Abstract
Non-alcoholic fatty liver disease (NAFLD) comprises fatty liver (steatosis), non-alcoholic steatohepatitis (NASH) and fibrosis/cirrhosis and may lead to end-stage liver failure or hepatocellular carcinoma. NAFLD is tightly associated with the most frequent metabolic disorders, such as obesity, metabolic syndrome, and type 2 diabetes mellitus (T2DM). Both multisystem diseases share several common mechanisms. Alterations of tissue communications include excessive lipid and later cytokine release by dysfunctional adipose tissue, intestinal dysbiosis and ectopic fat deposition in skeletal muscle. On the hepatocellular level, this leads to insulin resistance due to abnormal lipid handling and mitochondrial function. Over time, cellular oxidative stress and activation of inflammatory pathways, again supported by multiorgan crosstalk, determine NAFLD progression. Recent studies show that particularly the severe insulin resistant diabetes (SIRD) subgroup (cluster) associates with NAFLD and its accelerated progression and increases the risk of diabetes-related cardiovascular and kidney diseases, underpinning the critical role of insulin resistance. Consequently, lifestyle modification and certain drug classes used to treat T2DM have demonstrated effectiveness for treating NAFLD, but also some novel therapeutic concepts may be beneficial for both NAFLD and T2DM. This review addresses the bidirectional relationship between mechanisms underlying T2DM and NAFLD, the relevance of novel biomarkers for improving the diagnostic modalities and the identification of subgroups at specific risk of disease progression. Also, the role of metabolism-related drugs in NAFLD is discussed in light of the recent clinical trials. Finally, this review highlights some challenges to be addressed by future studies on NAFLD in the context of T2DM.
Keywords: Fatty liver, Type 2 diabetes, Insulin resistance, Biomarkers, Glucose-lowering drugs, Clinical trials
Highlights
-
•
T2DM and NAFLD are heterogeneous tightly associated diseases.
-
•
Insulin resistance is a common player in both NAFLD and T2DM.
-
•
Tissue cross-talk and multicellular network drives NAFLD initiation and progression.
-
•
The performance of NAFLD biomarkers is suboptimal in T2DM.
-
•
Antihyperglycemics, GLP-1ra and SGLT2i, have beneficial effects on NAFLD and CVD.
1. Introduction
Nonalcoholic fatty liver diseases (NAFLD) is currently defined by lipid deposition that exceeds >5% of hepatocytes, as assessed from liver biopsy, and/or by >5.6% hepatocellular fat content per liver weight, as assessed from magnetic resonance (MR) methods, in the absence of significant alcohol consumption and other causes of fatty liver [1,2]. NAFLD, which affects about 25% of the population [3], comprises a broad range of abnormalities ranging from simple fatty liver (steatosis) to non-alcoholic steatohepatitis (NASH), characterized by inflammation, necrosis, and hepatocellular ballooning, and progression to liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [2].
Some gene variants promote risk of NAFLD by altering lipid droplet formation and de novo lipogenesis (DNL), such as variants of patatin-like phospholipase domain-containing protein 3 (PNPLA3) and glucokinase regulatory protein [4], or by decreasing very-low-density lipoproteins (VLDL) export as shown for a missense mutation (E167K) in transmembrane 6 superfamily member 2 (TM6SF2) [5].
Nevertheless, NAFLD is tightly associated with common acquired metabolic diseases such as obesity and type 2 diabetes (T2DM). The mutual relationship between both diseases is illustrated by several epidemiological data. The prevalence of steatosis and NASH has been estimated to be 50 and 56%, respectively, in T2DM [6]. The age-adjusted relative risk of NAFLD is about 5.36fold higher in T2DM compared to healthy humans [7]. T2DM is also an emerging risk factor for NASH progression to advanced fibrosis, cirrhosis and HCC [8,9]. Diabetes was even a better predictor for HCC development in people with NASH and cirrhosis compared to other metabolic risk factors such as hyperlipidemia, body mass index (BMI) and hypertension [10]. Recently, a consensus panel has proposed to rename NAFLD a metabolic-dysfunction-associated fatty liver disease (MAFLD) based on the presence of overweight/obesity, T2DM and evidence of so-called “metabolic dysregulation” [11]. Future will tell, if this will help to better understand the multiple relationships between NAFLD and T2DM. In this context, NAFLD per se associates with more than double risk of incident diabetes pointing to specific liver-related mechanisms [12,13]. Moreover, multicenter studies in Skandinavia and Germany have recently found that diabetes can be stratified into subtypes (clusters) with different clinical and metabolic features [14,15]. The German Diabetes Study (GDS) found that only the severe insulin-resistant diabetes cluster (SIRD) had increased prevalence of NAFLD and its progression along with higher risk of diabetic-kidney disease and cardiovascular disease (CVD) [14]. Indeed, presence of T2DM in NAFLD results in a 2.3 and 2.8fold hazard ratio for overall and CVD-related mortality, respectively [16]. Whether NAFLD is also an independent risk factor for CVD [12,17] is currently under debate [18].
Despite discussions on causality, there is evidence for a bi- or even tridirectional relationship at least between subtypes of T2DM, NAFLD, and CVD, which could be linked by insulin resistance [12,19,20]. Several mechanisms of insulin resistance and cellular metabolism can contribute to NAFLD and its progression [20], but also help to develop diagnostic tools and treatment opportunities.
2. Mechanisms and consequences of metabolic alterations
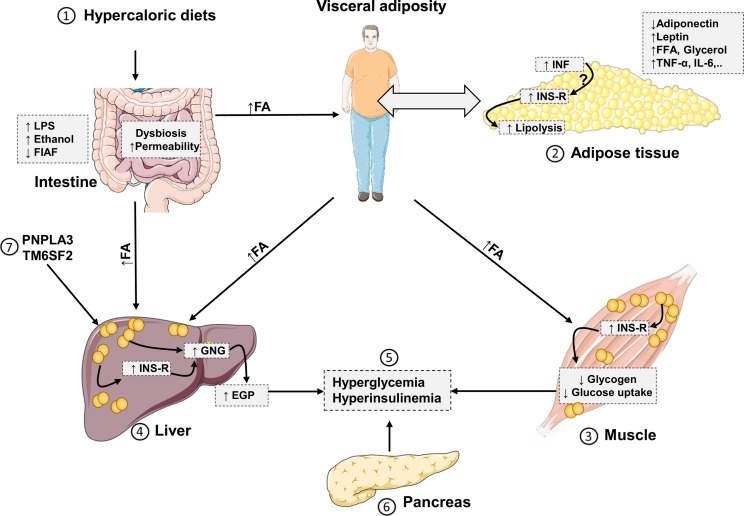
The two-hit hypothesis, which is based on exposure of the liver to a first hit “liver steatosis” followed by a second hit such as oxidative stress does not serve to explain the complexity of NAFLD. The current “multiple- or continuous-hit model” suggests the existence of different and continuous insults, which might be generated in individuals with T2DM or metabolic syndrome due to altered inter-organ crosstalk between liver, adipose tissue, intestine, and skeletal muscle (Fig. 1 ) [21]. These multiple insults would cooperatively and synergistically drive the development and progression of NAFLD, particularly in genetically predisposed individuals [22]. The earliest events initiating NAFLD may reside in hypercaloric energy-dense dietary habits [23]. A single oral intake of saturated fat has been shown to rapidly induce skeletal muscle, adipose tissue and hepatic insulin resistance along with 70% increased gluconeogenesis and upregulated inflammatory pathways [24]. Chronically positive energy balance will enlarge adipose tissue compartments and lead to dysfunctional adipose tissue with excessive lipolysis, overflow of free fatty acids (FFA) elevated gluconeogenesis and again insulin resistance [25].
Fig. 1.
Communication between hepatic and extrahepatic tissues during development of NAFLD 1) hypercaloric energy-dense diets could induce intestinal dysbiosis and promote fat storage in adipose tissues resulting in visceral adiposity. Other intestinal changes include intestinal permeability, which facilitates translocation of inflammatory LPS into the liver. Also, suppression of FIAF by gut dysbiosis promote fat storage in peripheral tissues 2) excess TAG in adipose tissue stimulate inflammation and insulin resistance. There is no consensus about if inflammation precedes insulin resistance or vice versa. Insulin resistance leads to increased lipolysis and release of FFA, which promote ectopic fat deposition in liver and muscle. Adipose tissue-derived cytokines and adipokines could also regulate insulin sensitivity in liver and muscle 3) increased fat storage in the skeletal muscle could be associated with increased insulin resistance leading to suppression of insulin-stimulated GLUT4-glucose uptake by myocytes and decrease of glycogenesis [25] 4) similarly, liver steatosis could be associated with hepatic insulin resistance i.e., increased gluconeogenesis and decreased glycogen synthesis. Importantly, metabolites of β-oxidation of FFA could allosterically activate gluconeogenesis-related enzymes [25] 5) hyperglycemia arise as a result of muscle and liver insulin resistance 6) pancreas secretes more insulin in response to peripheral and hepatic insulin resistance leading to hyperinsulinemia 7) genetic variants such as PNPLA3 and TM6SF2 interfere with lipid metabolism and export promoting liver steatosis. EGP, endogenous glucose production; FFA, free fatty acids; FIAF, fasting-induced adipocyte factor; GNG, gluconeogenesis; IL, interleukin; INF, inflammation; INS-R, insulin resistance; LPS, lipopolysaccharide; TNF, tumor necrosis factor.
2.1. Tissue-crosstalk
2.1.1. Adipose tissue
Early studies in rodents suggested that inflammation in adipose tissues is dispensable for local and systemic insulin resistance [26]. Inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6 were several-fold higher in subcutaneous and visceral tissue, as compared to liver in severely obese humans [27]. Mechanistically, inflammatory cytokines trigger insulin resistance through activation of cellular kinases such as c-Jun N-terminal kinase (JNK) and inhibitor kappa B kinase (IKKβ), which phosphorylate serine residues in insulin receptor substrate (IRS)-1 [28], resulting in increased lipolysis and FFA release into the circulation. On the contrary, a recent study demonstrated that obesity-induced insulin resistance preceded inflammation in adipose tissues of mice [29]. Indeed, adipose tissue inflammation might be a protective feedback mechanism as its local inhibition in mice induced ectopic lipid accumulation in liver, glucose intolerance, and systemic inflammation [30].
Besides released inflammatory cytokines and FFA, adipose tissue could still communicate with the liver and muscle through secretion of different adipokines such as adiponectin and leptin. Persons with NASH have lower serum adiponectin compared to those with NAFLD with or without normal liver enzymes [31]. By contrast, the circulating levels of leptin were higher in people with NAFLD and T2DM, probably due to increased leptin resistance, and were associated with disease severity [32].
2.1.2. Skeletal muscle
Increased FFA influx to skeletal muscle promotes accumulation of intramyocellular lipid (IMCL). Reduced mitochondrial oxidation contributes as well to IMCL as shown in aging and insulin-resistant humans [33]. Consequently, skeletal muscle exhibits insulin resistance, which often precedes the onset of T2DM and insulin resistance in the liver [34]. Lipid intermediate metabolites, in particular sn 1,2 diacylglycerols (DAG), link IMCL to skeletal muscle insulin resistance through activation of protein kinase C-theta (PKCθ) resulting in its translocation from cytoplasm to the plasma membrane [35]. Muscles of insulin resistant humans with obesity and T2DM showed increased DAG content and PKCθ activity as compared to healthy humans [35]. Mutation studies highlighted serine amino acid residue (Ser1101) of IRS1 to be a substrate for activated PKCθ [36]. As a consequence of skeletal muscle insulin resistance, postprandial energy storage shifts from glycogen synthesis in the muscle into triacylglycerol (TAG) in the liver, promoting NAFLD development [25].
2.1.3. Intestine
Gut microbiome is increasingly recognized as a modulator of liver pathogenesis through what is called the “gut-liver axis” [37]. Distinctive alterations of gut microbiome were reported in humans having NASH and T2DM [38]. The intestinal microbiome alters host metabolism by modulating the production of short-chain fatty acids (SCFA) e.g. butyrate, acetate, and propionate, which have beneficial effects on insulin sensitivity, lipid and glucose metabolism [38]. Also, intestinal dysbiosis could associate with increased intestinal permeability permitting translocation of bacterial lipopolysaccharide (LPS) into the systemic circulation, which could induce fat deposition in the liver, NASH progression, weight gain, and diabetes [39]. Moreover, intestinal microbiome could suppress the expression of fasting-induced adipocyte factor (FIAF) in intestinal epithelium, which functions as an inhibitor of circulating lipoprotein lipase, resulting in increased TAG storage in the peripheral tissues [22]. Also, ethanol-producing microbiome could increase blood alcohol concentration in NASH, which is metabolized in the liver generating high levels of reactive oxygen species (ROS). The last mechanism could explain the histological similarity between NASH and alcoholic steatohepatitis [22]. Furthermore, microbiota metabolize liver-derived primary bile acids into secondary bile acids. The latter are reabsorbed into bloodstream and may act as signaling molecules via a variety of receptors including farnesoid X receptor (FXR), which regulates the transcription of different metabolic genes involved in bile acid synthesis, transport, lipogenesis, and glucose homeostasis [40].
2.2. Hepatocellular mechanisms
2.2.1. Insulin resistance and increased DNL
Insulin resistance in both skeletal muscle and adipose tissues initiates liver steatosis by providing precursors and substrates for DNL and mitochondrial β-oxidation e.g. glucose, FFA and glycerol [25]. Although reesterification of FFA derived from diet and adipose tissue is the dominant contributor to TAG pool in the liver (59%), it did not increase in people with NAFLD. On the other side, DNL-derived FFA contribute by about 26%, which is severalfold higher as compared to individuals without NAFLD (10%) [41].
Later, insulin resistance of the liver develops, resulting in increased gluconeogenesis and elevation of endogenous glucose production (EGP) from the liver, which contributes to fasting hyperglycemia in individuals with T2DM [25]. Insulin signaling inhibits typically hepatic gluconeogenesis through Akt-induced phosphorylation of forkhead box (FOXO1) and induces lipogenesis through activation of sterol regulatory element-binding proteins (SREBP1C) and mammalian target of rapamycin complex (mTORC1) pathways. During hepatic insulin resistance, insulin does not suppress gluconeogenesis efficiently, while DNL is preserved or even increased. To explain this discrepancy, pathway-selective hepatic insulin resistance was postulated, which means that only one arm of insulin signaling is defective i.e. Akt/FOXO1 leading to reduced insulin-mediated suppression of gluconeogenesis, whereas insulin-activated SREBP-1C/mTORC1 pathway remains intact and activates lipogenesis [28]. Actually, DNL was reduced after induction of hepatic insulin resistance by feeding mice with a high-fat diet (HFD) [42], which challenges the concept of selective hepatic insulin resistance and suggest the existence of alternate mechanisms that contribute to increased DNL and gluconeogenesis in insulin-resistant liver, for example, hyperinsulinemia, insulin-independent re-esterification of adipose tissue-derived FFA, and increased acetyl CoA generation from β-oxidation of FFA, which allosterically activates pyruvate carboxylase enzyme, leading to enhanced gluconeogenesis [25]. In addition, increased blood glucose level can activate carbohydrate response element-binding protein (ChREBP) signaling pathway, thereby stimulating expression of several glycolytic genes, which provide additional metabolic precursors for DNL [43]. In line, ChREBP overexpression induced stearoyl-CoA desaturase 1 (Scd1) expression, an enzyme responsible for the biosynthesis of monounsaturated fatty acids (MUFA), resulting in increased liver fat content [44].
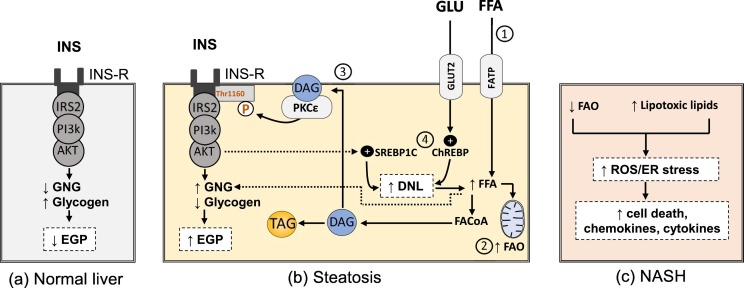
PKC epsilon (PKCε) is crucial in mediating hepatic insulin resistance and once activated by DAG, it translocates to the cell membrane, and phosphorylates specific threonine residue (Thr1160 in human and Thr1150 in mouse) on insulin receptor leading to destabilization of the active configuration of insulin receptor kinase and inhibition of its tyrosine kinase activity [45] (Fig. 2 ). In general, both hyperglycemia and toxic lipids such as ceramides, DAG, FFA, and cholesterol can induce deleterious effects on liver cells (glucolipotoxicity), which might initiate NAFLD progression from simple steatosis to NASH and fibrosis via various mechanisms, including cell death, oxidative stress, endoplasmic reticulum (ER) stress and mitochondrial disorders [46].
Fig. 2.
Altered signaling and pathogenic mechanisms in hepatocytes during NAFLD progression a) insulin signaling in healthy hepatocytes suppress endogenous glucose production through inhibition of gluconeogenesis and increasing glycogen synthesis b) steatotic hepatocytes are characterized by disturbed lipid metabolism i.e., 1) there is increase in FFA influx which enters the liver through fatty acid transport proteins (FATP) and leads to 2) increased mitochondrial fatty acid oxidation, 3) formation of lipid toxic intermediates e.g. sn 1,2 DAG which stimulates PKCε to phosphorylate Thr1160 in insulin receptor leading to hepatic insulin resistance i.e., increased gluconeogenesis and decreased glycogen synthesis. Again, FFA metabolites e.g. acetyl CoA could directly stimulate gluconeogenesis [25] 4) increased glucose enters the liver mainly through glucose transporter (GLUT2) and activates ChREBP pathway. Insulin-signaling activates SREBP1C pathway too. Both pathways increase DNL c) after progression to NASH, mitochondrial flexibility is lost leading to decreased fatty acid oxidation. Together with other toxic intermediate lipid, ER stress and ROS generation increase leading to hepatocytes death and release of inflammatory cytokines and chemokines. AKT, protein kinase B; ChREBP, carbohydrate response element-binding protein; DAG, sn 1,2 diacylglycerol; DNL, de novo lipogenesis; EGP, endogenous glucose production; ER, endoplasmic reticulum; FAO, fatty acid oxidation; FFA, free fatty acids; GLU, glucose; GNG, gluconeogenesis; INS, insulin; INS-R, insulin resistance; IR, insulin receptor; PI3K, phosphatidylinositol-3-kinase; PKC, protein kinase C; ROS, reactive oxygen species; SREBP1C; sterol regulatory element-binding proteins; TAG, triacylglycerol.
2.2.2. Abnormal mitochondrial function and ER stress
Alterations in the activity and abundance of oxidative phosphorylation (OXPHOS) proteins and antioxidant enzymes were described in various animal models of NAFLD [47]. Impaired hepatic mitochondrial function was evident as well in T2DM and NASH [48,49]. In a mouse model of choline-deficient diet-induced NAFLD, mitochondrial OXPHOS was increased at 12 weeks but lost at a later time point [50]. Also, the higher maximal respiration rate of liver mitochondria was severalfold higher in insulin-resistant obese individuals with fatty liver as compared to lean individuals [51]. These studies highlight the flexibility of mitochondria to adapt to increased metabolic inputs to keep energy homeostasis and to protect against the harmful effects of increased FFA and TAG in the liver. In NASH, mitochondrial flexibility was lost, which was associated with increased ROS production and exhaustion of protective antioxidant enzymes [51] (Fig. 2). Whether loss of mitochondrial flexibility is a cause or consequence for NAFLD progression remains obscure. Depletion of ATP due to mitochondrial disorders, together with hyperglycemia and lipid overload could be inducers for another signaling pathway, termed unfolded protein response “UPR”, which is adaptive response to resolve unfolded or misfolded proteins and to restore ER homeostasis [52,53]. Prolonged UPR stress can activate JNK and NF-kB signaling pathways, which are involved in insulin resistance, liver steatosis, and inflammation [52]. Furthermore, ER stress could increase insulin resistance through induction of lipin-2 expression, which is a phosphatase enzyme that catalyzes biosynthesis of DAG leading to activation of DAG/PKCε axis [54]. Interestingly, UPR could also increase liver steatosis through activation of SREBP-1c signaling pathway [53].
2.3. Intrahepatic communication during NAFLD progression
2.3.1. Cell death and cross-talk of non-parenchymal liver cells
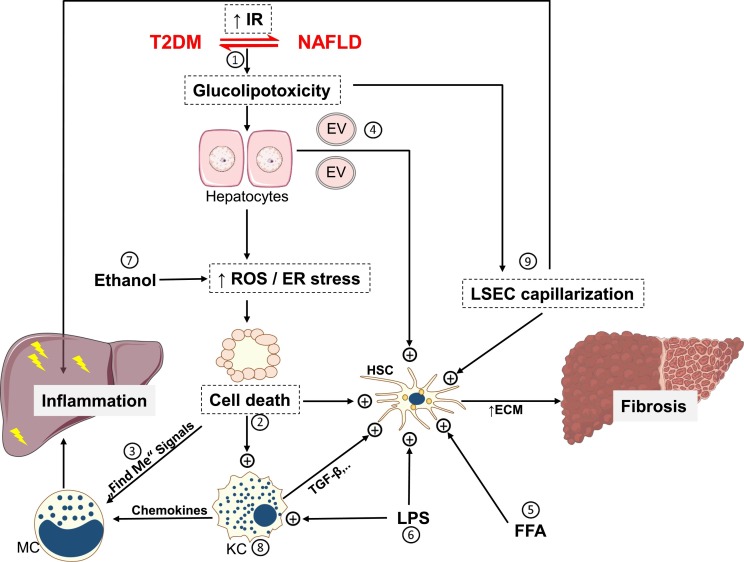
Elevated ROS and UPR are well-identified pathways that could induce hepatocytes death in NASH. Hepatocytes apoptosis and necroptosis are the main forms of cell death in human steatohepatitis and diet-induced mouse models of NASH [55]. The key fibrogenic liver cells, hepatic stellate cells (HSCs), usually exist in a quiescent state and get activated by engulfment of apoptotic bodies, the inflammatory milieu, or damage-associated molecular patterns (DAMPs) released from stressed and dying hepatocytes [56]. Interestingly, FFA-mediated lipotoxic effects stimulate hepatocytes to release extracellular vesicles (EVs) with distinctive microRNAs (miRNA) profile that increase the expression of fibrogenic genes in the surrounding HSCs [57] (Fig. 3 ). Free cholesterol could directly sensitize HSCs to the action of tumor growth factor (TGF)-β, a potent fibrogenic cytokine [58]. Treatment of human and rat immortalized HSCs cell lines with saturated fatty acid increased the expression of various profibrogenic genes [59]. Macrophages aggregate as well around dead hepatocytes forming a crown-like structure, a phenomenon that exists only in persons with NASH but not in those with simple steatosis [60]. Recruitment of more inflammatory cells from systemic circulation is facilitated by “Find me” signals that are released from dead cells [61]. Inhibition of inflammatory monocytes recruitment via inhibition of C-C chemokine receptors type 2 and type 5 (CCR2/CCR5) suppressed fibrogenesis and steatohepatitis in murine NASH [62]. Macrophages can modulate also hepatic insulin resistance and favor TAG accumulation in hepatocytes through secretion of IL-1β that downregulates peroxisome proliferator-activated receptor (PPAR) α, a key transcriptional pathway involved in fatty acid oxidation [63]. Liver sinusoidal cells (LSECs) are fenestrated cells that exist in close vicinity to hepatocytes, macrophages and HSCs, which, under physiological conditions, regulate lipid transport, maintain the quiescence of Kupffer cells, resident liver macrophages, and HSCs [64]. At early stage of NAFLD, LSECs lose their fenestrae, a process termed capillarization, which could favor liver steatosis and initiate HSCs activation [64] (Fig. 3). During NASH, LSECs display a pro-inflammatory phenotype that promotes steatohepatitis [64].
Fig. 3.
Multicellular cross-talks during NAFLD progression 1) insulin resistance is a potential nexus between T2DM and NAFLD that leads to glucolipotoxicity, which stimulates ROS generation and increases endoplasmic reticulum stress resulting in cell death e.g., apoptosis and necroptosis 2) dead cells activate HSC and Kupffer cells by various mechanisms including apoptotic bodies engulfment and DAMP release 3) “find me” signals released by dead cells stimulate inflammatory cells infiltration to the liver 4) lipid stressed HC could secrete EV that stimulate fibrogenic gene expression in HSC 5) free cholesterol and FFA could also directly activate HSC 6) intestine-derived LPS activate both HSC and KC 7) microbiota-derived ethanol precipitates in increased ROS generation 8) activated KC secrete TGF-β resulting in activation of HSC 9) defenestrated LSEC (LSEC capillarization) support HSC activation and acquire inflammatory phenotype that induces liver inflammation. As a result of HSC activation, ECM production increases, leading to liver fibrosis, which could progress further to cirrhosis or liver cancer [179]. DAMP, damage-associated molecular patterns; ECM, extracellular matrix; ER, endoplasmic reticulum; EV, extracellular vesicle; FFA, free fatty acids; HC, hepatocyte; HSC, hepatic stellate cells; KC, Kupffer cells; LPS, lipopolysaccharide; LSEC, liver sinusoidal endothelial cells; MC, monocytes; ROS, reactive oxygen species; TGF, tumor growth factor.
2.3.2. Autophagy
Autophagy is a self-degradative process which is used by the cells to remove misfolded proteins and damaged organelles [65]. Singh et al. showed that the cells can use autophagic process as lipolytic mechanism to mobilize lipids from intracellular lipid store, which is termed in this case “macrolipophagy” [66]. Various in vitro and in vivo studies showed that autophagy was decreased in fatty hepatocytes [67]. Impairment of autophagy by palmitic acid in macrophages induces inflammatory IL-1β production [68]. On the other side, selective loss of autophagic activity reduced liver fibrogenesis in cultured HSCs [69] and protected against diet-induced insulin resistance [70]. The results highlight that the net effect of autophagy on NAFLD might depend on the tissue or type of the cells that show autophagic dysfunction and the stage of NAFLD.
3. Diagnostic biomarkers
The liver biopsy is considered the gold standard for NAFLD diagnosis, especially for distinguishing steatosis from inflammation and fibrosis [2]. Nevertheless, this technique has several limitations not only resulting from the invasive procedure and rare post-interventional complications, but also due to the small tissue volume, which might not be representative for the whole liver and may not take into account inhomogeneous distribution of NAFLD-associated alterations. Novel imaging modalities such as ultrasound- or MR-based techniques are of increasing value, as recently reviewed [71], but are still not generally available, so that there is an urgent need for noninvasive screening tools.
3.1. Current biomarkers and indices
Biomarker-based scores such as the fatty liver index (FLI) have been developed to diagnose steatosis using routine laboratory and anthropometric data [2], but are of limited value, also due to the increasing availability of precise imaging tools [72].
Non-invasive detection of NASH and fibrosis remains challenging today. Biomarker-based panels such as aspartate aminotransferase (AST)/platelets count ratio index, fibrosis-4 (FIB-4) index, FibroTest, FibroSpect II, and NAFLD fibrosis score (NFS) offer variable diagnostic efficacy for assessing different stages of liver fibrosis [73,74]. Although combination of these panels could enhance their predictive values [73], they still suffer from limited accuracy even compared to the alanine aminotransferase (ALT) and AST, particularly in T2DM [75]. In this regard, transient elastography looks like a promising alternative in diabetes clinics for detection of liver fibrosis in NAFLD [74].
Numerous biomarkers have been developed to specifically track features of NASH progression and fibrosis. Cytokeratin (CK) 18, an intermediate filament protein that is cleaved during cell death to CK18 M30 and CK18 M65, has been intensively investigated as a surrogate of NASH-associated liver cell damage, but a recent meta-analysis of 25 studies reported a maximum sensitivity of 0.75 for NASH diagnosis [76] and suboptimal diagnostic value was shown in T2DM [75]. The ECM turnover marker, type III procollagen, can offer a diagnostic efficacy of 0.81 and 0.88 to detect moderate and advanced liver fibrosis in T2DM, respectively [77].
In 2016, the European Associations for the Study of the Liver, Obesity and Diabetes (EASL-EASO-EASD) jointly released recommendations for diagnosis and monitoring of disease severity in persons with suspected NAFLD and metabolic risk factors [78]. People with metabolic risk factors, such as T2DM, should undergo assessment by abdominal ultrasound, serum transaminases and fibrosis markers (e. g. FIB-4, NFS). Elevated transaminases or steatosis plus abnormal fibrosis test shall require referral to a specialist. This strategy has raised the question of a possible overreferral when adhering to these guidelines [79]. However, recent analyses show that a refined strategy of specific combining indices such as FLI and FIB-4 could reduce the number of people with T2DM for further diagnostic work up to a reasonable size [80]. This retrospective analysis also found that certain non-invasive biomarkers are consistently associated with different patterns of diabetes-related complications.
3.2. Novel biomarkers
Analyses of the plasma metabolomic of insulin-resistant individuals with NAFLD suggested that bile salts, e. g. glycocholate, taurocholate, and glycochenodeoxycholate allow to detect NASH in one study [81], while another study reported an association with insulin resistance but not with liver necroinflammation [82]. Also amino acids, specifically a glutamate-serine-glycine index, was associated with hepatic insulin resistance and transaminases and discriminated individuals with fibrosis grade 3–4 from those with grade 0–2 independently of BMI [83]. The diagnostic performance of a serum-based lipidomic analysis was substantially lower for NAFLD detection in T2DM compared to healthy individuals [84]. Nevertheless, certain sphingolipid species were recently found to be increased in insulin-resistant NASH and to correlate with hepatic oxidative stress and inflammation [85].
One circulating small noncoding RNA, miRNA-122, was found to be >5.7fold in steatosis and further doubled in NASH [86]. This miRNA was also higher in T2DM with NAFLD than in those without NAFLD and provided better prediction of NAFLD when combined with LDL and waist-to-hip ratio [87]. Moreover, extracellular vesicles (EV), which among other cargo also transport miRNA, may be promising biomarkers for NAFLD as shown by higher serum levels in people with NAFLD, obesity and diabetes [180].
Recently, metagenomics data derived from gut microbiota alterations allowed to detect advanced fibrosis in 86 NAFLD patients, of whom 23% had T2DM, with a robust diagnostic accuracy (AUROC: 0.936) [88].
4. Treatment of NAFLD in T2DM
The current guidelines recommend only lifestyle modification and - for certain groups - bariatric or metabolic surgery to treat NAFLD [1,2]. Although the numerous activities in this field, no pharmacological treatment is currently approved or expecting approval for the use in NAFLD with or without concomitant T2DM (Table 1, Table 2 ), except for obeticholic acid (OCA). Marketing authorization application for OCA has been submitted to the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA), awaiting FDA decision by June 2020 [89]. Against this background, current guidelines and recommendations primarily advise lifestyle modification (healthy nutrition and exercise) and weight loss in overweight/obese persons. The EASL-EASO-EASD guidelines recommend to consider pharmacological treatment in people with NASH when combined with fibrosis and in those with less severe disease, but high risk conditions for disease progression such as T2DM [78]. The American Association for the Study of Liver Diseases (AASLD) recommends to limit pharmacological treatment to those with biopsy-proven NASH and fibrosis [1].
Table 1.
Placebo-controlled double-blind RCTs investigating the effects of drugs approved for T2DM on NAFLD by MR-based methods or liver histology.
| Study | Patient characteristics | Intervention (n) | Dosage/duration | Metabolic effects |
Liver effects | |||
|---|---|---|---|---|---|---|---|---|
| BW | HbA1c | FBG | ||||||
| METa | Bugianesi et al., 2005 Ref. [110] |
Biopsy-proven NASH, 4–12% had T2DM | MET (55) vs VIT E (28) and WT-reducing diet (27) | MET 2 g, VIT E 800 IU daily/12 months | ↓ With MET and diet |
NA | ↓ Only with MET |
MET (but not VIT E and diet changes) ↓ liver fat (assessed histologically), necroinflammation, and fibrosis |
| Haukeland et al., 2009 Ref. [109] |
Biopsy-proven NAFLD, ~50% with prediabetes or T2DM, HbA1c = ~5.7 ± 0.6% | MET (24) vs PL (24) | Initial 0.5 mg and titrated to 2.5 or 3 g daily/6 months | ↓ | ↓ | ↓ | ↔ in liver steatosis (assessed by CT and histologically) or NAS as compared to PL | |
| DPP4i | Cui et al., 2016 Ref. [115] |
MRI-diagnosed NAFLD with prediabetes or controlled diabetes, HbA1c = ~5.7–8% | SITA (25) vs PL (25) | 100 mg daily/24 weeks | ↔ | ↔ | ↔ | ↔ in liver fat/fibrosis (measured by MRI-PDFF/MRE) as compared to PL |
| Joy et al., 2017 Ref. [116] |
T2DM with liver biopsy-proven NASH, HbA1c = ~7.9 ± 1% | SITA (6) vs PL (6) | 100 mg daily/24 weeks | ↔ | ↓ | NA | SITA was not better than PL in reducing liver fibrosis score or NAS | |
| Macauley et al., 2015 Ref. [117] |
T2DM on stable MET therapy with HbA1c ≤ 7.6% | VILDA (22) vs PL (22) | 50 mg twice daily/6 months | ↓ (p = 0.08) |
↓ | ↓ | VILDA ↓ liver fat (assessed by MRI) by 27% compared to BL and improved liver transaminases | |
| GLP-1ra | Armstrong et al., 2016 Ref. [100] |
Biopsy-confirmed NASH, 31–35% had T2DM, HbA1c = ~5.9 ± 0.7% |
LIRA (26) vs PL (26) | 1∙8 mg daily/48 weeks | ↓ | ↓ | ↓ | LIRA ↑ NASH resolution in 39% of patients compared to 9% in PL |
| Bizino et al., 2020 Ref. [121] |
T2DM with HbA1c = 7.0–10% | LIRA (24) vs PL (26) | 1∙8 mg daily/26 weeks | ↓ | ↔ | NA | ↔ in hepatic fat as assessed by MRI | |
| SGLT2i | Kahl et al., 2020 Ref. [127] |
Controlled T2DM with short known disease duration, HbA1c = ~6.6 ± 0.5% | EMP (42) vs PL (42) | 25 mg daily/24 weeks | ↓ | ↔ | ↓ | EMP resulted in PL-corrected absolute 1.8% ↓ in liver fat (assessed by MRS), ↓ circulating uric acid, ↑ serum adiponectin |
| Cusi et al., 2019 Ref. [129] |
Inadequately controlled T2DM, HbA1c = 7.7 ± 0.7% | CANA (26) vs PL (30) | Initial 100 mg and titrated to 300 mg daily/24 weeks | ↓ | ↓ | ↓ | CANA ↑ hepatic insulin sensitivity and ↓ liver fat (assessed by MRI-PDFF) in NAFLD patients by 6.9% vs 3.8% in PL. (p = 0.09) | |
| Latva-Rasku et al., 2019 Ref. [128] |
T2DM with HbA1c 6.5–10.5% and ≥3 months of stable treatment with MET and/or DPP4i | DAP (15) vs PL (16) | 10 mg daily/8 weeks | ↓ | ↓ | ↓ | ↓ liver fat (assessed by MRI-PDFF), ↓ liver volume, ↔ in EGP | |
| Eriksson et al., 2018 Ref. [130] |
T2DM with MRI-diagnosed NAFLD, HbA1c = ~7.38 ± 0.56% | DAP (21) and OM-3CA (20) as monotherapy or combined (22) vs PL (21) | DAP 10 mg, OM-3CA 4 g daily/12 weeks | ↓ Only in DAP monotherapy and combined |
↓ Only in DAP monotherapy |
↓ Only in DAP monotherapy and combined |
DAP, OM-3CA, and combined therapy ↓ liver fat (assessed by MRI-PDFF) by 13, 15, and 21% respectively compared to BL. Only combined therapy-mediated PDFF changes was significant compared to PL. Only DAP monotherapy ↓ hepatocytes injury biomarkers | |
| Glitazone | Aithal et al., 2008 Ref. [140] |
Biopsy-proven NASH without T2DM, HbA1c = ~5.8 ± 0.6% | PIO (31) vs PL (30) | PIO 30 mg daily/12 months | ↑ | ↓ | ↓ | PIO ↓ liver transaminases and improved histological features of NASH e.g. lobular inflammation, Mallory-Denk bodies, and fibrosis compared to PL |
| Sanyal et al., 2010 Ref. [139] |
Biopsy-proven NASH without T2DM | PIO (80) vs VIT E (84) or PL (83) | PIO 30 mg, VIT E 800 IU daily/96 weeks | ↑ Only with PIO |
NA | ↓ Only with PIO |
Only PIO ↑ resolution of definite NASH. Both VIT E and PIO ↓ liver fat (assessed histologically), ↓ lobular inflammation, and ↔ in liver fibrosis | |
| Cusi et al., 2016 Ref. [138] |
Biopsy-proven NASH with prediabetes or T2DM, HbA1c = ~7.1 ± 0.9% in patients with T2DM | PIO (50) vs PL (51) in combination with low caloric diet | 45 mg daily/18 months, followed by open-label 18 months extension with PIO | ↑ | ↓ Only in patients with T2DM |
↓ | PIO ↓ NAS by 2 points and ↑ NASH resolution in 58 and 52% of patients, respectively as compared to 17,7 and 19,6% in PL | |
Drug trials with liver-related outcomes in patients with NAFLD and T2DM or prediabetes. Retrospective studies were excluded. BL, baseline; BW, body weight; CAN, canagliflozin; CT, computed tomography; DAP, dapagliflozin; DPP4i, dipeptidyl peptidase inhibitors; EMP, empagliflozin; FBG, fasting blood glucose; GLP1-ra, glucagon-like peptide 1 receptor agonist; HbA1c, glycated haemoglobin; LIRA, liraglutide; MET, metformin; MRE, magnetic resonance elastography; MRI-PDFF, magnetic resonance imaging–estimated proton density fat fraction; MRS, magnetic resonance spectroscopy; NA, no data available; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; PIO, pioglitazone; NASH, non-alcoholic steatohepatitis; OM-3CA, omega-3 (n-3) carboxylic acids; PL, placebo; SGLT2i, sodium-glucose cotransporter 2 inhibitors; RCTs, randomized controlled trials; SITA, sitagliptin; T2DM, type 2 diabetes; VILDA; vildagliptin; VIT E, vitamin E; NAFLD, non-alcoholic fatty liver disease.
Only placebo-controlled, double-blind, randomized clinical trials are listed, except for metformin (the current first line therapy of T2DM), as few studies investigating the effects of metformin based on MR and liver histology are available.
Table 2.
Ongoing phase III randomized, placebo-controlled clinical trials on new treatment concepts for NAFLD.
| Drug | Mechanism | Trial identifier | Enrolled patients | Status | T2DM inclusion criteria | Primary endpoint | Primary completion date |
|---|---|---|---|---|---|---|---|
| Dapagliflozin | SGLT2 inhibitor | NCT03723252 | 100 | Recruiting | T2DM only with HbA1c < 9.5% | Improvement in scored liver histology over 1 year | 11/2021 |
| Elafibranor | PPARα/δ dual agonist | NCT02704403 | 2000 | Recruiting | T2DM only with HbA1c ≤ 9% | 1-% of patients with NASH resolution without fibrosis worsening at week 72 from BL 2-Long term liver-related outcomes (4 years) |
12/2021 |
| Saroglitazara | PPAR-α/γ agonist | NCT04193982 | 250 | Not yet recruiting | NA | Change in NFS at week 8, 16, and 24 | 07/2020 |
| MSDC-0602 | MPC inhibitor | NCT03970031 | 3600 | Not yet recruiting | T2DM, HbA1c > 6% | 1-Change in HbA1c at month 6 from BL 2-NASH resolution at 12 months |
12/2021 |
| Obeticholic Acid | FXR agonist | NCT03439254 | 919 | Active, not recruiting | T2DM only with HbA1c < 9.5% | % of patients with improvement of liver fibrosis by ≥1 stage with no worsening of NASH after 18 months | 06/2021 |
| Obeticholic acid | FXR agonist | NCT02548351 | 2480 | Active, not recruiting | T2DM only with HbA1c ≤ 9.5% | 1-Improvement of liver fibrosis by ≥1stage with no worsening of NASH OR achieving NASH resolution without worsening of liver fibrosis at month 18 from BL 2-Long term liver-related outcomes (7 years) |
10/2022 |
| Oltipraz | LXR-α inhibitor | NCT04142749 | 144 | Recruiting | T2DM with HbA1c ≤ 8% | Change in liver fat assessed by MRS at week 24 from BL | 10/2020 |
| Cenicriviroc | CCR2/5 dual antagonist | NCT03028740 | 2000 | Recruiting | T2DM with HbA1c ≤ 10% | 1-Improvement of liver fibrosis by ≥1 stage with no worsening of NASH after 12 months 2-Long term liver-related outcomes (5 years) |
10/2021 |
| Aramchol | SCD1 inhibitor | NCT04104321 | 2000 | Recruiting | T2DM with controlled glycemia or prediabetes | 1-NASH resolution with no worsening of fibrosis OR fibrosis improvement by ≥1 stage with no worsening of NASH at week 52 from BL 2-Long term liver-related outcomes (5 years) |
06/2022 |
| Resmetirom | THR-β agonist | NCT03900429 | 2000 | Recruiting | T2DM with HbA1c < 9% | 1-NASH resolution in patients with F2-F3 fibrosis after 52 weeks 2-Long term liver-related outcomes (4.5 years) |
06/2021 |
Phase III registered interventional trials on “clinicaltrials.gov” for NAFLD as accessed on 10th April 2020. BL, baseline; CCR2/5, C-C chemokine receptors type 2 and type 5; FXR, farnesoid X receptor; HbA1c, glycated haemoglobin; LXR, Liver X receptor; MPC, mitochondrial pyruvate carrier; NA, data not available; NAFLD, non-alcoholic fatty liver disease; NFS, NAFLD fibrosis score; PPAR, peroxisome proliferator-activated receptor; NASH, non-alcoholic steatohepatitis; SCD, stearoyl-CoA desaturase; SGLT, sodium-glucose cotransporter; THR, thyroid hormone receptor; T2DM, type 2 diabetes.
Only placebo-controlled, randomized clinical trials are listed, except for saroglitazar, a 4-arm active-controlled study.
4.1. Strategies to induce weight loss
4.1.1. Lifestyle modification
Weight management and physical exercise are key to the treatment of both NAFLD and T2DM. A proof-of-concept study showed that a hypocaloric diet with weight loss of ~8 kg within 12 weeks not only normalized fasting EGP and thereby hyperglycemia, but decreased hepatic TAG content down to normal concentrations in obese T2DM humans with NAFLD [90]. Subsequent studies in larger cohorts extended these results by demonstrating that a very low-caloric diet with weight loss of about 15% rapidly normalized liver fat content in 90 individuals with T2DM, which persisted for one year if weight loss was maintained [91]. Interestingly, Mediterranean, low-saturated fat and high plant-based protein diets also improve steatosis in NAFLD combined with T2DM despite minor or no relevant weight loss [92,93]. In addition, physical activity and exercise training interventions can also decrease liver fat content, which may not be exclusively depending on concomitant weight loss [94]. Although the beneficial effects of structured behavioral treatment to improve histological endpoints, likely extend beyond reduction of hepatic fat content to ameliorating the grade and stage of inflammation and fibrosis [95], only a minority of the people manages to adhere to dietary weight loss and exercise programs, which raises the issue of other therapeutic approaches [94].
4.1.2. Antiobesity drugs
Weight-loss inducing drugs could be an attractive option for persons with NAFLD with a BMI > 30 kg/m2 or >27 kg/m2 in the presence of at least one metabolic comorbidity, as an adjunct treatment to lifestyle modifications [96,97]. Currently, the most-popular weight-loss inducing medications associated with at least 5% body weight decrease in one year as compared to placebo are orlistat, the fixed combination of phentermine and topiramate or naltrexone and bupropion, and the glucagon-like peptide-1 (GLP-1) receptor agonist (GLP-1ra), liraglutide [97]. Of these drugs, orlistat treatment failed to improve liver histology when compared to placebo [98], while no reports are available for topiramate, naltrexone, bupropion and phentermine regarding hepatic endpoints in humans with NAFLD [99]. Only liraglutide treatment associated with the resolution of NASH without worsening of fibrosis in overweight/obese persons (mean values; liraglutide 34.2 vs. placebo 37.7 kg/m2) [100]. In addition, novel GLP-1ra and dual agonists with superior efficacy for weight loss are being tested in preclinical and early clinical trials.
4.1.3. Bariatric surgery
Bariatric or metabolic surgery is a therapeutic option to induce sustained weight loss partricularly in people with combined NAFLD and T2DM. In obese humans, bariatric surgery resulted in 85% resolution of NASH within one year [101] and in 77% complete remission of T2DM [102]. Surgical weight loss improves glucose metabolism and insulin sensitivity by different mechanisms such as increased GLP-1 secretion [103] and epigenetic modification [104,105].
4.2. Antihyperglycemic drugs
Several antihyperglycemic drug classes were or are currently being investigated in clinical trials and preclinical models to evaluate their efficacy for people with NAFLD and with or without T2DM.
4.2.1. Metformin
Metformin reduces body weight, hepatic gluconeogenesis - by yet unclear mechanisms [106], and the risk of macrovascular complications, which is still controversially discussed due to lack of optimally designed clinical trials [107,108]. Despite its action on hepatic metabolism, former small-scale studies reported conflicting results [109,110] (Table 1) and recent metaanalysis failed to demonstrate any effect of metformin on liver histology [111]. Nevertheless, epidemiological, observational and preclinical studies suggest that metformin may reduce the risk of HCC and also in T2DM, possibly by promoting apoptosis, but controlled clinical trials are not available [112].
4.2.2. Dipeptidyl peptidase-4 (DPP-4) inhibitors (DPP-4i)
DPP4 degrades incretins such as GLP-1 so that DPP-4i treatment increases the postprandial levels of endogenous GLP-1, which leads to lower glucose peaks after meals [113,114]. In individuals with NAFLD with prediabetes or recent onset diabetes, sitagliptin did not improve liver steatosis or liver fibrosis compared to placebo as assessed by MR-based techniques [115]. In line, another recent trial reported no benefits for sitagliptin versus placebo on liver fibrosis or steatohepatitis in T2DM [116] (Table 1). In contrast, a moderate reduction in liver fat content was observed with vildagliptin in individuals with T2DM [117].
4.2.3. Incretin mimetics
The actions of endogenous GLP-1 are mimicked by GLP-1 receptor agonists (GLP-1ra), which effectively reduce blood glucose levels and also reduce the risk for CVD in T2DM [118]. In individuals with NAFLD and T2DM, liraglutide in combination with metformin reduced liver, subcutaneous, and visceral fat [119]. Also, liraglutide improved hepatic steatosis measured by MR-based methods as well as resolved biopsy-proven NASH without worsening of fibrosis [100,120] (Table 1). However, a recent subanalysis did not detect an effect of liraglutide on liver fat content quantified by 1H-MRS [121] (Table 1). Respective trials with semaglutide and dulaglutide are still waiting for results (NCT02970942, NCT03648554). In an animal model of T2DM, exenatide also counteracted HCC development by suppression of STAT3-regulated genes [122].
Glucose-dependent insulinotropic polypeptide (GIP) represents the second important incretin with proposed beneficial effects on peripheral energy metabolism [123]. Tirzepatide, a novel dual agonist for GIP and GLP-1 receptors with probably greater efficacy than GLP-1ra [124], decreased transaminases and surrogate markers of liver injury paralleled by increased circulating adiponectin levels in people with T2DM [125]. A clinical trial on NASH resolution is currently ongoing in persons with NASH with or without T2DM (NCT04166773).
4.2.4. Sodium glucose cotransporter-2 inhibitors (SGLT2i)
SGLT2i inhibit reabsorption of glucose in the proximal renal tubule resulting in glucosuria and calory loss, thus effectively reducing blood glucose level and body weight in T2DM. Moreover, SGLT2is show beneficial effects on cardiovascular risk and progression of nephropathy [118,126]. Most, but not all recent randomized controlled trials (RCTs) showed that SGLT2i treatment resulted in reduction in liver fat content compared to placebo [[127], [128], [129], [130], [131]] (Table 1). In an open-label pilot study in liver-biopsy proven cohort of NASH with T2DM, treatment with empagliflozin for 6 months reduced liver steatosis, ballooning and fibrosis and induced NASH resolution in approximately half of those persons [132]. Animal models further suggested anti-inflammatory, anti-oxidant and pro-apoptotic actions of SGLT2i with cardio-protective effects as well as inhibition of NASH progression and tumor growth of HCC [133,134]. The dual SGLT1/2i, licogliflozin, is expected to block both intestinal and renal glucose (re)absorption [135] and currently investigated to evaluate its efficacy on resolution of NASH as monotherapy and as combination therapy with the FXR agonist, tropifexor, in people with NASH and fibrosis (NCT04065841). First results hint at improvement of transaminases and reduction of steatosis by licogliflozin in NASH [136].
4.2.5. Peroxisome proliferator-activated receptor (PPAR) agonists
PPARs is a family of nuclear receptors composed of multiple isoforms with wide tissue distribution [137]. The PPAR-γ agonist pioglitazone had convincing efficacy on NASH resolution in individuals with and without T2DM, but with conflicting results on fibrosis [[138], [139], [140]] (Table 1). Of note, pioglitazone has been withdrawn in many European countries because of its disadvantageous safety profile [181]. However, pioglitazone exerts beneficial effects on cardiovascular outcomes in persons with T2DM and a history of CVD [141].
It has been proposed that non-PPAR-γ dependent mechanisms, as the inhibition of the mitochondrial pyruvate carrier (MPC), might mediate the beneficial effects of pioglitazone on NAFLD [142]. However, a novel drug with PPAR-γ sparing effects and MPC binding activity did not improve histological components of NASH in individuals with T2DM compared to placebo [143].
Elafibranor is a dual agonist for PPAR-α and PPAR-δ without PPAR-γ stimulation [144]. Based on a post-hoc analysis, NASH was resolved without worsening of fibrosis in a higher percentage of people with and without T2DM in the elafibranor group as compared to the placebo [145] and a follow-up study on these findings has been initiated (Table 2). Recent announcements on an interim analysis state that elafibranor treatment failed to resolve NASH and improve fibrosis when compared to placebo [146].
Saroglitazar is another dual PPAR-α/γ agonist with higher affinity for PPAR-α. In a mouse model of NASH, saroglitazar reduced liver steatosis, inflammation and prevented fibrosis development and a respective clinical trial is ongoing [147] (Table 2). Lanifibranor is a pan-PPAR agonist for PPAR-α, β, and γ receptors focused on treatment of liver fibrosis and NASH (NCT03008070). In an animal model of liver fibrosis, lanifibranor decreased hepatic collagen deposition [148].
4.3. Antiinflammatory drugs
Several other strategies for pharmacological targeting of NASH have emerged over the last few years; however, these strategies are not specifically designed for T2DM, but for the whole NAFLD collective. They comprise antiinflammatory drugs, but also modulators of other pathways, which are briefly summarized in the following sections.
4.3.1. Farnesoid X receptor (FXR) agonists
FXR can be activated by bile acids in a negative feedback mechanism to suppress bile acid synthesis [40]. OCA is a potent semisynthetic and selective FXR agonist approved for treatment of primary biliary cholangitis [149]. OCA treatment resulted in histologic improvement of NASH in people with and without T2DM compared to placebo [150,151]; however, concerns were raised about OCA-induced changes in plasma lipoprotein profile [152]. Tropifexor, another potent FXR agonist [153], is currently being tested in combinatorial approaches of NASH treatment (cenicriviroc and licogliflozin; NCT03517540).
4.3.2. Liver X receptor alpha (LXR-α) inhibitors
Oltipraz is a synthetic dithiolethione with antisteatotic effects by inhibiting the activity of LXR-α. It activates adenosine monophosphate activated protein kinase (AMPK) and inactivates S6K1, affecting LXR-α thus reducing lipogenesis and increasing lipid oxidation [154]. A recent 24-week phase 2 clinical trial found decreased steatosis measured by 1H-MRS with oltipraz compared to placebo treatment [154] and a respective phase 3 clinical trial is ongoing (Table 2).
4.3.3. Chemokine receptors (CCR) 2/5 antagonists
Chemokine receptors type 2 (CCR2) and type 5 (CCR5) are expressed on various inflammatory and fibrogenic cells [155]. Cenicriviroc is a CCR2/CRR5 dual antagonist that reduced insulin resistance, liver inflammation and fibrosis in diet-induced models of NASH [62]. In recent RCTs in a NASH cohort with fibrosis, cenicriviroc treatment did not improve NAS but may reduce liver fibrosis [156,157]. Currently, there is an ongoing clinical trial to evaluate the effects of cenicriviroc on fibrosis in NASH (Table 2).
4.4. Modulation of lipid metabolism
4.4.1. Lipid lowering drugs
Both people with obesity, metabolic syndrome or T2DM as well as those with NAFLD are at increased risk for dyslipidemia. Statins, inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, are generally safe and have unmet efficacy to decreased serum LDL and prevent cardiovascular outcomes, despite slightly increasing the risk of T2DM [2]. Use of statins associated with a 46% lower relative risk of hepatic decompensation and mortality in cirrhosis and a trend towards lower fibrosis progression in non-cirrhotic liver diseases [158]. However, the data on liver histology ist limited [159] so that statins are not currently recommended for the management of NAFLD [2]. The inhibitor of intestinal cholesterol absorption, ezetimibe, decreased the histological NAS, but not consistently liver fat content in an analysis of the few available studies [160]. Fenofibrate, a PPAR-α agonist, does not affect or even increase liver fat content or volume [161,162]. Nevertheless, the combination of statins with certain anti-NASH drugs, such as OCA, could be beneficial to counteract drug-related increases in LDL during long-term treatment.
4.4.2. Acetyl CoA carboxylase (ACC) 1/2 inhibitors
Inhibition of DNL in NAFLD may be achieved by inhibition of ACC as this enzyme catalyzes the conversion of acetyl CoA into malonyl CoA, which acts as a substrate for fatty acid synthesis and inhibitor for fatty acid β-oxidation [163]. In a recent clinical trial in individuals with NASH with and without T2DM, treatment with the dual ACC1 and ACC2 inhibitor GS-0976 decreased liver steatosis without improvement of fibrosis [164]. The major concern of this strategy is that decreased DNL in NAFLD might channel lipids towards other harmful directions, e.g. increased blood lipids [163].
4.4.3. Stearoyl-CoA desaturase (SCD)1 inhibitors
SCD1 is a key enzyme for hepatic lipogenesis that catalyzes the conversion of saturated fatty acids to MUFA and its downregulation protected mice from high carbohydrate-induced liver steatosis [165]. A clinical trial with aramchol, an inhibitor of SCD1, showed a reduction in liver fat content, resolution of NASH and improvement of liver fibrosis in persons with NAFLD with prediabetes or T2DM [166]. These results paved the way for a respective phase 3 clinical trial (Table 2).
4.5. Modulation of energy metabolism
4.5.1. Mitochondrial uncouplers (protonophores)
Mitochondrial uncoupling describes any process that uncouples the electron transport from ATP synthesis in mitochondria [167]. 2,4-Dinitrophenol (DNP) was widely used for the treatment of obesity before its discontinuation due to life-threatening serious adverse events [168]. To overcome the side effects of DNP, improved formulas of DNP were recently developed to decrease the toxic to effective dose ratio (DNP-methyl ether (DNPME) and controlled-release mitochondrial protonophore (CRMP)) [169,170]. Oral CRMP targets mainly the liver due to the first-pass effect and decreased hepatic insulin resistance, hepatic steatosis and liver fibrosis in a methionine-choline deficient rat model of NASH [170]. CRMP was also effective in nonhuman primates with diet-induced NAFLD for reduction of hepatic steatosis and EGP [171].
4.5.2. Thyroid hormone receptor (THR)-β agonists
Thyroid hormone deficiency has been associated with NAFLD development [172]. Thyroid hormone analogues decreased liver fat, liver transaminases, and inflammatory and fibrosis markers in animal models of NASH [172]. Resmetirom is a liver-targeted highly selective THR-β agonist which showed efficacy in reducing liver fat content in NASH with and without T2DM [173]. Currently, there is ongoing clinical trial for evaluation of long-term outcomes of resmetirom and its efficacy on NASH resolution (Table 2).
4.5.3. Vitamin E
Vitamin E is a potent antioxidant [174]. In NASH without T2DM, vitamin E improved NAS without worsening of fibrosis [139]. Despite concerns regarding adverse effects of long term vitamin E usage, treatment with vitamin E for ≥2 years reduced the risk of liver failure in a NASH cohort with advanced fibrosis and with or without T2DM [175].
4.6. Modulation of the microbiome
Therapeutic manipulation of intestinal microbiome is still in its infancy. Rodent data showed efficacy for fecal microbiota transplantation in improving NAFLD in a diet-induced NASH model [176]. Also, modulation of the intestinal microbiota by antibiotic treatment reduced liver transaminases in NAFLD [177]. The use of prebiotics and probiotics in obese NAFLD/NASH is currently not supported by high-quality clinical studies with MR- or biopsy-based endpoints [178].
5. Summary and outlook
The evidence for shared pathophysiological mechanisms between T2DM and NAFLD will help to develop strategies for detecting and treating both diseases and preventing their leading complication, CVD. Altered lipid and energy metabolism, insulin resistance, low-grade inflammation and intestinal dysbiosis represent key targets. In addition to weight loss by lifestyle modification or bariatric surgery, GLP-1ra and SGLT2i are promising antihyperglycemic concepts with beneficial effects on NAFLD and CVD. In addition, many metabolism-based drugs are currently studied comprising PPAR agonists, endocrine dual co-agonists to modulators of hepatic metabolism or microbiota.
Nevertheless, several roadblocks need to be overcome to reduce the burden of NAFLD in T2DM. First, there is still lack of preclinical animal models that encapsulate essential features of human NASH and diabetes. Second, available biomarkers lack diagnostic efficacy to identify NAFLD progression. Innovative strategies such as cluster analysis already enabled detection of a diabetes subtype (SIRD) with high risk of NAFLD [14]. Combination with computational integration of multiomics data shall identify specific disease signatures and pave the way to precision medicine and targeted management of T2DM and NAFLD. Finally, studies on so-called endpoints are scarce, which may be due to the need of long-term studies to evaluate liver-related mortality, to the current neglect to accept CVD morbidity and mortality as NAFLD outcome and to ongoing discussions on the relevance of surrogate markers.
CRediT authorship contribution statement
Bedair Dewidar:Conceptualization, Writing - original draft, Writing - review & editing.Sabine Kahl:Conceptualization, Writing - original draft, Writing - review & editing.Kalliopi Pafili:Writing - original draft, Writing - review & editing.Michael Roden: Conceptualization, Supervision, Writing - original draft, Writing - review & editing.
Declaration of competing interest
BD, SK and KP declare no conflicts of interest. MR is on the scientific advisory boards of Allergan, Astra-Zeneca, Bristol-Myers Squibb, Eli Lilly, Gilead Sciences, Inventiva, Intercept Pharma, Novartis, NovoNordisk, Servier Laboratories, Target Pharmasolutions, and Terra Firma and receives investigator-initiated support from Boehringer Ingelheim, Nutricia/Danone and Sanofi–Aventis.
Acknowledgments
The research of the authors is supported in part by grants from the German Federal Ministry of Health (BMG) and the Ministry of Culture and Science of the State North Rhine-Westphalia (MKW NRW) to the German Diabetes Center (DDZ), the German Federal Ministry of Education and Research (BMBF) to German Center for Diabetes Research (DZD e.V.). MR is further supported by grants from the European Funds for Regional Development (EFRE-0400191), EUREKA Eurostars-2 (E! 113230 DIA-PEP) and the German Science Foundation (DFG; CRC/SFB 1116/2 B12) and the Schmutzler Stiftung. SK is further supported by grants from the German Diabetes Association (DDG) and DZD.
This article is published as part of a supplement sponsored by Gilead Sciences, Inc.
References
- 1.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatol Baltim Md. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. 10.1016/j.jhep.2015.11.004. [DOI] [PubMed]
- 3.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol Baltim Md. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Lammert F. Clinical genomics of NAFLD. The liver, John Wiley & Sons, Ltd; 2020, p. 509–20. 10.1002/9781119436812.ch41. [DOI]
- 5.Kozlitina J., Smagris E., Stender S., Nordestgaard B.G., Zhou H.H., Tybjærg-Hansen A. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portillo-Sanchez P., Bril F., Maximos M., Lomonaco R., Biernacki D., Orsak B. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab. 2015;100:2231–2238. doi: 10.1210/jc.2015-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wild S.H., Morling J.R., McAllister D.A., Kerssens J., Fischbacher C., Parkes J. Type 2 diabetes and risk of hospital admission or death for chronic liver diseases. J Hepatol. 2016;64:1358–1364. doi: 10.1016/j.jhep.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Alexander M., Loomis A.K., van der Lei J., Duarte-Salles T., Prieto-Alhambra D., Ansell D. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17:95. doi: 10.1186/s12916-019-1321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koehler E.M., Plompen E.P.C., Schouten J.N.L., Hansen B.E., Darwish Murad S., Taimr P. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatol Baltim Md. 2016;63:138–147. doi: 10.1002/hep.27981. [DOI] [PubMed] [Google Scholar]
- 10.Yang J.D., Ahmed F., Mara K.C., Addissie B.D., Allen A.M., Gores G.J. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatol Baltim Md. 2019 doi: 10.1002/hep.30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eslam M., Newsome P.N., Anstee Q.M., Targher G., Gomez M.R., Zelber-Sagi S. A new definition for metabolic associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A., Byrne C.D., Bonora E., Targher G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care. 2018;41:372–382. doi: 10.2337/dc17-1902. [DOI] [PubMed] [Google Scholar]
- 13.Byrne C.D., Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Zaharia O.P., Strassburger K., Strom A., Bönhof G.J., Karusheva Y., Antoniou S. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol. 2019;7:684–694. doi: 10.1016/S2213-8587(19)30187-1. [DOI] [PubMed] [Google Scholar]
- 15.Ahlqvist E., Storm P., Käräjämäki A., Martinell M., Dorkhan M., Carlsson A. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 16.Stepanova M., Rafiq N., Younossi Z.M. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59:1410–1415. doi: 10.1136/gut.2010.213553. [DOI] [PubMed] [Google Scholar]
- 17.Targher G., Lonardo A., Byrne C.D. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol. 2018;14:99–114. doi: 10.1038/nrendo.2017.173. [DOI] [PubMed] [Google Scholar]
- 18.Henson J.B., Roden M., Targher G., Corey K.E. Is NAFLD not a risk factor for cardiovascular disease: not yet time for a change of heart. Hepatol Baltim Md. 2020 doi: 10.1002/hep.31156. [DOI] [PubMed] [Google Scholar]
- 19.Tilg H., Moschen A.R., Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14:32–42. doi: 10.1038/nrgastro.2016.147. [DOI] [PubMed] [Google Scholar]
- 20.Gastaldelli A., Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep. 2019;1:312–328. doi: 10.1016/j.jhepr.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rotman Y, Kapuria D. Non-alcoholic fatty liver disease. The liver, John Wiley & Sons, Ltd; 2020, p. 670–81. 10.1002/9781119436812.ch52. [DOI]
- 22.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Nseir W., Hellou E., Assy N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J Gastroenterol WJG. 2014;20:9338–9344. doi: 10.3748/wjg.v20.i28.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernández E.Á., Kahl S., Seelig A., Begovatz P., Irmler M., Kupriyanova Y. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J Clin Invest. 2017;127:695–708. doi: 10.1172/JCI89444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roden M., Shulman G.I. The integrative biology of type 2 diabetes. Nature. 2019;576:51–60. doi: 10.1038/s41586-019-1797-8. [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 27.Moschen A.R., Molnar C., Geiger S., Graziadei I., Ebenbichler C.F., Weiss H. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–1264. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- 28.Mu W., Cheng X.-F., Liu Y., Lv Q.-Z., Liu G.-L., Zhang J.-G. Potential nexus of non-alcoholic fatty liver disease and type 2 diabetes mellitus: insulin resistance between hepatic and peripheral tissues. Front Pharmacol. 2018;9:1566. doi: 10.3389/fphar.2018.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimobayashi M., Albert V., Woelnerhanssen B., Frei I.C., Weissenberger D., Meyer-Gerspach A.C. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. 2018;128:1538–1550. doi: 10.1172/JCI96139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wernstedt Asterholm I., Tao C., Morley T.S., Wang Q.A., Delgado-Lopez F., Wang Z.V. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arvaniti V.A., Thomopoulos K.C., Tsamandas A., Makri M., Psyrogiannis A., Vafiadis G. Serum adiponectin levels in different types of non alcoholic liver disease. Correlation with steatosis, necroinflammation and fibrosis. Acta Gastroenterol Belg. 2008;71:355–360. [PubMed] [Google Scholar]
- 32.Cernea S., Roiban A.L., Both E., Huţanu A. Serum leptin and leptin resistance correlations with NAFLD in patients with type 2 diabetes. Diabetes Metab Res Rev. 2018;34 doi: 10.1002/dmrr.3050. [DOI] [PubMed] [Google Scholar]
- 33.Petersen MC, Samuel VT, Petersen KF, Shulman GI. Non-alcoholic fatty liver disease and insulin resistance. The liver, John Wiley & Sons, Ltd; 2020, p. 455–71. 10.1002/9781119436812.ch37. [DOI]
- 34.Rothman D.L., Magnusson I., Cline G., Gerard D., Kahn C.R., Shulman R.G. Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1995;92:983–987. doi: 10.1073/pnas.92.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szendroedi J., Yoshimura T., Phielix E., Koliaki C., Marcucci M., Zhang D. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci U S A. 2014;111:9597–9602. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Soos T.J., Li X., Wu J., Degennaro M., Sun X. Protein kinase C theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101) J Biol Chem. 2004;279:45304–45307. doi: 10.1074/jbc.C400186200. [DOI] [PubMed] [Google Scholar]
- 37.Tripathi A., Debelius J., Brenner D.A., Karin M., Loomba R., Schnabl B. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canfora E.E., Meex R.C.R., Venema K., Blaak E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15:261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- 39.Brandl K., Schnabl B. Intestinal microbiota and nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2017;33:128–133. doi: 10.1097/MOG.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han C.Y. Update on FXR biology: promising therapeutic target? Int J Mol Sci. 2018;19 doi: 10.3390/ijms19072069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Softic S., Cohen D.E., Kahn C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci. 2016;61:1282–1293. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vatner D.F., Petersen M.C., Li X., Rogers J.C., Cline G., Samuel V. Evidence against pathway-selective hepatic insulin resistance in mice. Diabetes. 2018;67 doi: 10.2337/db18-1766-P. [DOI] [Google Scholar]
- 43.Roden M. Mechanisms of disease: hepatic steatosis in type 2 diabetes—pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab. 2006;2:335–348. doi: 10.1038/ncpendmet0190. [DOI] [PubMed] [Google Scholar]
- 44.Benhamed F., Denechaud P.-D., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J Clin Invest. 2012;122:2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen M.C., Madiraju A.K., Gassaway B.M., Marcel M., Nasiri A.R., Butrico G. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J Clin Invest. 2016;126:4361–4371. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mota M., Banini B.A., Cazanave S.C., Sanyal A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049–1061. doi: 10.1016/j.metabol.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simões I.C.M., Fontes A., Pinton P., Zischka H., Wieckowski M.R. Mitochondria in non-alcoholic fatty liver disease. Int J Biochem Cell Biol. 2018;95:93–99. doi: 10.1016/j.biocel.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 48.Pérez-Carreras M., Del Hoyo P., Martín M.A., Rubio J.C., Martín A., Castellano G. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatol Baltim Md. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- 49.Schmid A.I., Szendroedi J., Chmelik M., Krssák M., Moser E., Roden M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care. 2011;34:448–453. doi: 10.2337/dc10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teodoro J.S., Rolo A.P., Duarte F.V., Simões A.M., Palmeira C.M. Differential alterations in mitochondrial function induced by a choline-deficient diet: understanding fatty liver disease progression. Mitochondrion. 2008;8:367–376. doi: 10.1016/j.mito.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Koliaki C., Szendroedi J., Kaul K., Jelenik T., Nowotny P., Jankowiak F. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Lebeaupin C., Vallée D., Hazari Y., Hetz C., Chevet E., Bailly-Maitre B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2018;69:927–947. doi: 10.1016/j.jhep.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Kapoor A., Sanyal A.J. Endoplasmic reticulum stress and the unfolded protein response. Clin Liver Dis. 2009;13:581–590. doi: 10.1016/j.cld.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Ryu D., Seo W.-Y., Yoon Y.-S., Kim Y.-N., Kim S.S., Kim H.-J. Endoplasmic reticulum stress promotes LIPIN2-dependent hepatic insulin resistance. Diabetes. 2011;60:1072–1081. doi: 10.2337/db10-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akazawa Y., Nakao K. To die or not to die: death signaling in nonalcoholic fatty liver disease. J Gastroenterol. 2018;53:893–906. doi: 10.1007/s00535-018-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 2014;147:765–783.e4. 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed]
- 57.Lee Y.-S., Kim S.Y., Ko E., Lee J.-H., Yi H.-S., Yoo Y.J. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci Rep. 2017;7:3710. doi: 10.1038/s41598-017-03389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teratani T, Tomita K, Suzuki T, Oshikawa T, Yokoyama H, Shimamura K, et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology 2012;142:152–164.e10. 10.1053/j.gastro.2011.09.049. [DOI] [PubMed]
- 59.Duan N.-N., Liu X.-J., Wu J. Palmitic acid elicits hepatic stellate cell activation through inflammasomes and hedgehog signaling. Life Sci. 2017;176:42–53. doi: 10.1016/j.lfs.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 60.Ioannou G.N., Haigh W.G., Thorning D., Savard C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J Lipid Res. 2013;54:1326–1334. doi: 10.1194/jlr.M034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwabe R.F., Tabas I., Pajvani U.B. Mechanisms of fibrosis development in NASH. Gastroenterology. 2020 doi: 10.1053/j.gastro.2019.11.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krenkel O., Puengel T., Govaere O., Abdallah A.T., Mossanen J.C., Kohlhepp M. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatol Baltim Md. 2018;67:1270–1283. doi: 10.1002/hep.29544. [DOI] [PubMed] [Google Scholar]
- 63.Lefere S., Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: crosstalk with metabolism. JHEP Rep. 2019;1:30–43. doi: 10.1016/j.jhepr.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hammoutene A., Rautou P.-E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J Hepatol. 2019;70:1278–1291. doi: 10.1016/j.jhep.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 65.Glick D., Barth S., Macleod K.F. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khambu B., Yan S., Huda N., Liu G., Yin X.-M. Autophagy in non-alcoholic fatty liver disease and alcoholic liver disease. Liver Res. 2018;2:112–119. doi: 10.1016/j.livres.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X., de Carvalho Ribeiro M., Iracheta-Vellve A., Lowe P., Ambade A., Satishchandran A. Macrophage-specific hypoxia-inducible factor-1α contributes to impaired autophagic flux in nonalcoholic steatohepatitis. Hepatol Baltim Md. 2019;69:545–563. doi: 10.1002/hep.30215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hernández-Gea V., Ghiassi-Nejad Z., Rozenfeld R., Gordon R., Fiel M.I., Yue Z. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938–946. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim K.H., Jeong Y.T., Oh H., Kim S.H., Cho J.M., Kim Y.-N. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 71.Hannah W.N., Harrison S.A. Noninvasive imaging methods to determine severity of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatol Baltim Md. 2016;64:2234–2243. doi: 10.1002/hep.28699. [DOI] [PubMed] [Google Scholar]
- 72.Kahl S., Straßburger K., Nowotny B., Livingstone R., Klüppelholz B., Keßel K. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PloS One. 2014;9 doi: 10.1371/journal.pone.0094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iqbal U., Perumpail B.J., Akhtar D., Kim D., Ahmed A. The epidemiology, risk profiling and diagnostic challenges of nonalcoholic fatty liver disease. Medicines. 2019;6 doi: 10.3390/medicines6010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castera L. Non-invasive tests for liver fibrosis in NAFLD: creating pathways between primary healthcare and liver clinics. Liver Int Off J Int Assoc Study Liver. 2020;40(Suppl. 1):77–81. doi: 10.1111/liv.14347. [DOI] [PubMed] [Google Scholar]
- 75.Bril F., McPhaul M.J., Caulfield M.P., Clark V.C., Soldevilla-Pico C., Firpi-Morell R.J. Performance of plasma biomarkers and diagnostic panels for nonalcoholic steatohepatitis and advanced fibrosis in patients with type 2 diabetes. Diabetes Care. 2020;43:290–297. doi: 10.2337/dc19-1071. [DOI] [PubMed] [Google Scholar]
- 76.He L., Deng L., Zhang Q., Guo J., Zhou J., Song W. Diagnostic value of CK-18, FGF-21, and related biomarker panel in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Res Int. 2017;2017:9729107. doi: 10.1155/2017/9729107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bril F., Leeming D.J., Karsdal M.A., Kalavalapalli S., Barb D., Lai J. Use of plasma fragments of propeptides of type III, V, and VI procollagen for the detection of liver fibrosis in type 2 diabetes. Diabetes Care. 2019;42:1348–1351. doi: 10.2337/dc18-2578. [DOI] [PubMed] [Google Scholar]
- 78.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL–EASD–EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016;59:1121–40. 10.1007/s00125-016-3902-y. [DOI] [PubMed]
- 79.Blond E., Disse E., Cuerq C., Drai J., Valette P.-J., Laville M. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease in severely obese people: do they lead to over-referral? Diabetologia. 2017;60:1218–1222. doi: 10.1007/s00125-017-4264-9. [DOI] [PubMed] [Google Scholar]
- 80.Ciardullo S., Muraca E., Perra S., Bianconi E., Zerbini F., Oltolini A. Screening for non-alcoholic fatty liver disease in type 2 diabetes using non-invasive scores and association with diabetic complications. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2019-000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kalhan S.C., Guo L., Edmison J., Dasarathy S., McCullough A.J., Hanson R.W. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;60:404–413. doi: 10.1016/j.metabol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Legry V., Francque S., Haas J.T., Verrijken A., Caron S., Chávez-Talavera O. Bile acid alterations are associated with insulin resistance, but not with NASH, in obese subjects. J Clin Endocrinol Metab. 2017;102:3783–3794. doi: 10.1210/jc.2017-01397. [DOI] [PubMed] [Google Scholar]
- 83.Gaggini M., Carli F., Rosso C., Buzzigoli E., Marietti M., Della Latta V. Altered amino acid concentrations in NAFLD: impact of obesity and insulin resistance. Hepatol Baltim Md. 2018;67:145–158. doi: 10.1002/hep.29465. [DOI] [PubMed] [Google Scholar]
- 84.Bril F., Millán L., Kalavalapalli S., McPhaul M.J., Caulfield M.P., Martinez-Arranz I. Use of a metabolomic approach to non-invasively diagnose non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2018;20:1702–1709. doi: 10.1111/dom.13285. [DOI] [PubMed] [Google Scholar]
- 85.Apostolopoulou M., Gordillo R., Koliaki C., Gancheva S., Jelenik T., De Filippo E. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis. Diabetes Care. 2018;41:1235–1243. doi: 10.2337/dc17-1318. [DOI] [PubMed] [Google Scholar]
- 86.Cermelli S., Ruggieri A., Marrero J.A., Ioannou G.N., Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PloS One. 2011;6 doi: 10.1371/journal.pone.0023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ye D., Zhang T., Lou G., Xu W., Dong F., Chen G. Plasma miR-17, miR-20a, miR-20b and miR-122 as potential biomarkers for diagnosis of NAFLD in type 2 diabetes mellitus patients. Life Sci. 2018;208:201–207. doi: 10.1016/j.lfs.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 88.Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 2017;25:1054–1062.e5. 10.1016/j.cmet.2017.04.001. [DOI] [PMC free article] [PubMed]
- 89.Intercept's hotly anticipated NASH drug has to wait for its FDA hearing, thanks to COVID-19. FiercePharma n.d. https://www.fiercepharma.com/pharma/after-earlier-fda-delay-intercept-s-nash-hearing-pushed-back-due-to-covid-19 (accessed May 25, 2020).
- 90.Petersen K.F., Dufour S., Befroy D., Lehrke M., Hendler R.E., Shulman G.I. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taylor R., Al-Mrabeh A., Zhyzhneuskaya S., Peters C., Barnes A.C., Aribisala B.S. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for β cell recovery. Cell Metab. 2018;28:667. doi: 10.1016/j.cmet.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 92.Properzi C., O’Sullivan T.A., Sherriff J.L., Ching H.L., Jeffrey G.P., Buckley R.F. Ad libitum Mediterranean and low-fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatol Baltim Md. 2018;68:1741–1754. doi: 10.1002/hep.30076. [DOI] [PubMed] [Google Scholar]
- 93.Markova M, Pivovarova O, Hornemann S, Sucher S, Frahnow T, Wegner K, et al. Isocaloric diets high in animal or plant protein reduce liver fat and inflammation in individuals with type 2 diabetes. Gastroenterology 2017;152:571–585.e8. 10.1053/j.gastro.2016.10.007. [DOI] [PubMed]
- 94.Romero-Gómez M., Zelber-Sagi S., Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 95.Bellentani S., Dalle Grave R., Suppini A., Marchesini G., Network Fatty Liver Italian. Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatol Baltim Md. 2008;47:746–754. doi: 10.1002/hep.22009. [DOI] [PubMed] [Google Scholar]
- 96.Garvey W.T., Mechanick J.I., Brett E.M., Garber A.J., Hurley D.L., Jastreboff A.M. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients With Obesity executive summary complete guidelines available at https://www.aace.com/publications/guidelines. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2016;22:842–884. doi: 10.4158/EP161356.ESGL. [DOI] [PubMed] [Google Scholar]
- 97.Khera R., Murad M.H., Chandar A.K., Dulai P.S., Wang Z., Prokop L.J. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315:2424–2434. doi: 10.1001/jama.2016.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang H., Wang L., Cheng Y., Xia Z., Liao Y., Cao J. Efficacy of orlistat in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep. 2018;9:90–96. doi: 10.3892/br.2018.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pan C.S., Stanley T.L. Effect of weight loss medications on hepatic steatosis and steatohepatitis: a systematic review. Front Endocrinol. 2020;11:70. doi: 10.3389/fendo.2020.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet Lond Engl. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 101.Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology 2015;149:379–88; quiz e15–16. 10.1053/j.gastro.2015.04.014. [DOI] [PubMed]
- 102.Buchwald H., Avidor Y., Braunwald E., Jensen M.D., Pories W., Fahrbach K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 103.Laursen T.L., Hagemann C.A., Wei C., Kazankov K., Thomsen K.L., Knop F.K. Bariatric surgery in patients with non-alcoholic fatty liver disease - from pathophysiology to clinical effects. World J Hepatol. 2019;11:138–149. doi: 10.4254/wjh.v11.i2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahrens M., Ammerpohl O., von Schönfels W., Kolarova J., Bens S., Itzel T. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 2013;18:296–302. doi: 10.1016/j.cmet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 105.Gancheva S., Ouni M., Jelenik T., Koliaki C., Szendroedi J., Toledo F.G.S. Dynamic changes of muscle insulin sensitivity after metabolic surgery. Nat Commun. 2019;10:4179. doi: 10.1038/s41467-019-12081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rena G., Hardie D.G., Pearson E.R. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bergmark B.A., Bhatt D.L., McGuire D.K., Cahn A., Mosenzon O., Steg P.G. Metformin use and clinical outcomes among patients with diabetes mellitus with or without heart failure or kidney dysfunction: observations from the SAVOR-TIMI 53 trial. Circulation. 2019;140:1004–1014. doi: 10.1161/CIRCULATIONAHA.119.040144. [DOI] [PubMed] [Google Scholar]
- 108.Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A.W. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 109.Haukeland J.W., Konopski Z., Eggesbø H.B., von Volkmann H.L., Raschpichler G., Bjøro K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 110.Bugianesi E., Gentilcore E., Manini R., Natale S., Vanni E., Villanova N. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 111.Li Y., Liu L., Wang B., Wang J., Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep. 2013;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mantovani A., Targher G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: spotlight on nonalcoholic fatty liver disease. Ann Transl Med. 2017;5:270. doi: 10.21037/atm.2017.04.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Omar B., Ahrén B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes. 2014;63:2196–2202. doi: 10.2337/db14-0052. [DOI] [PubMed] [Google Scholar]
- 114.Home P. Cardiovascular outcome trials of glucose-lowering medications: an update. Diabetologia. 2019;62:357–369. doi: 10.1007/s00125-018-4801-1. [DOI] [PubMed] [Google Scholar]
- 115.Cui J., Philo L., Nguyen P., Hofflich H., Hernandez C., Bettencourt R. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2016;65:369–376. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Joy T.R., McKenzie C.A., Tirona R.G., Summers K., Seney S., Chakrabarti S. Sitagliptin in patients with non-alcoholic steatohepatitis: a randomized, placebo-controlled trial. World J Gastroenterol. 2017;23:141–150. doi: 10.3748/wjg.v23.i1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Macauley M., Hollingsworth K.G., Smith F.E., Thelwall P.E., Al-Mrabeh A., Schweizer A. Effect of vildagliptin on hepatic steatosis. J Clin Endocrinol Metab. 2015;100:1578–1585. doi: 10.1210/jc.2014-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zelniker T.A., Wiviott S.D., Raz I., Im K., Goodrich E.L., Furtado R.H.M. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022–2031. doi: 10.1161/CIRCULATIONAHA.118.038868. [DOI] [PubMed] [Google Scholar]
- 119.Yan J., Yao B., Kuang H., Yang X., Huang Q., Hong T. Liraglutide, sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatol Baltim Md. 2019;69:2414–2426. doi: 10.1002/hep.30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petit J.-M., Cercueil J.-P., Loffroy R., Denimal D., Bouillet B., Fourmont C. Effect of Liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: the Lira-NAFLD study. J Clin Endocrinol Metab. 2017;102:407–415. doi: 10.1210/jc.2016-2775. [DOI] [PubMed] [Google Scholar]
- 121.Bizino M.B., Jazet I.M., de Heer P., van Eyk H.J., Dekkers I.A., Rensen P.C.N. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre-specified secondary study on ectopic fat accumulation. Diabetologia. 2020;63:65–74. doi: 10.1007/s00125-019-05021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou M., Mok M.T., Sun H., Chan A.W., Huang Y., Cheng A.S. The anti-diabetic drug exenatide, a glucagon-like peptide-1 receptor agonist, counteracts hepatocarcinogenesis through cAMP-PKA-EGFR-STAT3 axis. Oncogene. 2017;36:4135–4149. doi: 10.1038/onc.2017.38. [DOI] [PubMed] [Google Scholar]
- 123.Coskun T., Sloop K.W., Loghin C., Alsina-Fernandez J., Urva S., Bokvist K.B. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3–14. doi: 10.1016/j.molmet.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Finan B, Ma T, Ottaway N, Müller TD, Habegger KM, Heppner KM, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med 2013;5:209ra151. 10.1126/scitranslmed.3007218. [DOI] [PubMed]
- 125.Hartman M.L., Sanyal A.J., Loomba R., Wilson J.M., Nikooienejad A., Bray R. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43:1352–1355. doi: 10.2337/dc19-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zelniker T.A., Wiviott S.D., Raz I., Im K., Goodrich E.L., Bonaca M.P. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Lond Engl. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 127.Kahl S., Gancheva S., Straßburger K., Herder C., Machann J., Katsuyama H. Empagliflozin effectively lowers liver fat content in well-controlled type 2 diabetes: a randomized, double-blind, phase 4, placebo-controlled trial. Diabetes Care. 2020;43:298–305. doi: 10.2337/dc19-0641. [DOI] [PubMed] [Google Scholar]
- 128.Latva-Rasku A., Honka M.-J., Kullberg J., Mononen N., Lehtimäki T., Saltevo J. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: a randomized, double-blind, placebo-controlled study with 8-week treatment in type 2 diabetes patients. Diabetes Care. 2019;42:931–937. doi: 10.2337/dc18-1569. [DOI] [PubMed] [Google Scholar]
- 129.Cusi K., Bril F., Barb D., Polidori D., Sha S., Ghosh A. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21:812–821. doi: 10.1111/dom.13584. [DOI] [PubMed] [Google Scholar]
- 130.Eriksson J.W., Lundkvist P., Jansson P.-A., Johansson L., Kvarnström M., Moris L. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923–1934. doi: 10.1007/s00125-018-4675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kuchay M.S., Krishan S., Mishra S.K., Farooqui K.J., Singh M.K., Wasir J.S. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT trial) Diabetes Care. 2018;41:1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 132.Lai L.-L., Vethakkan S.R., Nik Mustapha N.R., Mahadeva S., Chan W.-K. Empagliflozin for the treatment of nonalcoholic steatohepatitis in patients with type 2 diabetes mellitus. Dig Dis Sci. 2020;65:623–631. doi: 10.1007/s10620-019-5477-1. [DOI] [PubMed] [Google Scholar]
- 133.Jojima T., Wakamatsu S., Kase M., Iijima T., Maejima Y., Shimomura K. The SGLT2 inhibitor canagliflozin prevents carcinogenesis in a mouse model of diabetes and non-alcoholic steatohepatitis-related hepatocarcinogenesis: association with SGLT2 expression in hepatocellular carcinoma. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20205237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun X., Han F., Lu Q., Li X., Ren D., Zhang J. Empagliflozin ameliorates obesity-related cardiac dysfunction by regulating Sestrin2-mediated AMPK-mTOR signaling and redox homeostasis in high-fat induced obese mice. Diabetes. 2020 doi: 10.2337/db19-0991. [DOI] [PubMed] [Google Scholar]
- 135.Tsimihodimos V., Filippas-Ntekouan S., Elisaf M. SGLT1 inhibition: pros and cons. Eur J Pharmacol. 2018;838:153–156. doi: 10.1016/j.ejphar.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 136.LIK066 (licogliflozin), an SGLT1/2 inhibitor, robustly decreases ALT and improves markers of hepatic and metabolic health in patients with nonalcoholic fatty liver disease: interim analysis of a 12-week, randomized, placebo-controlled, phase 2a study n.d. http://www.natap.org/2019/AASLD/AASLD_75.htm (accessed April 20, 2020).
- 137.Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cusi K., Orsak B., Bril F., Lomonaco R., Hecht J., Ortiz-Lopez C. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 139.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aithal G.P., Thomas J.A., Kaye P.V., Lawson A., Ryder S.D., Spendlove I. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 141.Zhou Y., Huang Y., Ji X., Wang X., Shen L., Wang Y. Pioglitazone for the primary and secondary prevention of cardiovascular and renal outcomes in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis. J Clin Endocrinol Metab. 2020;105 doi: 10.1210/clinem/dgz252. [DOI] [PubMed] [Google Scholar]
- 142.Finck B.N. Targeting metabolism, insulin resistance, and diabetes to treat nonalcoholic steatohepatitis. Diabetes. 2018;67:2485–2493. doi: 10.2337/dbi18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harrison S.A., Alkhouri N., Davison B.A., Sanyal A., Edwards C., Colca J.R. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase IIb study. J Hepatol. 2019 doi: 10.1016/j.jhep.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 144.Hong F., Xu P., Zhai Y. The opportunities and challenges of peroxisome proliferator-activated receptors ligands in clinical drug discovery and development. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19082189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016;150:1147–1159.e5. 10.1053/j.gastro.2016.01.038. [DOI] [PubMed]
- 146.S.A G. GENFIT: announces results from interim analysis of RESOLVE-IT phase 3 trial of elafibranor in adults with NASH and fibrosis. GlobeNewswire News Room 2020. http://www.globenewswire.com/news-release/2020/05/11/2031418/0/en/GENFIT-Announces-Results-from-Interim-Analysis-of-RESOLVE-IT-Phase-3-Trial-of-Elafibranor-in-Adults-with-NASH-and-Fibrosis.html (accessed June 3, 2020).
- 147.Jain M.R., Giri S.R., Bhoi B., Trivedi C., Rath A., Rathod R. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int Off J Int Assoc Study Liver. 2018;38:1084–1094. doi: 10.1111/liv.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boubia B., Poupardin O., Barth M., Binet J., Peralba P., Mounier L. Design, synthesis, and evaluation of a novel series of indole sulfonamide peroxisome proliferator activated receptor (PPAR) α/γ/δ triple activators: discovery of lanifibranor, a new antifibrotic clinical candidate. J Med Chem. 2018;61:2246–2265. doi: 10.1021/acs.jmedchem.7b01285. [DOI] [PubMed] [Google Scholar]
- 149.Keitel V., Dröge C., Häussinger D. Targeting FXR in cholestasis. Handb Exp Pharmacol. 2019;256:299–324. doi: 10.1007/164_2019_231. [DOI] [PubMed] [Google Scholar]
- 150.Younossi Z.M., Ratziu V., Loomba R., Rinella M., Anstee Q.M., Goodman Z. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet Lond Engl. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 151.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet Lond Engl. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Siddiqui M.S., Van Natta M.L., Connelly M.A., Vuppalanchi R., Neuschwander-Tetri B.A., Tonascia J. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J Hepatol. 2020;72:25–33. doi: 10.1016/j.jhep.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tully D.C., Rucker P.V., Chianelli D., Williams J., Vidal A., Alper P.B. Discovery of tropifexor (LJN452), a highly potent non-bile acid FXR agonist for the treatment of cholestatic liver diseases and nonalcoholic steatohepatitis (NASH) J Med Chem. 2017;60:9960–9973. doi: 10.1021/acs.jmedchem.7b00907. [DOI] [PubMed] [Google Scholar]
- 154.Kim W., Kim B.G., Lee J.S., Lee C.K., Yeon J.E., Chang M.S. Randomised clinical trial: the efficacy and safety of oltipraz, a liver X receptor alpha-inhibitory dithiolethione in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45:1073–1083. doi: 10.1111/apt.13981. [DOI] [PubMed] [Google Scholar]
- 155.Tacke F. Cenicriviroc for the treatment of non-alcoholic steatohepatitis and liver fibrosis. Expert Opin Investig Drugs. 2018;27:301–311. doi: 10.1080/13543784.2018.1442436. [DOI] [PubMed] [Google Scholar]
- 156.Friedman S.L., Ratziu V., Harrison S.A., Abdelmalek M.F., Aithal G.P., Caballeria J. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatol Baltim Md. 2018;67:1754–1767. doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ratziu V., Sanyal A., Harrison S.A., Wong V.W.-S., Francque S., Goodman Z. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR study. Hepatol Baltim Md. 2020 doi: 10.1002/hep.31108. [DOI] [PubMed] [Google Scholar]
- 158.Kim RG, Loomba R, Prokop LJ, Singh S. Statin use and risk of cirrhosis and related complications in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2017;15:1521–1530.e8. 10.1016/j.cgh.2017.04.039. [DOI] [PMC free article] [PubMed]
- 159.van den Berg E.H., Wolters A.A.B., Dullaart R.P.F., Moshage H., Zurakowski D., de Meijer V.E. Prescription of statins in suspected non-alcoholic fatty liver disease and high cardiovascular risk, a population-based study. Liver Int. 2019;39:1343–1354. doi: 10.1111/liv.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee H.Y., Jun D.W., Kim H.J., Oh H., Saeed W.K., Ahn H. Ezetimibe decreased nonalcoholic fatty liver disease activity score but not hepatic steatosis. Korean J Intern Med. 2019;34:296–304. doi: 10.3904/kjim.2017.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fabbrini E., Mohammed B.S., Korenblat K.M., Magkos F., McCrea J., Patterson B.W. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95:2727–2735. doi: 10.1210/jc.2009-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Oscarsson J, Önnerhag K, Risérus U, Sundén M, Johansson L, Jansson P-A, et al. Effects of free omega-3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non-alcoholic fatty liver disease: a double-blind, randomized, placebo-controlled study. J Clin Lipidol 2018;12:1390–1403.e4. 10.1016/j.jacl.2018.08.003. [DOI] [PubMed]
- 163.Kim C-W, Addy C, Kusunoki J, Anderson NN, Deja S, Fu X, et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab 2017;26:394–406.e6. 10.1016/j.cmet.2017.07.009. [DOI] [PMC free article] [PubMed]
- 164.Lawitz EJ, Coste A, Poordad F, Alkhouri N, Loo N, McColgan BJ, et al. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2018;16:1983–1991.e3. 10.1016/j.cgh.2018.04.042. [DOI] [PubMed]
- 165.Miyazaki M., Flowers M.T., Sampath H., Chu K., Otzelberger C., Liu X. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 166.One-year results of the global phase 2b randomized placebo-controlled ARREST trial of aramchol, a stearoyl CoA desaturasemodulator in NASH patients n.d. http://www.natap.org/2018/AASLD/AASLD_222.htm (accessed April 20, 2020).
- 167.Mookerjee S.A., Divakaruni A.S., Jastroch M., Brand M.D. Mitochondrial uncoupling and lifespan. Mech Ageing Dev. 2010;131:463–472. doi: 10.1016/j.mad.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Goedeke L., Perry R.J., Shulman G.I. Emerging pharmacological targets for the treatment of nonalcoholic fatty liver disease, insulin resistance, and type 2 diabetes. Annu Rev Pharmacol Toxicol. 2019;59:65–87. doi: 10.1146/annurev-pharmtox-010716-104727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Perry R.J., Kim T., Zhang X.-M., Lee H.-Y., Pesta D., Popov V.B. Reversal of hypertriglyceridemia, fatty liver disease and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell Metab. 2013;18:740–748. doi: 10.1016/j.cmet.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Perry R.J., Zhang D., Zhang X.-M., Boyer J.L., Shulman G.I. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science. 2015;347:1253–1256. doi: 10.1126/science.aaa0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Goedeke L., Peng L., Montalvo-Romeral V., Butrico G.M., Dufour S., Zhang X.-M. Controlled-release mitochondrial protonophore (CRMP) reverses dyslipidemia and hepatic steatosis in dysmetabolic nonhuman primates. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aay0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sinha R.A., Bruinstroop E., Singh B.K., Yen P.M. Nonalcoholic fatty liver disease and hypercholesterolemia: roles of thyroid hormones, metabolites, and agonists. Thyroid Off J Am Thyroid Assoc. 2019;29:1173–1191. doi: 10.1089/thy.2018.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Harrison S.A., Bashir M.R., Guy C.D., Zhou R., Moylan C.A., Frias J.P. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Lond Engl. 2019;394:2012–2024. doi: 10.1016/S0140-6736(19)32517-6. [DOI] [PubMed] [Google Scholar]
- 174.Kim K.-S., Lee B.-W., Kim Y.J., Lee D.H., Cha B.-S., Park C.-Y. Nonalcoholic fatty liver disease and diabetes: part II: treatment. Diabetes Metab J. 2019;43:127–143. doi: 10.4093/dmj.2019.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vilar-Gomez E., Vuppalanchi R., Gawrieh S., Ghabril M., Saxena R., Cummings O.W. Vitamin E improves transplant-free survival and hepatic decompensation among patients with nonalcoholic steatohepatitis and advanced fibrosis. Hepatol Baltim Md. 2020;71:495–509. doi: 10.1002/hep.30368. [DOI] [PubMed] [Google Scholar]
- 176.Zhou D., Pan Q., Shen F., Cao H.-X., Ding W.-J., Chen Y.-W. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7:1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gangarapu V., Ince A.T., Baysal B., Kayar Y., Kılıç U., Gök Ö. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:840–845. doi: 10.1097/MEG.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 178.Tarantino G., Finelli C. Systematic review on intervention with prebiotics/probiotics in patients with obesity-related nonalcoholic fatty liver disease. Future Microbiol. 2015;10:889–902. doi: 10.2217/fmb.15.13. [DOI] [PubMed] [Google Scholar]
- 179.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim A, Shah AS, Nakamura T. Extracellular Vesicles: A Potential Novel Regulator of Obesity and Its Associated Complications. Children. 2018;5 doi: 10.3390/children5110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sumida Y, Seko Y, Yoneda M. Novel Antidiabetic Medications for Non-Alcoholic Fatty Liver Disease With Type 2 Diabetes Mellitus. Hepatol Res. 2017;47:266–280. doi: 10.1111/hepr.12856. [DOI] [PubMed] [Google Scholar]