Google Scholar]; (c) Yang X; Shan G; Rao Y Synthesis of 2-Aminophenols and Heterocycles by Ru-Catalyzed C–H Mono- and Dihydroxylation. Org. Lett 2013, 15, 2334–2337. [DOI] [PubMed] [Google Scholar]; (d) Yang Y; Lin Y; Rao Y Ruthenium(II)-Catalyzed Synthesis of Hydroxylated Arenes with Ester as an Effective Directing Group. Org. Lett 2012, 14, 2874–2877. [DOI] [PubMed] [Google Scholar]; (e) Thirunavukkarasu VS; Hubrich J; Ackermann L Ruthenium-Catalyzed Oxidative C(sp2)-H Bond Hydroxylation: Site-Selective C-O Bond Formation on Benzamides. Org. Lett. 2012, 14, 4210–4213. [DOI] [PubMed] [Google Scholar]; (f) Shan G; Han X; Lin Y; Yu S; Rao Y Broadening the Catalyst and Reaction Scope of Regio-and Chemoselective C-H Oxygenation: a Convenient and Scalable Approach to 2-Acylphenols by Intriguing Rh(II) and Ru(II) Catalysis. Org. Biomol. Chem 2013, 11, 2318–2322. [DOI] [PubMed] [Google Scholar]; (g) Liu W; Ackermann L Ortho- and Para-Selective Ruthenium-Catalyzed C(sp2)–H Oxygenations of Phenol Derivatives. Org. Lett 2013, 15, 3484–3486. [DOI] [PubMed] [Google Scholar]; (h) Yang F; Rauch K; Kettelhoit K; Ackermann L Aldehyde-Assisted Ruthenium(II)-Catalyzed C–H Oxygenations. Angew. Chem., Int. Ed 2014, 53, 11285–11288. [DOI] [PubMed] [Google Scholar]
- (17). Control experiments confirmed the requirement of a specific Ru-catalysis for this transformation, as well as excess of PIFA for further oxidation. See Supporting Information.
- (18).For selected examples, see:Southgate EH; Pospech J; Fu J; Holycross DR; Sarlah D Dearomative Dihydroxylation with Arenophiles. Nat. Chem 2016, 8, 922–928.Okumura M; Nakamata Huynh SM; Pospech J; Sarlah D Arenophile-mediated Dearomative Reduction. Angew. Chem., Int. Ed 2016, 55, 15910–15914.(c) Okumura M; Shved AS; Sarlah D Palladium-Catalyzed Dearomative syn-1,4-Carboamination. J. Am. Chem. Soc 2017, 139, 17787–17790.Hernandez LW; Klöckner U; Pospech J; Hauss L; Sarlah D Nickel-Catalyzed Dearomative trans-1,2-Carboamination. J. Am. Chem. Soc 2018, 140, 4503–4507.Wertjes WC; Okumura M; Sarlah D Palladium-Catalyzed Dearomative syn-1,4-Carboamination. J. Am. Chem. Soc 2019, 141, 163–167.
- (19).(a) Hamrock SJ; Sheridan RS. Para photoaddition of N-methyltriazolinedione to benzene. Synthesis of Energy-Rich Azo Compounds Comprising Benzene + Nitrogen. J. Am. Chem. Soc 1989, 111, 9247. [Google Scholar]; (b) Kjell DP; Sheridan RS Photochemical Cycloaddition of N-methyltriazolinedione to Naphthalene. J. Am. Chem. Soc 1984, 106, 5368. [Google Scholar]; (c) Kjell DP; Sheridan RS A Photochemical Diels–Alder Reaction of N-methyltriazolinedione. J. Photochem 1985, 28, 205–214. [Google Scholar]; (d) Hamrock SJ; Sheridan R S. Photochemical Diels–Alder Addition of N-methyltriazolinedione to Phenanthrene. Tetrahedron Lett. 1988, 29, 5509–5512. [Google Scholar]
- (20).(a) Männig D; Nöth H Catalytic Hydroboration with Rhodium Complexes. Angew. Chem. Int. Ed. Engl 1985, 24, 878–879. [Google Scholar]; (b) Evans DA; Fu GC; Hoveyda AH Rhodium(I)-Catalyzed Hydroboration of Olefins. The Documentation of Regio- and Stereochemical Control in Cyclic and Acyclic Systems. J. Am. Chem. Soc 1988, 110, 6917–6918. [Google Scholar]; (c) Evans DA; Fu GC; Hoveyda AH Rhodium(I)- and Iridium(I)-Catalyzed Hydroboration Reactions: Scope and Synthetic Applications. J. Am. Chem. Soc 1992, 114, 6671–6679. [Google Scholar]
- (21).For an insightful perspective on stability of borates, see: Hall DG Structure, Properties, and Preparation of Boronic Acid Derivatives Boronic Acids; Wiley-VCH Verlag GmbH & Co. KGaA, 2011; pp 1–133. [Google Scholar]
- (22).For recent reviews on the C(sp2)–C(sp3) bond formation, see:Chemler SR; Trauner D; Danishefsky SJ The B-Alkyl Suzuki–Miyaura Cross-Coupling Reaction: Development, Mechanistic Study, and Applications in Natural Product Synthesis. Angew. Chem., Int. Ed 2001, 40, 4544–4568.Jana R; Pathak TP; Sigman MS Advances in Transition Metal (Pd, Ni, Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners. Chem. Rev 2011, 111, 1417–1492.Cherney AH; Kadunce NT; Reisman SE Enantioselective and Enantiospecific Transition-Metal-Catalyzed Cross-Coupling Reactions of Organo-metallic Reagents to Construct C–C Bonds. Chem. Rev 2015, 115, 9587–9652.
- (23).(a) Zweifel G; Arzoumanian H; Whitney CC A Convenient Stereoselective Synthesis of Substituted Alkenes via Hydroboration-Iodination of Alkynes. J. Am. Chem. Soc 1967, 89, 3652–3653. [Google Scholar]; (b) For a recent review, see: Armstrong, R. J.; Aggarwal VK 50 Years of Zweifel Olefination: a Transition-Metal-Free Coupling. Synthesis 2017, 49, 3323–3336. [Google Scholar]
- (24).(a) Rasmussen JK; Krepski LR; Heilmann SM; Smith HK II; Tumey ML A Convenient Synthesis of 1,2-Bis-[trimethylsiloxy]alkenes from α-Diketones. Synthesis 1983, 457–459. [Google Scholar]; (b) Boudjouk P; So JH Organic Sonochemistry. Ultrasonic Acceleration of the Reaction of Dicarbonyls with Trimethylchlorosilane in the Presence of Zinc. Synth. Commun 1986, 16, 775–778. [Google Scholar]
- (25).Sonawane RP; Jheengut V; Rabalakos C; Larouche-Gauthier R; Scott HK; Aggarwal VK Enantioselective Construction of Quaternary Stereogenic Centers from Tertiary Boronic Esters: Methodology and Applications. Angew. Chem., Int. Ed 2011, 50, 3760–3763. [DOI] [PubMed] [Google Scholar]
- (26).For beneficial use of pot economy in organic synthesis, see: Hayashi Y Pot Economy and One-Pot Synthesis. Chem. Sci 2016, 7, 866–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Isayama S; Mukaiyama T A New Method for Preparation of Alcohols from Olefins with Molecular Oxygen and Phenylsilane by the Use of Bis(acetylacetonato)cobalt(II). Chem. Lett 1989, 18, 1071–1074. [Google Scholar]
- (28).Obradors CL; Martinez RM; Shenvi RA Ph(i-PrO)SiH2: An Exceptional Reductant for Metal-Catalyzed Hydrogen Atom Transfers. J. Am. Chem. Soc 2016, 138, 4962–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).(a) Moore HW Bioactivation as a Model for Drug Design Bioreductive Alkylation. Science 1977, 197, 527–532. [DOI] [PubMed] [Google Scholar]; (b) Moore HW, Czerniak R Naturally Occurring Quinones as Potential Bioreductive Alkylating Agents. Med. Res. Rev 1981, 1, 249–280. [DOI] [PubMed] [Google Scholar]
- (30).(a) Gaudiano G; Frigerio M; Bravo P; Koch TH Reduction of Daunomycin in Dimethyl Sulfoxide. Long-Lived Semiquinones and Quinone Methide and Formation of an Enolate at the 14-Position via the Quinone Methide. J. Am. Chem. Soc 1992, 114, 3107–3113. [Google Scholar]; (b) Gaudiano G; Frigerio M; Sangsurasak C; Bravo P; Koch TH Reduction of Daunomycin and 11-Deoxydaunomycin with Sodium Dithionite in DMSO. Formation of Quinone Methide Sulfite Adducts and the First NMR Characterization of an Anthracycline Quinone Methide. J. Am. Chem. Soc 1992, 114, 5546–5553. [Google Scholar]; (c) Schweitzer BA; Koch TH Synthesis and Redox Chemistry of 5-Deoxydaunomycin. A Long-Lived Hydroquinone Tautomer. J. Am. Chem. Soc 1993, 115, 5440–5445. [Google Scholar]
- (31).(a) Kleyer DL; Koch TH Spectroscopic Observation of the Tautomer of 7-Deoxydaunomycinone from Elimination of Daunosamine from Daunomycin Hydroquinone. J. Am. Chem. Soc 1983, 105, 2504–2505. [Google Scholar]; (b) Bird DM; Gaudiano G; Koch TH Leucodaunomycins, New Intermediates in the Redox Chemistry of Daunomycin. J. Am. Chem. Soc 1991, 113, 308–315. [Google Scholar]; (c) Schweitzer BA; Koch TH Glycosidic Cleavage from Anaerobic Saponification of the Heptaacetate of Daunomycin Hydroquinone. J. Am. Chem. Soc 1993, 115, 5446–5452. [Google Scholar]; (d) See also ref 30. [Google Scholar]
- (32).Gaudiano G; Koch TH Reaction of the Quinonemethide from Reductive Glycosidic Cleavage of Daunomycin with Molecular Oxygen. Evidence for Semiquinonemethide Formation. J. Am. Chem. Soc 1990, 112 (25), 9423–9425. [Google Scholar]
- (33).(a) Ref 8i. For similar hydroxylation at C-7 in other anthracyclinones, see:Kende AS; Tsay Y-G; Mills JE Total Synthesis of (±)-Daunomycinone and (±)-Carminomycinone. J. Am. Chem. Soc 1976, 98, 1967–1969.Wong CM; Schwenk R; Popien D; Ho TL The Total Synthesis of Daunomycinone. Can. J. Chem 1973, 51, 466–467.Smith TH; Fujiwara AN; Lee WW; Wu HY; Henry DW Synthetic Approaches to Adriamycin. 2. Degradation of daunorubicin to a nonasymmetric tetracyclic ketone and refunctionalization of the A ring to adriamycin. J. Org. Chem 1977, 42, 3653–3660.Kimball SD; Walt DR; Johnson F Anthracyclines and Related Substances. 3. Regiospecific Total Synthesis of 11-Deoxydaunomycinone. J. Am. Chem. Soc 1981, 103, 1561–1563.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.