Abstract
Background
The prevalence of infections due to nontuberculous mycobacteria (NTM) is increasing worldwide, yet little is known about the epidemiology and pathophysiology of these ubiquitous environmental organisms. Pulmonary disease due to Mycobacterium avium complex is most prevalent, but many other NTM species can cause disease in virtually any organ system. As NTM becomes an increasingly common cause of morbidity and mortality, more information is needed about the epidemiology of NTM disease.
Methods
We conducted a retrospective chart review of all patients with cultures that grew NTM at a Midwestern tertiary hospital from 1996 to 2017. Information on demographics, medical history, clinical findings, treatment, and outcome was obtained from medical records of all NTM isolates. American Thoracic Society/Infectious Diseases Society of America criteria were used to define pulmonary NTM infections.
Results
We identified 1064 NTM isolates, 365 of which met criteria for NTM infection. Pulmonary cases predominated (185 of 365; 50.7%), followed by skin/soft tissue (56 of 365; 15.3%), disseminated (40 of 365; 11%), and lymphatic (28 of 365; 7.7%) disease. Mycobacterium avium complex was the most common species (184 of 365; 50.4%). Individuals aged >50 years were most affected (207 of 365; 56.7%). Common comorbidities included structural lung disease (116 of 365; 31.8%), use of immunosuppressive medications (78 of 365; 21.4%), malignancy (59 of 365; 16.2%), and human immunodeficiency virus (42 of 365; 11.5%).
Conclusions
This large cohort provides information on the demographics, risk factors, and disease course of patients with pulmonary and extrapulmonary NTM infections. Most patients had medical comorbidities that resulted in anatomic, genetic, or immunologic risk factors for NTM infection. Further population-based studies and increased disease surveillance are warranted to further characterize NTM infection prevalence and trends.
Keywords: atypical mycobacteria, nontuberculous mycobacteria
We describe demographics, comorbidities, clinical presentation, radiographic and microbiologic information, treatment, and outcomes of patients with nontuberculous mycobacterial infections at a Midwestern tertiary care center over 2 decades. The findings shared similar patterns to other population-based studies on NTM infection.
Nontuberculous mycobacteria (NTM), generally defined as mycobacteria not in the Mycobacterium tuberculosis complex (MTC) and other than Mycobacterium leprae, represent a diverse and emerging group of pathogens that cause disease in both immunocompromised and immunocompetent people [1]. Nontuberculous mycobacteria are ubiquitous in the environment and are thought to be acquired through exposure to water, soil, animals, plants, and food sources [2, 3]. Nontuberculous mycobacteria infections can involve any organ system but most commonly manifest as pulmonary disease, skin/soft tissue infection (SSTI), and lymphadenitis [1]. Higher rates of infection are seen in patients with underlying anatomic/structural organ abnormalities (eg, cystic fibrosis, bronchiectasis, emphysema) or immunodeficiency (eg, human immunodeficiency virus [HIV], organ transplant, immunosuppressive medications) [2, 3]. Often, NTM infections present insidiously and mimic other conditions, resulting in delayed diagnosis and significant morbidity and mortality [4]. Treatment methods and success rates vary depending on the organism and disease site.
The incidence of NTM infection is likely increasing at a steep rate [5]. Brode et al [6] found that although rates of tuberculosis are declining in many countries, infection and death from NTM are rising. In the 1980s, NTM disease prevalence was reported at 1.6 to 1.8 per 100 000 people, whereas recent studies have shown a prevalence of 14.1 per 100 000 in North America [4]. These infections disproportionately affect the elderly, suggesting that NTM disease will become even more common as the population ages [4]. With increasing NTM-related morbidity and mortality worldwide, more epidemiologic information is needed to guide diagnosis and management. In the United States, population-based data are lacking, partly because NTM are not reportable to public health authorities [7]. Much of the available data comes from laboratory-based surveillance, which does not provide the clinical or radiographic information needed to distinguish true infection from colonization or contamination. In this study, thorough medical record review was performed on all patients with NTM culture isolates at a Midwestern tertiary hospital from 1996 to 2017. We collected clinical, radiographic, and microbiologic data from medical records to identify cases of true NTM infection and further characterize patient demographics, clinical manifestations, risk factors, treatment, and outcomes for these increasingly common infections.
METHODS
Study Design
We conducted a retrospective medical record review of all patients with at least 1 positive NTM culture at the University of Iowa Hospitals and Clinics in Iowa City, Iowa between January 1, 1996 and May 31, 2017. Patients of all ages were eligible for inclusion. Complete paper records of final, definitive identifications for all positive mycobacterial cultures were kept through the study period and were used to identify patients for chart review. All chart review data were obtained from hospital electronic health records. The study was approved by the hospital’s institutional review board. During the period covered by this review, the number of hospital beds stayed essentially the same at approximately 820, but the number of clinics expanded from approximately 30 to 300. The University of Iowa serves a population of several million as the only academic institution in the state.
Laboratory Methods
Samples were decontaminated with reagents created by an in-house reagent laboratory, and primary samples and positive cultures were stained with both Auramine-Rhodamine and Ziehl-Neelsen. Blood cultures were collected in BACTEC 12B/13A bottles, BACTEC MYCO/F Lytic bottles, or (rarely) Isolator tubes transferred to primary media (Lowenstein-Jensen and 7H10 or 7H11 media). Other broth cultures were performed on the BACTEC MGIT 960 system. Sterile material was ground and transferred to these solid media. Solid media were incubated at 37°C in 5%–10% CO2. Skin, connective tissue, joint, and bone specimens were also incubated at 30°C, with an X-factor disk included to recover Mycobacterium haemophilum. Standard incubation was for ≥6 weeks; positive acid-fast bacillus smears that were culture negative at 6 weeks were held for an additional 4 weeks, and if Mycobacterium ulcerans was suspected, cultures were incubated for 8 weeks. Mycobacterium xenopi, which grows only slightly faster at 42°C versus 37°C, is recovered similarly to Mycobacterium avium complex (MAC) under these conditions [8], so primary culture at higher temperature was not performed. Review of archived laboratory procedures reveals minor changes in culture technique over the study period that would not be expected to affect diagnostic yield or identification of NTM significantly. After 2014, bone marrow cultures were additionally incubated in BACTEC MYCO/F Lytic bottles. After 2013, the institution transitioned from cotton swabs, from which mycobacteria were essentially never recovered, to Copan eSwabs, from which mycobacteria are rarely recovered. Throughout the study period, laboratory policy was that tissue or fluid should be pursued over swabs as the preferred diagnostic sample.
The AccuProbe system was used to identify M tuberculosis to complex level, M avium/intracellulare to complex level, and Mycobacterium gordonae (after 2006). If isolates were initially probe-positive for MTC and Mycobacterium bovis infection was suspected, further identification was made by biochemical or genetic methods. After 2014, some MAC isolates were further identified as Mycobacterium chimaera using whole genome sequencing at National Jewish Hospital. Otherwise, MTC and MAC were not routinely identified to the species level. After probes were performed, rapidly growing mycobacteria were identified to group level by 3-day arylsulfatase and standardized growth studies. Subsequently, definitive identification was performed by sending samples to ARUP Laboratories for identification of slow-growers other than MTC, the State Hygienic Laboratory at the University of Iowa and the Centers for Disease Control and Prevention for MTC with susceptibility testing, and the University of Texas Health Science Center at Tyler for identification of rapidly growing NTM by gene sequencing.
Data Collection
Review of the electronic medical record was performed on all patients with at least 1 NTM culture isolate, as well as on patients whose cultures grew M bovis. Although M bovis is a subspecies of the MTC, an exception was made to include these cases in the study because they were clinically significant, iatrogenic infections from use of intravesical bacillus Calmette-Guerin (BCG) for the treatment of bladder cancer. After detailed chart review, cases were excluded from primary data analysis for 1 of 3 reasons: (1) insufficient information in the medical record to diagnose infection (ie, limited/absent documentation of comorbid conditions, clinical presentation, treatment, or outcome of NTM infection), (2) NTM culture isolate identified as a contaminant (defined as a single positive culture without clinical or radiographic evidence of disease), or (3) NTM culture isolate represented colonization (defined as repeat positive cultures from the same specimen site without associated clinical or radiographic evidence of disease). All M gordonae isolates were considered contaminants and excluded, because M gordonae is the least pathogenic mycobacterial species and a frequent specimen contaminant in the hospital environment [9]. For patients who had multiple NTM culture isolates from the same illness episode, only the first isolate was included in data analysis.
Information collected during chart review included patient demographics, relevant comorbid medical conditions (eg, HIV, chronic lung disease, malignancy, transplant recipient), relevant exposures (eg, recent travel, drug or tobacco use, recent healthcare interventions), and chronic medications, specifically focusing on immunosuppressive medications defined as systemic corticosteroids at doses equivalent to ≥15 mg/day prednisone for ≥1 month, tumor necrosis factor inhibitors, and a variety of non-corticosteroid immunosuppressive agents including immunophilin-binding drugs, antimetabolites, and alkylating agents. We collected information on the clinical presentation and longitudinal course of NTM infection, microbiologic data, and any available laboratory, radiographic, and histopathologic data. Finally, all available notes were reviewed to determine the treatment regimen and clinical outcome for each patient. Clinical outcomes were grouped into 3 categories based on the most recent information in the medical record at the end of the study period: resolution of infection, chronic/persistent infection, and death. Cause of death was most commonly determined by clinical judgment of the primary medical team or expert consultants caring for the patient at the time of death, as documented in the medical record. In 4 cases, autopsy records were available to determine cause of death.
Pulmonary Nontuberculous Mycobacteria Infection Definition
Cases of pulmonary NTM infection were diagnosed using the American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) diagnostic criteria [9], which require clinical symptoms and signs of disease, radiographic evidence of disease, and mycobacterial cultures to formulate diagnostic criteria NTM lung disease. Clinical and microbiologic criteria consist of positive culture results from ≥2 separate expectorated sputum samples or ≥1 bronchial wash or lavage. A single investigator collected data from the medical record, reviewed documentation by the patient’s primary team and/or expert consultants (eg, pulmonology, infectious diseases), and synthesized this information to identify cases of pulmonary NTM infection according to ATS/IDSA guidelines.
Extrapulmonary Nontuberculous Mycobacteria Infection Definition
We defined extrapulmonary infection as ≥1 positive culture from an ordinarily sterile site with associated clinical evidence of disease. Categories of extrapulmonary NTM infection included SSTI, lymphadenitis, isolated bacteremia, ocular, sinus, genitourinary, gastrointestinal, intraperitoneal, intraarticular, and bone infections, as well as disseminated disease. Classification into a given category meant that all available microbiologic and clinical information indicated that infection was limited to that single organ system. Disseminated disease was defined as clinical, microbiologic, and/or radiographic evidence of NTM infection in at least 2 nonadjacent organ systems.
Data Analysis
Descriptive statistics were used to examine the frequency and distribution of variables of interest. Multivariable logistic regression analyses were performed to assess for factors associated with disseminated mycobacterial infection and mortality from mycobacterial infection. We reached our final model using a backwards elimination approach, retaining predetermined potential confounders (age and gender) and significant covariates (P < .05 on the Wald test). Model fit was assessed using the Hosmer-Lemeshow goodness-of-fit test. All statistical analyses were performed using STATA 14 (STATA Corporation, College Station, TX).
RESULTS
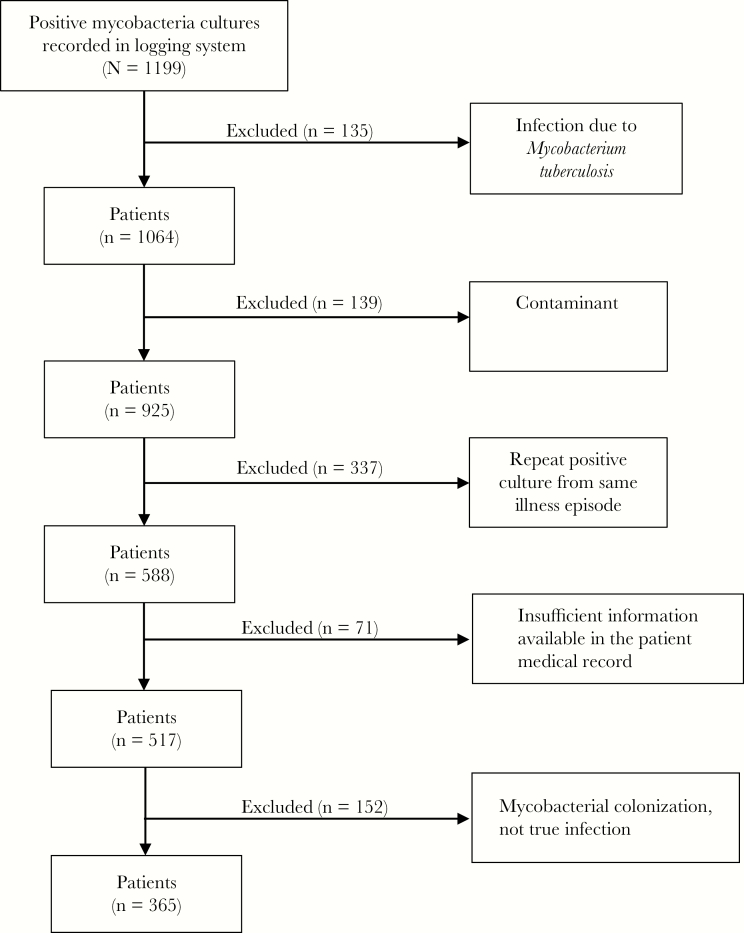
Between 1996 and 2017, the hospital laboratory identified 1064 positive NTM cultures, of which 139 were contaminants, 152 represented colonization, 337 were repeat positive cultures from the same patient and illness, and 71 lacked sufficient information in the medical record to determine whether infection was present (Figure 1). After exclusions, a total of 365 cases met criteria for infection.
Figure 1.
Flowchart of patient selection. A logging system containing the medical record numbers of all patients between 1996 and 2017 at the University of Iowa Hospitals and Clinics with cultures positive for Mycobacterium tuberculous or nontuberculous mycobacteria (NTM) was reviewed. Medical record review was performed on all patients with cultures positive for NTM or Mycobacterium bovis. Infection was defined using the American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) NTM lung disease guidelines. Contaminant was defined as a single positive culture without clinical or radiographic evidence of disease. Colonization was defined as ≥2 positive cultures without clinical or radiographic evidence of disease. Cases were excluded if medical records lacked sufficient information to diagnose infection according to ATS/IDSA guidelines. Cases of chronic NTM infection with multiple positive cultures were included only once in data analysis.
The age of patients meeting disease criteria (n = 365) ranged from 1 to 95 years, with a median age of 55 years. Approximately half (193 of 365; 52.9%) were female. Three hundred twenty-two (88.2%) of the 365 patients were white, 15 (4.1%) were black, 10 (2.7%) were Hispanic, and 6 (1.6%) were Asian. Pulmonary disease predominated (185 of 365; 50.7%), followed by SSTI (56 of 365; 15.3%), disseminated disease (40 of 365; 11.0%), and lymphadenitis (28 of 365; 7.7%) (Table 1). Mycobacterium tuberculosis complex was the most common NTM isolate (184 of 365; 50.4%), followed by Mycobacterium chelonae (54 of 365; 14.8%), Mycobacterium kansasii (21 of 365; 5.8%), Mycobacterium abscessus (20 of 365; 5.5%), and Mycobacterium fortuitum (19 of 365; 5.2%). Females were disproportionately affected by MAC (106 of 184; 57.6%) and M abscessus (14 of 20; 70%), whereas males were more commonly affected by M kansasii (15 of 21; 71.4%).
Table 1.
Frequency of Nontuberculous Mycobacteria Species by Disease Site
| Organism | Pulmonary | SSTI | Lymphatic | Disseminated | Othera | Total |
|---|---|---|---|---|---|---|
| Mycobacterium avium complex | 127 | 2 | 26 | 25 | 4 | 184 (50) |
| Mycobacterium chimaera b | 4 | 4 (1) | ||||
| Mycobacterium chelonae | 10 | 19 | 2 | 23 | 54 (15) | |
| Mycobacterium kansasii | 15 | 5 | 1 | 21 (6) | ||
| Mycobacterium abscessus | 14 | 3 | 1 | 2 | 20 (5) | |
| Mycobacterium fortuitum | 4 | 10 | 5 | 19 (5) | ||
| Mycobacterium bovis | 2 | 7 | 9 (2) | |||
| Mycobacterium marinum | 8 | 1 | 9 (2) | |||
| Mycobacterium xenopi | 4 | 1 | 1 | 0 | 6 (2) | |
| Mycobacterium mucogenicum | 2 | 4 | 6 (2) | |||
| Mycobacterium NOS | 5 | 2 | 2 | 6 | 15 (4) | |
| Combinationc | 5 | 4 | 1 | 10 (3) | ||
| Otherd | 1 | 4 | 1 | 2 | 8 (2) | |
| Total | 185 (51) | 56 (15) | 28 (8) | 40 (11) | 56 (15) | 365 |
Abbreviations: SSTI, skin/soft tissue infection; NOS, not otherwise specified.
NOTE: Values represent number of cases (%).
aIncludes ocular (n = 19), bacteremia (n = 15), sinus (n = 4), genitourinary (n = 6), intraperitoneal (n = 4), intraarticular (n = 4), bone (n = 2), and gastrointestinal (n = 2).
b Mycobacterium chimaera is a member of the M avium complex but was separated for clarity.
cIncludes M avium complex/M chelonae (n = 6), M avium complex/M kansasii (n = 1), M chelonae/M abscessus (n = 1), M fortuitum/M kansasii (n = 1), and M mucogenicum/Mycobacterium phocaicum (n = 1).
dIncludes Mycobacterium neoaurum (n = 2), Mycobacterium terrae complex (n = 2), Mycobacterium obuense (n = 1), Mycobacterium porcinum (n = 1), Mycobacterium scrofulaceum (n = 1), and Mycobacterium smegmatis (n = 1).
Overall, 60 (60 of 365; 16.4%) patients died during the study period, and NTM infection was considered the primary cause of death in 63.3% (38 of 60) of cases. The majority of deaths from NTM resulted from pulmonary and disseminated infections, with case-fatality rates of 10.8% and 50% during the study period, respectively (Table 2). Patients who deceased from mycobacterial infection were compared with patients with NTM infection who deceased from other causes, recovered from infection, or remained stable with chronic infection. Patients whose outcomes were unknown (n = 50) were excluded from analysis. The factors associated with significantly increased odds of mortality from mycobacterial infection were as follows: disseminated mycobacterial infection (odds ratio [OR], 5.0; 95% confidence interval [CI], 1.6–15.0; P = .005), concomitant HIV infection (OR, 5.5; 95% CI, 1.4–21.6; P = .014), history of immunosuppressive therapy (OR, 4.9; 95% CI, 1.9–12.9; P = .001), and history of unintentional weight loss at presentation (OR, 4.0; 95% CI, 1.5–10.4; P = .005).
Table 2.
Demographic and Clinical Characteristics of Patients by Infection Site
| Characteristic | Pulmonary NTM (n = 185) | Disseminated NTM (n = 40) | Extrapulmonary NTMa (n = 140) |
|---|---|---|---|
| Age (Years) | |||
| Median | 63 | 42 | 44 |
| Range | 3–95 | 1–87 | 1–88 |
| Gender | |||
| Female | 101 (55) | 13 (33) | 79 (56) |
| Male | 84 (45) | 27 (68) | 61 (44) |
| Race | |||
| White | 168 (91) | 31 (78) | 123 (88) |
| Black | 4 (2) | 7 (18) | 4 (3) |
| Hispanic | 3 (2) | 1 (3) | 6 (4) |
| Asian | 6 (3) | 0 | 0 |
| Comorbidities | |||
| Structural lung diseaseb | 112 (61) | 0 | 4 (3) |
| Solid malignancy | 36 (19) | 7 (18) | 16 (11) |
| Hematologic malignancy | 12 (6) | 9 (23) | 15 (11) |
| Solid organ transplant | 8 (4) | 2 (5) | 4 (3) |
| Hematopoietic stem cell transplant | 6 (3) | 1 (3) | 4 (3) |
| HIV | 15 (8) | 26 (65) | 1 (1) |
| Iatrogenicc | 1 (1) | 9 (23) | 50 (34) |
| Severe neutropeniad | 2 (1) | 5 (13) | 3 (2) |
| Risk factors | |||
| Current smoker | 101 (55) | 20 (50) | 34 (24) |
| Immunosuppressive medicatione | 34 (18) | 6 (15) | 38 (27) |
| IV drug use | 6 (3) | 5 (13) | 5 (4) |
| Treatmentf | |||
| Medical | 129 (70) | 28 (70) | 89 (64) |
| Surgical | 13 (7) | 5 (13) | 92 (66) |
| Outcome | |||
| Resolved | 60 (32) | 8 (20) | 120 (86) |
| Persistent infection | 60 (32) | 3 (8) | 4 (3) |
| Deceased from NTM | 20 (11) | 20 (50) | 3 (2) |
Abbreviations: HIV, human immunodeficiency virus; IV, intervenous; NTM, nontuberculous mycobacteria.
NOTE: Values represent number of patients (%).
aNondisseminated, extrapulmonary infections.
bChronic obstructive pulmonary disease, bronchiectasis, cystic fibrosis, interstitial lung disease.
cInfections directly related to diagnostic and therapeutic procedures performed on the patient.
dAbsolute neutrophil count ≤500 cells/mm3.
eSystemic corticosteroids at doses equivalent to ≥15 mg/day prednisone ≥1 month, tumor necrosis factor inhibitors, and noncorticosteroid immunosuppressive agents including immunophilin-binding drugs, antimetabolites, and alkylating agents.
fIndicates whether patients’ treatment course included antimicrobial therapy or surgical intervention. Patients could be included in more than 1 category.
Pulmonary Disease
Pulmonary disease accounted for 50.7% (185 of 365) of infections, the majority of which were caused by MAC (127 of 185; 68.6%), then M kansasii (15 of 185; 8.1%) and M abscessus (14 of 185; 7.6%). Demographics of patients with pulmonary disease were similar to the overall study population (Table 2). Sixty-percent (112 of 185; 60.5%) of patients with pulmonary NTM infection had underlying structural lung disease, including 56 (56 of 185; 30.3%) with chronic obstructive pulmonary disease, 49 (49 of 185; 26.5%) with bronchiectasis, 14 (14 of 185; 7.6%) with cystic fibrosis, 11 (11 of 185; 5.9%) with interstitial lung disease, and 5 (5 of 185; 2.7%) with history of lung transplant. The majority (101 of 185; 54.6%) of patients with pulmonary disease were cigarette smokers, and 18.4% (34 of 185) were taking ≥1 immunosuppressive medication at the time of diagnosis. When looking specifically at cases of MAC pulmonary disease (MAC-PD) (n = 127), women were more commonly affected (76 of 127; 59.8%). Less than half (35 of 76; 46.1%) of women with MAC-PD smoked cigarettes, compared with 68.6% (35 of 51) of men with MAC-PD.
The most common initial manifestations of pulmonary NTM infection were cough, hemoptysis, weight loss, and fever. The most common radiographic findings in patients with pulmonary NTM infection were fibrocavitary disease (observed in 52 patients), nodular/bronchiectatic disease (observed in 48 patients), and dense consolidation or solitary, noncavitating mass (observed in 23 patients). Multifocal bronchiectasis alone was observed in 33 patients with pulmonary NTM infection, but the chronicity of radiographic bronchiectasis and whether it preceded the diagnosis of NTM infection could not always be determined. In women with MAC-PD (n = 76), nodular/bronchiectatic radiographic findings were observed 50 patients, and fibrocavitary changes were observed in 19 patients. For men with MAC-PD (n = 51), 27 patients had nodular/bronchiectatic radiographic findings, and 15 had fibrocavitary changes.
Of the patients who received medical treatment (117 of 185; 63.2%), antibiotic regimens typically included macrolide antibiotics (98 of 117; 83.8%), ethambutol (101 of 117; 86.3%), and rifamycin antibiotics (93 of 117; 79.5%). The majority (80 of 117; 68.4%) of patients who received treatment were on anti-mycobacterial medications for ≥12 months, with 1 patient on treatment for 11 years. Approximately one third (60 of 185, 32.4%) of patients with pulmonary NTM had full resolution of infection, 32.4% (60 of 185) had chronic infection, and 18.9% (35 of 185) died. Of the 35 patients who died, NTM was identified as the underlying cause of death in 57.1% (20 of 35) of cases.
Skin/Soft Tissue Infection
There were 56 (56 of 365; 15.3%) SSTIs, the majority (33 of 56; 58.9%) of which occurred in females. Mycobacterium chelonae was the most common organism (19 of 56; 33.9%), followed by M fortuitum (10 of 56; 17.9%) and Mycobacterium marinum (8 of 56; 14.3%). Approximately half (29 of 56; 51.8%) of SSTIs occurred after documented injury to the skin. Fourteen SSTIs (14 of 56; 25%) were iatrogenic infections and occurred at the site of injections, lines and catheters, surgical wounds, or medical implants. There were 15 cases of nonhealthcare-associated trauma preceding SSTI, including gardening injuries, chemical burns, intravenous drug use, tattoos, pedicures, and injuries while cleaning fish tanks. Three of the 15 cases of nonhealthcare-associated trauma involved self-inflicted injuries in patients who were diagnosed with factitious disorder imposed on self (ie, Munchausen syndrome). In all 3 cases, patients presented with recurrent, nonhealing SSTIs that were eventually attributed to self-injection of foreign substances including feces and pond water.
The clinical manifestations of SSTIs were varied and included papules, plaques, nodules, abscesses, cellulitis, ulcers, panniculitis, and 1 case of necrotizing fasciitis. The majority (30 of 56; 53.6%) of SSTIs involved the upper extremities, 15 (15 of 56; 26.8%) involved the trunk, and 11 (11 of 56; 19.6%) involved the lower extremities. Thirty-five (35 of 56; 62.5%) patients required surgical intervention to achieve disease resolution, ranging from simple surgical excision to limb amputation. Full disease resolution was achieved in 83.9% (47 of 56) of cases. One patient with a severe M abscessus SSTI at the exitsite of a percutaneous gastrostomy tube died from septic shock attributed to NTM infection.
Disseminated Disease
Of the 365 patients diagnosed with NTM infection, 40 (11.0%) had disseminated infection. Mycobacterium avium complex was identified in 25 of the 40 cases (62.5%), and an additional 4 patients with disseminated infection had cultures growing a combination of MAC and M chelonae. Cultures were obtained from a variety of sources, including bone marrow, stool, cerebrospinal fluid, peritoneal fluid, prostate tissue, abscesses, and blood. The factors associated with significantly increased odds of disseminated mycobacterial infection were HIV infection (OR, 32.8; 95% CI, 12.8–84.2; P < .001) and having a history of night sweats at presentation (OR, 4.3; 95% CI, 1.6–12.0; P = .005). The presenting signs and symptoms of disseminated disease most commonly included fever (25 of 40; 62.5%), weight loss (14 of 40; 35%), and night sweats (13 of 40; 32.5%). Eleven patients with disseminated NTM had pulmonary involvement of disease; only 1 of these patients had fibrocavitary lung disease on imaging, whereas the rest had a multifocal, reticulonodular pattern.
Ninety-percent (36 of 40) of disseminated NTM infection developed in severely immunocompromised patients, including 26 (26 of 40; 65%) patients with HIV, all of whom had MAC infection. For all 42 patients in this study with HIV, the median CD4 count at the time of NTM infection diagnosis was 23 cells/mm3 (interquartile range, 6–69). Other comorbidities in patients with disseminated infection included idiopathic CD4 lymphopenia, Mendelian susceptibility to mycobacterial diseases, acute leukemia, and severe neutropenia, defined as absolute neutrophil count <500 cells/mm3. As previously reported by Marra et al [10], there were 4 cases of disseminated infection caused by M chimaera, a MAC subspecies, in patients who had been exposed to contaminated heater-cooler units used during cardiothoracic surgery. There were 2 cases of disseminated infection from M bovis after intravesical BCG therapy for bladder cancer.
Lymphadenitis
There were 28 cases of NTM lymphadenitis, and the majority (26 of 28; 92.9%) of patients were ≤6 years of age. Twenty (71.4%) of the 28 patients were female. Only 2 adult patients were diagnosed with isolated lymphadenitis, both of whom were severely immunocompromised. Twenty-six (92.9%) of the 28 cases were caused by MAC; the remaining 2 cases were caused by Mycobacterium scrofulaceum and M xenopi. All patients presented with a progressively enlarging head/neck mass, often with overlying erythematous skin changes. Swelling was painless in 24 of 28 cases. Fever was reported in only 2 patients. All cases of lymphadenitis occurred in the head/neck region and involved either cervical (16 of 28; 57.1%), submandibular (7 of 28; 25.0%), or preauricular (5 of 28; 17.9%) lymph nodes. All patients required surgical excision of the infected node, and 8 (8 of 28; 28.6%) received adjuvant anti-mycobacterial therapy; the median duration of therapy in these patients was 2 months. All cases of lymphadenitis fully resolved with treatment.
DISCUSSION
This study represents one of the largest retrospective chart reviews on NTM infections in a cohort of patients diagnosed at a Midwestern facility over the course of 2 decades. By reviewing medical records, we were able to correlate NTM cultures with clinical and radiographic data to identify clinically significant NTM infection using the ATS/IDSA diagnostic criteria [11].
The findings in this study were consistent with US NTM disease patterns reported in the literature, particularly regarding patient demographics, risk factors, organism, and organ involvement. We identified MAC as the most common organism and pulmonary disease as the most common site of infection. These findings reflect the fact that MAC-PD is the most prevalent NTM infection worldwide [7, 11, 12, 13]. The prevalence of MAC compared with other NTM species is not completely understood but may be a result of its ability to form biofilms, high innate chlorine and biocide resistance, and tolerance of extraordinarily low nutrient levels and temperature extremes. These features allow MAC to persist in both treated and untreated water sources, which then presumably leads to human exposure through drinking, bathing, and swimming [14]. There are 2 prototypical phenotypes for MAC-PD: (1) slowly progressive nodular bronchiectatic disease in postmenopausal, nonsmoking females (“Lady Windermere syndrome”) [15] and (2) rapidly progressive fibrocavitary disease in older males with underlying smoking-related lung disease [16]. In accordance with these classic phenotypes, we found that MAC-PD was more common in women, the majority of whom were nonsmokers, whereas most men with MAC-PD were smokers. In this study population, nodular/bronchiectatic changes were the most common radiographic finding in MAC-PD for both men and women.
After lung disease, we identified SSTIs as the most frequent manifestation of NTM infection. The incidence of cutaneous NTM appears to be increasing [17]. Although NTM SSTIs typically remain localized to the skin/soft tissues, they typically have a protracted clinical course requiring surgical interventions and multidrug regimens. Similar to findings from a recent retrospective case series on cutaneous NTM by Philips et al [18], we found that SSTIs were most commonly caused by rapid-growing NTM species (ie, M chelonae, M fortuitum), as well as the slow-grower M marinum. One significant finding from our cohort was that 1 in 4 SSTIs were considered nosocomial infections. We also identified iatrogenic infections of the bloodstream, eye, urinary tract, joint, bone, and intraperitoneal space, as well as disseminated disease from M chimaera. Prevention of nosocomial NTM is challenging when so little is known on the mechanism of disease acquisition and pathophysiology [11]. Currently, water sources, particularly tap water, pose the greatest risk for healthcare-associated NTM infections, particularly SSTIs. The use of hospital tap water during any sterile or nonsterile procedure should be avoided to prevent healthcare-associated NTM outbreaks [11].
The majority of patients in our cohort were >50 years old, reflecting the strong association between aging and NTM infection [7, 12]. This association may be due to decreased efficiency of cell-mediated immunity, as well structural and functional changes in organ systems that occur with aging [19]. Regardless of the affected organ system, most patients in our cohort had pre-existing structural, immunologic, or genetic abnormalities that conferred increased risk for infection, such as structural lung disease in patients who acquired pulmonary infections, physical tissue trauma preceding the development of SSTIs, and patients with HIV who developed disseminated NTM. Increased prevalence of NTM infection in patients with predisposing factors is well documented in the literature [1]. Szymanski et al [20] suggested that even in otherwise healthy patients with NTM pulmonary disease, there are multiple underlying genetic variants that may increase susceptibility to infection.
Although aging, medical comorbidities, and immunodeficiencies are major risk factors for NTM infection, Al-Houqani et al [21] demonstrated that these factors only partly account for the increasing prevalence of NTM disease. There are likely unknown environmental and/or pathogen-specific factors that are also contributing to increased disease prevalence. This concept is demonstrated in the epidemiology of NTM lymphatic infections. Mycobacterial lymphadenitis is a relatively common pediatric infection that typically occurs in otherwise healthy children. The prevailing theory is that children acquire NTM from drinking water and/or frequent hand-to-mouth contact with NTM in soil [11, 22]. Historically, M scrofulaceum was the most common cause of NTM lymphadenitis in the United States until approximately 1975, when rates of M scrofulaceum sharply declined and MAC became the predominant cause of infection [14]. Primm et al [14] proposed that this shift may have resulted from the implementation of the Clean Water Act in 1972, when increased use of chlorination of public water supplies allowed chlorine-resistant NTM species (ie, MAC) to persist, whereas the more chloride-susceptible M scrofulaceum became less prevalent [14, 23]. This is reflected in our cohort, where almost all cases of lymphadenitis were caused by MAC and only 1 was from M scrofulaceum. Environmental and pathogen-specific factors may also explain the geographical differences in NTM species worldwide [14]. Similar to other North American-based studies, we found that M chelonae, M kansasii, M abscessus, and M fortuitum were the most common causes of infection after MAC, whereas Mycobacterium malmoense, M xenopi, and M scrofulaceum predominate in other parts of the world [7, 11, 24]. In addition to worldwide differences in NTM species, Spaulding et al [24] demonstrated that there are likely differences in geographical distribution even within the United States. They found that MAC is more frequently identified in NTM isolates in the South and Northeast, whereas other regions, such as Iowa, have proportionally higher frequencies of M abscessus/M chelonae isolates.
Limitations of this study are similar to the limitations of other epidemiologic studies of NTM and include lack of consensus on diagnostic criteria for extrapulmonary disease and microbiologic challenges with accurate identification and speciation of mycobacteria. Positive cultures for NTM must be interpreted with caution, with consideration of specimen source and organism virulence. When synthesizing information from the medical records of patients with positive NTM cultures, it can be difficult to differentiate between cases of transient infection, true infection, colonization, and contamination, particularly in complex, systemically unwell patients with multiple medical comorbidities. Our findings were also limited by the retrospective nature of the study, the fact that data were collected from a single institution, and the fact that cases identified using nonculture methods (ie, polymerase chain reaction-based methods) were excluded from the study.
Conclusions
This retrospective chart review represents one of the largest reviews of clinical, radiographic, and microbiologic findings of NTM infections, providing a broad overview of the demographics and clinical manifestations of pulmonary and extrapulmonary NTM infections and contributing to the body of knowledge on this clinically significant yet poorly understood class of organisms. Nontuberculous mycobacteria infections pose a serious threat to patients and may risk development of antimicrobial resistance, particularly given that these diseases are notoriously challenging to recognize, diagnose, and treat in a timely manner, if at all [25]. Even within NTM disease states, (1) decisions to manage medically versus surgically and (2) antimicrobial therapeutic selections vary widely, making understanding the epidemiology of infection critical to improving patient outcomes. The most pressing issue in understanding NTM disease is the need for comprehensive surveillance efforts and systematic epidemiologic studies, which could be more readily performed if isolation of NTM or diagnosis of NTM disease were reportable to public health authorities, such as is required in the states of Oregon and Tennessee. In addition, further studies are needed to validate the use of the ATS/IDSA diagnostic criteria as well as to develop guidelines for the diagnosis and treatment of extrapulmonary infections.
Acknowledgments
Author contributions. All authors contributed to the design and implementation of the research, analysis of the results, and writing of the manuscript. C. E. H. and K. A. W. performed this work while at The University of Iowa Hospitals and Clinics.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Koh WJ. Nontuberculous mycobacteria – overview. Microbiol Spectr 2017; 5:TNM17-0024-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Honda JR, Virdi R, Chan ED. Global environmental nontuberculous mycobacteria and their contemporaneous man-made and natural niches. Front Microbiol 2018; 9:2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung J, Ince D, Ford BA, Wanat KA. Cutaneous infections due to nontuberculous mycobacterium: recognition and management. Am J Clin Dermatol 2018; 19:867–78. [DOI] [PubMed] [Google Scholar]
- 4. Mirsaeidi M, Machado RF, Garcia JG, Schraufnagel DE. Nontuberculous mycobacterial disease mortality in the United States, 1999-2010: a population-based comparative study. PLoS One 2014; 9:e91879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park SC, Kang MJ, Han CH, et al. Prevalence, incidence, and mortality of nontuberculous mycobacterial infection in Korea: a nationwide population-based study. BMC Pulm Med 2019; 19:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brode SK, Daley CL, Marras TK. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. Int J Tuberc Lung Dis 2014; 18:1370–7. [DOI] [PubMed] [Google Scholar]
- 7. Cassidy PM, Hedberg K, Saulson A, et al. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis 2009; 49:e124–9. [DOI] [PubMed] [Google Scholar]
- 8. Marx CE, Fan K, Morris AJ, et al. Laboratory and clinical evaluation of Mycobacterium xenopi isolates. Diagn Microbiol Infect Dis 1995; 21:195–202. [DOI] [PubMed] [Google Scholar]
- 9. Arnow PM, Bakir M, Thompson K, Bova JL. Endemic contamination of clinical specimens by Mycobacterium gordonae. Clin Infect Dis 2000; 31:472–6. [DOI] [PubMed] [Google Scholar]
- 10. Marra AR, Diekema DJ, Edmond MB. Mycobacterium chimaera infections associated with contaminated heater-cooler devices for cardiac surgery: outbreak management. Clin Infect Dis 2017; 65:669–74. [DOI] [PubMed] [Google Scholar]
- 11. Griffith DE, Aksamit T, Brown-Elliott BA, et al. ; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 12. O’Brien RJ, Geiter LJ, Snider DE Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis 1987; 135:1007–14. [DOI] [PubMed] [Google Scholar]
- 13. Good RC, Snider DE Jr. Isolation of nontuberculous mycobacteria in the United States. J Infect Dis 1982; 146:829–33. [DOI] [PubMed] [Google Scholar]
- 14. Primm TP, Lucero CA, Falkinham JO 3rd. Health impacts of environmental mycobacteria. Clin Microbiol Rev 2004; 17:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reich JM, Johnson RE. Mycobacterium avium complex pulmonary infection presenting as isolated lingular or middle lob pattern: the lady Windermere Syndrome. Chest 1992;101:1605–9. [DOI] [PubMed] [Google Scholar]
- 16. Kwon YS, Koh WJ, Daley CL. Treatment of Mycobacterium avium complex pulmonary disease. Tuberc Respir Dis (Seoul) 2019; 82:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc 2013; 88:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Philips RC, Hoyer PE, White SM, et al. Cutaneous nontuberculous mycobacteria infections: a retrospective case series of 78 patients from the Texas Gulf Coast Region. J Am Acad Dermatol 2019; 81:730–9. [DOI] [PubMed] [Google Scholar]
- 19. Gardner ID. The effect of aging on susceptibility to infection. Rev Infect Dis 1980; 2:801–10. [DOI] [PubMed] [Google Scholar]
- 20. Szymanski EP, Leung JM, Fowler CJ, et al. Pulmonary nontuberculous mycobacterial infection. a multisystem, multigenic disease. Am J Respir Crit Care Med 2015; 192:618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Houqani M, Jamieson F, Mehta M, et al. Aging, COPD, and other risk factors do not explain the increased prevalence of pulmonary Mycobacterium avium complex in Ontario. Chest 2012; 141:190–7. [DOI] [PubMed] [Google Scholar]
- 22. Haverkamp MH, Arend SM, Lindeboom JA, et al. Nontuberculous mycobacterial infection in children: a 2-year prospective surveillance study in the Netherlands. Clin Infect Dis 2004; 39:450–6. [DOI] [PubMed] [Google Scholar]
- 23. Falkinham JO., 3rd Factors influencing the chlorine susceptibility of Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum. Appl Environ Microbiol 2003; 69:5685–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spaulding AB, Lai YL, Zelazny AM, Olivier KN, Kadri SS, Prevots DR, Adjemian J. Geographic distribution of nontuberculous mycobacterial species identified among clinical isolates in the United States, 2009–2013. Ann Am Thorac Soc 2017; 14:1655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGrath EE, Anderson PB. Increased prevalence of non-tuberculous mycobacteria infection. Lancet 2007; 370:28. [DOI] [PubMed] [Google Scholar]