The advent of biologics has evolved the treatment options for patients with inflammatory bowel disease (IBD). However, there are issues with nonadherence, especially with infusible biologics, as patients have to physically come to the hospital.1 Nonadherence is associated with higher rate of loss of response to biologic therapy, hospitalization, and increased health care cost.2 , 3
The adherence to infusible biologic (infliximab, inflectra, renflexis, and vedolizumab) is of increased concern during the time of the COVID-19 pandemic. Since the national emergency declared in March 2020 for COVID-19 pandemic, patients are avoiding going to the hospital for regular care because they are fearful of contracting COVID-19, which has resulted in a >50% drop in hospital volume.4 The effect of COVID-19 on the adherence of infusible biologic is unknown.
The aims of our study were to evaluate whether adherence to scheduled infusions of biologics declined after the declaration of national emergency and to determine factors associated with reduced adherence during the COVID-19 crisis. To understand the clinical consequence of nonadherence to biologics infusion, we also explored the association between adherence status and subsequent 6-month and 12-month corticosteroid requirements using 2019 data.
Methods
We conducted a nationwide retrospective cohort study using US national Veterans Affairs health care system data from the Veterans Affairs Informatics and Computing Infrastructure. The eligible IBD patients were identified from the Veterans Affairs health care system using a previously validated algorithm (Supplementary Material). To be included in the cohort, a patient must have received an infusible biologic between January 3, 2020 and March 13, 2020 (ie, the 10-week period before March 13, 2020, which is the date the United States declared a national emergency).
The outcome was adherence with infusible biologics, which was defined as receiving an infusion within 10 weeks of the prior infusion. We chose 10 weeks because the maximum interval for infusion treatments is 8 weeks; we added 2 weeks to account for logistical challenges. Any patient that did not receive an infusion after 10 weeks was considered nonadherent. IBD patients from the same months in 2019 were also included as a benchmark to determine baseline adherence rate. Inclusion criteria and adherence in 2019 was defined in the same way as that in 2020.
We used logistical regression with adherence as the outcome variable. Only patients in the 2020 cohort were included to identify factors associated with adherence during the COVID-19 crisis. Candidate predictors included age, sex, race, IBD type, geographical region, concomitant thiopurine use, and Charlson Comorbidity Index. We did a backward selection and kept only the variables with P < .05 in the final model.
We also conducted 2 secondary analyses. For patients in 2019, we defined an index date that was 10 weeks after their last infusion between January 3, 2019 and March 13, 2019. We then looked at the incidence of any oral or intravenous corticosteroid use by adherence status within the 6-month and 12-month periods after the index date, respectively. Chi-square test was used for P values. In addition, we examined the weekly number of infusible biologics in Veterans Affairs Informatics and Computing Infrastructure between January 1, 2019 and May 31, 2020.
Results
We identified 2510 and 2516 patients in 2019 and 2020, respectively. The characteristics were similar in both groups (Table 1 ). Adherence was 84.6% in 2019 and 73.6% in 2020 (P < .0001 for the difference). In the final logistic regression model, higher Charlson Comorbidity Index was associated with lower adherence (odds ratio, 0.95 for 1-unit increase in Charlson Comorbidity Index; 95% confidence interval, 0.90–0.99; P = .048). The range of adherence to infusible biologics was from 68.4% to 77.3% across the different geographical locations in 2020. Compared with the Midwest, patients from the Southeastern region were less compliant (odds ratio, 0.635; 95% confidence interval, 0.487–0.828; P = .0008).
Table 1.
Patient Characteristics in 2019 and 2020
| Characteristic | 2020 |
2019 |
P value | ||
|---|---|---|---|---|---|
| n | Column % | n | Column % | ||
| All | 2516 | 100.0 | 2510 | 100.0 | — |
| Concomitant thiopurine | .435 | ||||
| Yes | 325 | 12.9 | 343 | 13.7 | |
| No | 2191 | 87.1 | 2167 | 86.3 | |
| Age | .922 | ||||
| <50 y | 1035 | 41.1 | 1046 | 41.7 | |
| 50–65 y | 705 | 28.0 | 700 | 27.9 | |
| >65 y | 776 | 30.8 | 764 | 30.4 | |
| Sex | .832 | ||||
| Male | 2250 | 89.4 | 2240 | 89.2 | |
| Female | 266 | 10.6 | 270 | 10.8 | |
| Race | .622 | ||||
| White | 873 | 34.7 | 907 | 36.1 | |
| Black | 164 | 6.5 | 157 | 6.3 | |
| Other | 14 | 0.6 | 18 | 0.7 | |
| Unknown | 1465 | 58.2 | 1428 | 56.9 | |
| IBD diagnosis | .586 | ||||
| Crohn’s disease | 1354 | 53.8 | 1370 | 54.6 | |
| Ulcerative colitis | 1162 | 46.2 | 1140 | 45.4 | |
| Geographic region | .762 | ||||
| Continental | 369 | 14.7 | 369 | 14.7 | |
| Midwest | 644 | 25.6 | 650 | 25.9 | |
| North Atlantic | 601 | 23.9 | 598 | 23.8 | |
| Pacific | 411 | 16.3 | 435 | 17.3 | |
| Southeast | 491 | 19.5 | 458 | 18.2 | |
| Charlson Comorbidity index | .942 | ||||
| 0 | 1558 | 61.9 | 1551 | 61.8 | |
| 1 | 451 | 17.9 | 443 | 17.6 | |
| 2–3 | 329 | 13.1 | 338 | 13.5 | |
| 4–5 | 109 | 4.3 | 108 | 4.3 | |
| 6–7 | 48 | 1.9 | 43 | 1.7 | |
| 8+ | 21 | 0.8 | 27 | 1.1 | |
| Compliance | < .0001 | ||||
| Yes | 1851 | 73.6 | 2123 | 84.6 | |
| No | 665 | 26.4 | 387 | 15.4 | |
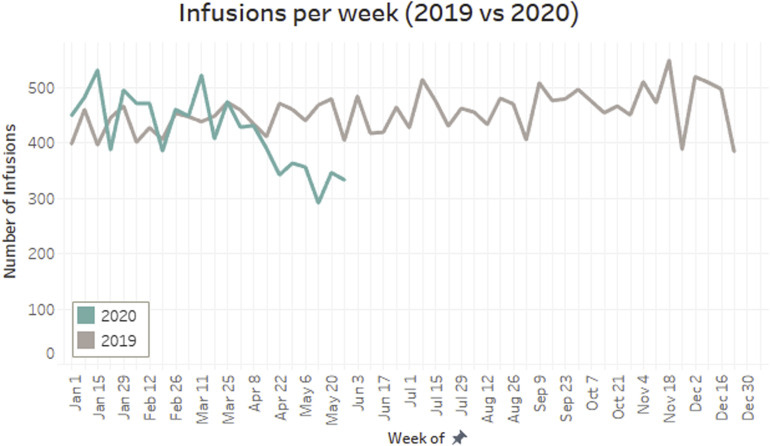
In 2019, the 6-month incidence of any oral or intravenous corticosteroid use for those who adhered and who did not adhere were 18.2% and 23.5% (P = .014), and for 12 months it was 25.2% and 33.1% (P < .001), respectively. In Supplementary Figure 1, the weekly number of infusions remained stable in 2019 and started to decrease in late March 2020 and the decreasing trend continued through May 2020.
Supplementary Figure 1.
Number of Infusions per week for patients with IBD in 2019 and 2020.
Discussion
To our knowledge, this study is the first that evaluated the impact of the COVID-19 pandemic on the adherence of infusible biologics in patients with IBD. We found that adherence decreased from 84.6% in 2019 to 73.6% during the 2020 COVID-19 crisis. This reduction translated into a precipitous and persistent drop in the weekly number of infusions since late March 2020.
Recent studies have found that anti–tumor necrosis factor medications do not increase the risk of getting COVID-19 or the worse outcomes of COVID-19.5 , 6 Despite these findings and the importance of biologics in inducing and maintaining disease remission, our study found that nonadherence to infusible biologics increased by 70% after the COVID-19 pandemic began among patients with IBD nationwide. This is particularly relevant because scheduled maintenance therapy is superior to intermittent therapy to preserve remission and prevent formation of antibodies targeting the drug and infusion reactions.7 Our exploratory analysis also confirmed a significant association between nonadherence to biologics infusion and the subsequent risk of corticosteroid requirement among US veterans.
Major strengths of the study include the use of a nationwide study cohort and using geographically diverse sample patient population. We were able to calculate adherence accurately, as infusion visits are completely captured in the medical records. This study captures recent data with the end point of May 2020. Thus, physicians can review the records of patients who were supposed to get their infusion in the last 3 months to evaluate whether treatment gaps occurred. As the Veterans Affairs health care system does not routinely use home infusions, we could not assess the impact of home infusion vs infusion center.
In conclusion, using a nationwide Veterans Affairs database, we found that nonadherence to infusible biologics increased by 70% (or 11.0% point) after the COVID-19 pandemic began and almost one-quarter of patients had gaps of more than 10 weeks between infusions. Higher comorbidities and geographical locations were associated with increased nonadherence to infusible biologics. Quality improvement efforts, such as education programs for patients about the relative safety of these biologic medications and employment of social distancing within infusion centers, are needed to assure adherence to therapy despite the COVID-19 pandemic.
CRediT Authorship Contributions
Nabeel Khan, MD (Conceptualization: Equal; Methodology: Equal; Supervision: Lead; Writing – review & editing: Equal). Dhruvan Patel, MD (Conceptualization: Equal; Methodology: Supporting; Validation: Equal; Writing – original draft: Lead; Writing – review & editing: Lead). Dawei Xie, PhD (Formal analysis: Equal; Methodology: Equal; Writing – review & editing: Equal). Tyler Pernes, BA (Methodology: Supporting; Validation: Equal). James Lewis, MD (Writing – review & editing: Equal). Yu-Xiao Yang, MD (Formal analysis: Equal; Methodology: Equal; Writing – review & editing: Equal).
Footnotes
Conflicts of interest These authors disclose the following: Nabeel Khan has received research funding from Pfizer, Luitpold, and Takeda Pharmaceuticals. James Lewis has served as a consultant for Merck, AbbVie, Lilly, Janssen, Johnson & Johnson Consumer Inc, and Takeda; has served on Data Safety Monitoring Boards for Pfizer, Gilead, and UCB; and has received research support from Takeda and Nestle Health Science. The remaining authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dxdoi.org/10.1053/j.gastro.2020.06.044.
Supplementary Methods
To be included in the study, patients must meet all of the following criteria: (1) one inpatient or outpatient International Classification of Diseases 9th revision or 10th revision diagnosis code for ulcerative colitis (555.xx), (K50.xx) and/or Crohn’s disease (556.xx), (K51.xx); (2) at least one outpatient/inpatient/telemedicine visit in the Veterans Affairs health care system between January 1, 2020 and May 15, 2020; (3) at least one outpatient pharmacy claim for any of the following IBD medications in groups 1–5 ([1] 5-aminosalicylate compounds, [2] thiopurines, [3] anti–tumor necrosis factor agents, [4] combination of thiopurines and anti–tumor necrosis factor and [5] vedolizumab); and (4) at least 2 different prescriptions of 1 distinct IBD medication in groups 1–5.
References
- 1.Wentworth B.J., et al. Inflamm Bowel Dis. 2018;24:2053–2061. doi: 10.1093/ibd/izy102. [DOI] [PubMed] [Google Scholar]
- 2.Van der Have M., et al. J Crohn Colitis. 2016;10:549–555. doi: 10.1093/ecco-jcc/jjw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter C.T., et al. Adv Ther. 2011;28:671. doi: 10.1007/s12325-011-0048-7. [DOI] [PubMed] [Google Scholar]
- 4.Wong L.E., et al. 2020 May 14. NEJM Catal Innov Care Deliv. [Google Scholar]
- 5.Khan N., et al. Gastroenterology. 2020 In press. [Google Scholar]
- 6.Brenner E.J., et al. Gastroenterology. 2020 In press. [Google Scholar]
- 7.Strand V., et al. BioDrugs. 2017;31:299–316. doi: 10.1007/s40259-017-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]