Summary
Since the outbreak in 2019, researchers are trying to find effective drugs against the SARS-CoV-2 virus based on de novo drug design and drug repurposing. The former approach is very time consuming and needs extensive testing in humans, whereas drug repurposing is more promising, as the drugs have already been tested for side effects, etc. At present, there is no treatment for COVID-19 that is clinically effective, but there is a huge amount of data from studies that analyze potential drugs.
We developed CORDITE to efficiently combine state-of-the-art knowledge on potential drugs and make it accessible to scientists and clinicians. The web interface also provides access to an easy-to-use API that allows a wide use for other software and applications, e.g., for meta-analysis, design of new clinical studies, or simple literature search. CORDITE is currently empowering many scientists across all continents and accelerates research in the knowledge domains of virology and drug design.
Subject Areas: Medicine, Virology, Drugs, Bioinformatics, Biological Database
Graphical Abstract
Highlights
-
•
CORDITE aggregates all available knowledge on potential drugs for SARS-CoV-2
-
•
CORDITE represents the largest curated database for drugs and interactions
-
•
CORDITE makes the data accessible via a webserver and an open API
-
•
CORDITE enables easy meta-analyses, design of new clinical studies, or literature search
Medicine; Virology; Drugs; Bioinformatics; Biological Database
Introduction
Coronaviruses can cause major outbreaks with fatal pneumonia, e.g., the severe acute respiratory syndrome coronavirus (SARS-CoV) in 2003 with a fatality rate of around 10% (Lee et al., 2003) or the Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012 with a fatality rate of 35% (de Groot et al., 2013). In 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused the disease COVID-19 and led to a pandemic (Zhu et al., 2020). The common symptoms of COVID-19 include fever, cough, shortness of breath, and dyspnea. A more severe progression of the COVID-19 disease may lead to pneumonia and severe acute respiratory syndromes. Unfortunately, there are no specific drugs or vaccines approved for the treatment of COVID-19 yet (Huang et al., 2020).
Traditionally, de novo drug design and drug repurposing are the common strategies scientists use to combat viral diseases. However, designing a new drug is very time consuming and needs extensive testing before clinical use in humans. In contrast, the repurposing of approved drugs or drugs in clinical trials is more promising, in particular as these have already been tested for side effects, toxicity, bioavailability, etc. At the moment, there is a huge amount of data from studies that analyze potential drugs based on computer simulations, in vitro studies, case studies, or clinical trials. However, none of the proposed treatments for COVID-19 have been proven to be clinically effective yet.
Potential drugs can target viral proteins or human proteins. At the time of writing, the most important viral target molecules that have been investigated for drug repurposing are SARS-CoV-2 spike protein, main protease (3CLpro, Mpro), papain-like proteinase (PLpro), and RNA-dependent RNA polymerase (RdRp) (Ou et al., 2020; Zhang et al., 2020), whereas the main targets on the human cell membrane are angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) (Aronson and Ferner, 2020). Besides these targets, several other candidates have been investigated as potential targets. Such investigations occurred on both viral and human sides, e.g., the methyltransferase and furin, respectively (Bestle et al., 2020; Khan et al., 2020). Also, several potential drugs were announced at the beginning of the pandemic, which later proved to be insufficiently effective or even harmful to patients, such as hydroxychloroquine or chloroquine phosphate. For instance, Gautret et al., 2020 reported a significant decrease in viral load in patients treated with hydroxychloroquine compared with controls. However, others have shown that these potential drugs can have severe side effects such as cardiac toxicity and also increased mortality (Mehra et al., 2020). Nevertheless, some of those studies showing higher mortality were later retracted because of a lack of transparency concerning the data (Mehra et al., 2020b). Other potential drug candidates, such as a combination of lopinavir with ritonavir (also known as Kaletra), were first considered promising candidates (Jeon et al., 2020) but later shown to be ineffective for SARS-CoV-2 (Cao et al., 2020). Nevertheless, many new drug candidates have been proposed by using different computational approaches, which address different viral and human targets (Hufsky et al., 2020; Sadegh et al., 2020).
The SARS-CoV-2 virus uses the human receptor ACE2 for cell entry, which is also used by SARS (Gralinski and Menachery, 2020; Wan et al., 2020; Xu et al., 2020; Zhou et al., 2020). It is expressed as a membrane-bound protein in the lungs, heart, kidneys, stomach, spleen, intestine, bone marrow, kidney, liver, brain, testis, and placenta (Shenoy et al., 2011; Donoghue et al., 2000; Soler et al., 2008). Moreover, Hoffmann et al. (2020) showed that SARS-CoV-2 depends on TMPRSS2, which therefore can serve as a target in therapy as well. TMPRSS2 primes the spike protein of SARS-Cov-2 to enable cell entry on ACE2. The authors further suggested that ACE2 binding to the spike protein might have a higher affinity compared with SARS. This may explain the infection of the upper respiratory system by SARS-CoV-2, which has not been observed in SARS, because in the upper respiratory system less ACE2 is expressed.
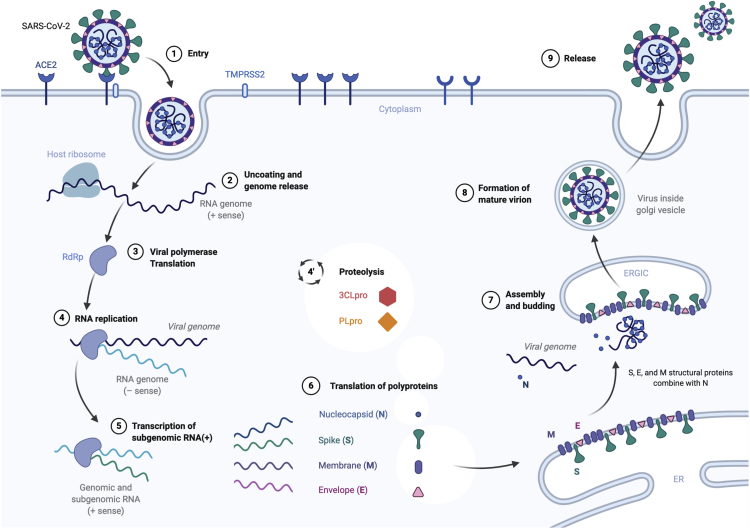
Structurally, each virion is approximately 50–200 nm in diameter (Chen et al., 2020). Like other coronaviruses, SARS-CoV-2 has four structural polyproteins, known as the S (spike), E (envelope), M (membrane), and N (nucleocapsid) proteins. The N protein holds the RNA genome, whereas the S, E, and M proteins together are part of the viral envelope. Upon successful attachment of the virus to the membrane of the host cell via the spike protein, the virus fuses with the membrane (Wu et al., 2020; Hoffmann et al., 2020). Next, the endosome sees a drop in pH and the genomic RNAss(+) is released into the cell's cytoplasm (Yang et al., 2004). As shown in Figure 1, upon uncoating and release of the RNA + sense viral genome in the cytoplasm, the host ribosome translates the viral RNA replicase or the viral RNA-dependent RNA polymerase (RdRp) (Ahn et al., 2012; Yin et al., 2020). This triggers proteolysis using the main protease of SARS-CoV-2 (also named 3CLpro) and the papain-like proteinase (PLpro) and enables the replicate-transcriptase complex to transcript subgenomic RNAs(+) (Anand et al., 2003; Chen et al., 2014). Pre-genomic RNAss(−) are replicated into genomic RNAss(+), whereas the former translation of subgenomic RNA(+) results in the aforementioned polyproteins. Essentially, the virus RNA uses the host cell to create new viral RNA genomes and to assemble new viral particles. These are the aforementioned S, E, M, and N structural proteins. They transit to the endoplasmic reticulum and are recruited for the assembly and building of new virions into the Golgi apparatus and burgeon out of the apparatus to then form mature virions (Guo et al., 2020). The N protein allows the viral genome to be extremely tightly packed into future viral capsids. In the end, new virions are released out of the host cell to infiltrate other cells in the host organism (He et al., 2020). A simplified illustration of the replication cycle of the SARS-CoV-2 virus is shown in Figure 1.
Figure 1.
Overview of the Replication Cycle of SARS-CoV2 Inside a Human Cell
The main targets that are investigated for drug repurposing are reported.
The knowledge from the life cycle of the SARS-CoV-2 virus offers a great potential to find suitable drugs for the already known targets. To combine the large amounts of current knowledge, we centralize the aggregated information from published articles and preprints about potential drugs, targets, and their interactions into CORDITE (CORona Drug InTEractions database), which includes a web interface and an application programming interface (API).
Results
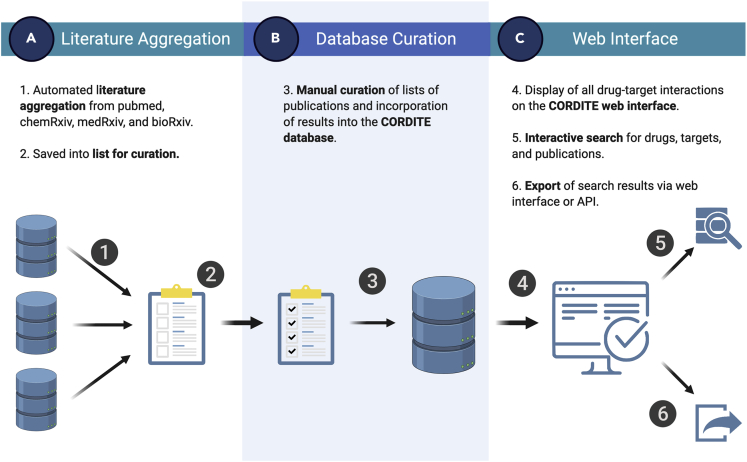
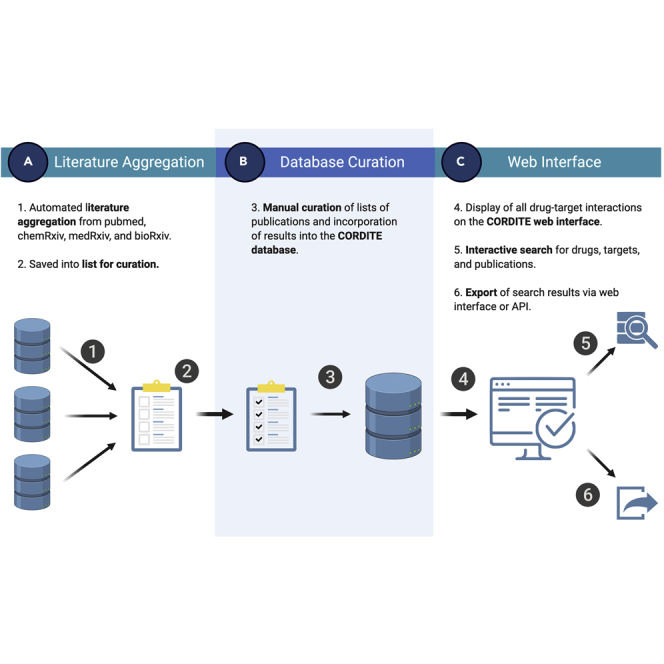
CORDITE integrates many functionalities to enable users to access, sort, and download relevant data to conduct meta-analyses, to design new clinical trials, or even to conduct a curated literature search. CORDITE automatically incorporates publications from PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), bioRxiv (https://www.biorxiv.org/), chemRxiv (https://www.chemrxiv.org/), and medRxiv (https://www.medrxiv.org/) that report information on computational, in vitro, or case studies on potential drugs for COVID-19 (see Figure 2). Besides original research, we also included reviews and comments in the database. The information from the articles and preprints are manually curated by moderators and can be accessed via the web interface or the open API. Users can directly access the publications, interactions, drugs, targets, and clinical trials. The interactions and the effectiveness of certain drugs are also shown as positive/negative results in a transparent way. Thus, the user can easily see the contradictory results of different studies and can take these into account for, e.g., meta-analyses. This access may offer easier integration for future software or apps that may need curated data.
Figure 2.
Depiction of All Processing Steps within the CORDITE Workflow.
(A) Literature aggregation.
(B) database curation.
(C) web interface.
Moreover, we include registered clinical trials from the National Institutes of Health (https://clinicaltrials.gov/) for COVID-19. The CORDITE database is updated through manual curation. The current summary for interactions, drugs, etc., is reported in Table 1. An introductory video for the use of CORDITE can be found on the website.
Table 1.
Summary Table of all Entry Types at Date of Submission
| Type | Numbers |
|---|---|
| Interactions | 849 |
| Targets | 25 |
| Drugs | 639 |
| Publications | 324 |
| Clinical trials | 247 |
Discussion
CORDITE provides an easy-to-use web interface and also access to an easy-to-use API that allows a wide use for other software and applications, e.g., for meta-analysis, design of new clinical studies, or simple literature search. CORDITE is currently the largest curated database for drug interactions for SARS-CoV-2, and therefore it offers the opportunity to accelerate research in the knowledge domains of virology and drug design. Because of the popularity and impact of the CORDITE website and database, we plan to continue hosting and managing CORDITE throughout the COVID-19 pandemic. Moreover, we will continuously improve the website and database to establish a standard tool for future diseases. We believe our efforts are essential to help researchers and clinicians to get an overview of existing studies and to accelerate the global search for new treatments.
Limitations of the Study
CORDITE aggregates available knowledge on drugs for COVID-19; however, in particular the preprints have not been peer reviewed. Thus, CORDITE cannot guarantee the reliability of the studies and their results, in particular the effectiveness of the drugs.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Dominik Heider (dominik.heider@uni-marburg.de).
Materials Availability
This study did not generate new unique data.
Data and Code Availability
This study did not generate data. The curated data can be accessed freely via the API.
Availability: CORDITE is available at https://cordite.mathematik.uni-marburg.de.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors thank Hagen Dreβler for technical assistance.
Author Contributions
R.M. developed and implemented the database and the API. H.F.L. developed and implemented the web interface and curated approximately 2.3% of the data. M.W. developed the automated literature crawler and curated approximately 11.3% of the data. G.H. created Figure 1 and the visual identity of the web interface and contributed to the initial draft. A.-C.H. contributed to the project design, created Figure 2, and curated approximately 7.4% of the data. D.H. designed and supervised the project, wrote the manuscript, and curated approximately 79.0% of the data. All authors revised the final manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101297.
Supplemental Information
References
- Ahn D.G., Choi J.K., Taylor D.R., Oh J.W. Biochemical characterization of a recombinant SARS coronavirus nsp12 RNA-dependent RNA polymerase capable of copying viral RNA templates. Arch. Virol. 2012;157:2095–2104. doi: 10.1007/s00705-012-1404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- Aronson J.K., Ferner R.E. Drugs and the renin-angiotensin system in covid-19. BMJ. 2020;369:m1313. doi: 10.1136/bmj.m1313. [DOI] [PubMed] [Google Scholar]
- Bestle D., Heindl M.R., Limburg H., Lam van T.V., Pilgram O., Moulton H., Stein D.A., Hardes K., Eickmann M., Dolnik O. TMPRSS2 and furin are both essential for proteolytic activation and spread of SARS-CoV-2 in human airway epithelial cells and provide promising drug targets. BioRxiv. 2020 doi: 10.1101/2020.04.15.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787-1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Yang X., Zheng Y., Yang Y., Xing Y., Chen Z. SARS coronavirus papain-like protease inhibits the type I interferon signaling pathway through interaction with the STING-TRAF3-TBK1 complex. Protein Cell. 2014;5:369–381. doi: 10.1007/s13238-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R.J., Baker S.C., Baric R.S., Brown C.S., Drosten C., Enjuanes L., Fouchier R.A., Galiano M., Gorbalenya A.E., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the coronavirus study group. J. Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. https://pubmed.ncbi.nlm.nih.gov/32205204/ [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gralinski L.E., Menachery V.D. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12:E135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K.S., Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil. Med. Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Deng Y., Li W. Coronavirus disease 2019: what we know? J. Med. Virol. 2020;92:719–725. doi: 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufsky F., Lamkiewicz K., Almeida A., Aouacheria A., Arighi C., Bateman A., Baumbach J., Beerenwinkel N., Brandt C., Cacciabue M. Computational strategies to combat COVID-19: useful tools to accelerate SARS-CoV-2 and coronavirus research. Preprint. 2020 doi: 10.20944/preprints202005.0376.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S., Shum D., Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother. 2020;64:e00819-20. doi: 10.1128/AAC.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R.J., Jha R.K., Amera G.M., Jain M., Singh E., Pathak A., Singh R.P., Muthukumaran J., Singh A.K. Targeting SARS-CoV-2: a systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2'-O-ribose methyltransferase. J. Biomol. Struct. Dyn. 2020;20:1–14. doi: 10.1080/07391102.2020.1753577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31180-6/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mehra M.R., Ruschitzka F., Patel A.N. Retraction—hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadegh S., Matschinske J., Blumenthal D.B., Galindez G., Kacprowski T., List M., Nasirigerdeh R., Oubounyt M., Pichlmair A., Rose T.D., Salgado-Albarrán M. Exploring the SARS-CoV-2 virus-host-drug interactome for drug repurposing. arXiv. 2020 doi: 10.1038/s41467-020-17189-2. https://arxiv.org/abs/2004.12420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy V., Qi Y., Katovich M.J., Raizada M.K. ACE2, a promising therapeutic target for pulmonary hypertension. Curr. Opin. Pharmacol. 2011;11:150–155. doi: 10.1016/j.coph.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler M.J., Barrios C., Oliva R., Batlle D. Pharmacologic modulation of ACE2 expression. Curr. Hypertens. Rep. 2008;10:410–414. doi: 10.1007/s11906-008-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W., Mao C., Luan X., Shen D.D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M., Chang S. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368:1499–1504. doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate data. The curated data can be accessed freely via the API.
Availability: CORDITE is available at https://cordite.mathematik.uni-marburg.de.