Since the end of February, Bergamo’s province in Northern Italy has become the second epicenter worldwide after Wuhan, China of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Papa Giovanni XXIII Hospital was selected as the tertiary referral center for COVID-19, with 500 dedicated beds and more than 1500 hospitalization so far. In the first 2 months’ experience, mounting evidence was collected that coagulopathy is one of the possible predictors of poor outcomes in COVID-19 patients.1 Although the most affected organ is the lung in terms of both pneumonia1 and vascular disease such as pulmonary embolism. A recent study reported ischemic involvement at abdominal imaging in some patients with COVID-19 and gastrointestinal symptoms.2
Methods
We retrospectively collected clinical records of SARS-CoV-2–positive patients diagnosed with intestinal ischemia upon emergency department admission or during their hospitalization in the period from March 1 to April 30, 2020 in Papa Giovanni XXIII Hospital. Those patients were compared with patients diagnosed with intestinal ischemia in a pre–COVID-19 period from January 1 to February 29, 2020. The protocol was approved by the local ethical committee.
Results
We found that 7 patients fulfilled clinical and radiologic criteria for intestinal ischemia (Table 1 ). All patients presented with gastrointestinal (GI) symptoms. Six were evaluated with abdominal contrast-enhanced computed tomography scan. Two demonstrated evidence of ischemic colitis with marked wall edema and layered enhancement involving either the whole large bowel or the right colon. Four had evidence of ischemia (thin wall with reduced enhancement or signs of pneumatosis intestinalis) involving either the small bowel or the right transverse colon (Supplementary Figure 1). Among the latter 4, thromboembolic filling defects in the inferior vena cava and superior mesenteric vein were found in 1. Two of the 6 patients also showed unexplained splenic infarcts. One additional patient was diagnosed with ischemic colitis through endoscopy (Supplementary Figure 2). D-dimer was tested in 6 patients and results were markedly elevated in all of them. Six of 7 patients also underwent a thoracic computed tomography scan that showed pulmonary thromboembolism in 1 patient. Four of 7 patients (57%) died either in the first 48 hours after admission (n = 3) or after surgery (n = 1)
Table 1.
Characteristics of Patients With Intestinal Ischemia During COVID-19 Infection
| Patient no. | Age, y | Sex | Ongoing ACT/APT | Entry symptoms | CT/endoscopy findings | D-dimer | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 85 | Male | ASA + DOAC | Low GI bleeding | CT and endoscopy: ischemic colitis | Not done | Discharged |
| 2 | 71 | Female | ASA | Loss of appetite, vomiting, low GI bleeding | Endoscopy: ischemic colitis | 10 N | Discharged |
| 3 | 69 | Male | No | Diarrhea, fever, and dyspnea | CT: ischemic colitis (right colon) | 8 N | Death |
| 4 | 79 | Female | LMWH | Abdominal pain | CT: SB ischemia with SB occlusion; splenic infarcts | 8 N | Discharged |
| 5 | 63 | Male | No | Lower limb pain | CT: right and transverse colon ischemia; splenic infarcts | >70 N | Death |
| 6 | 62 | Male | No | Abdominal pain and vomiting | CT: SB ischemia with SMV and IVC thrombosis | >70 N | Death |
| 7 | 83 | Female | LMWH | Dyspnea and abdominal pain | CT: SB and colon ischemia | 3 N | Death |
ACT, anticoagulant therapy; APT, antiplatelet therapy; ASA, acetylsalicylic acid; CT, computed tomography, DOAC, direct oral anticoagulant; GI, gastrointestinal; IVC, inferior vena cava; LMWH, low-molecular-weight heparin; SB, small bowel; SMV, superior mesenteric vein.
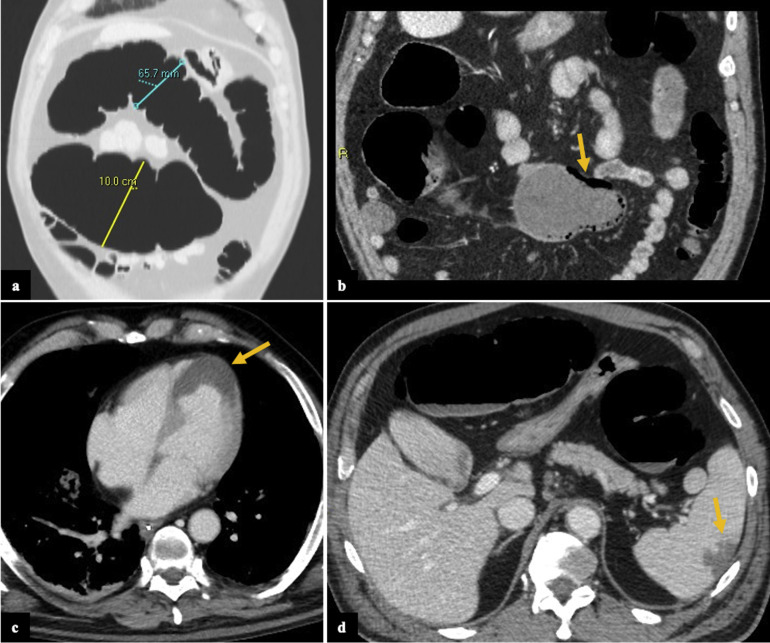
Supplementary Figure 1.
Computed tomography (CT) scan findings of patient 5. Axial (A) and coronal (B) images show pathologic dilation of the transverse colon (measuring up to 10 cm in caliber, A) associated with thin wall and parietal pneumatosis (arrow, B). Also, the ascending colon was involved (not completely shown). There were no signs of pneumoperitoneum or main mesenteric vessels thrombosis; the small bowel loops were normal. Concomitantly, a myocardial infarct (arrow, C) and a focal ischemic lesion in the spleen were noted (arrow, D). Patient was admitted to intensive care unit and died 1 day after the CT scan.
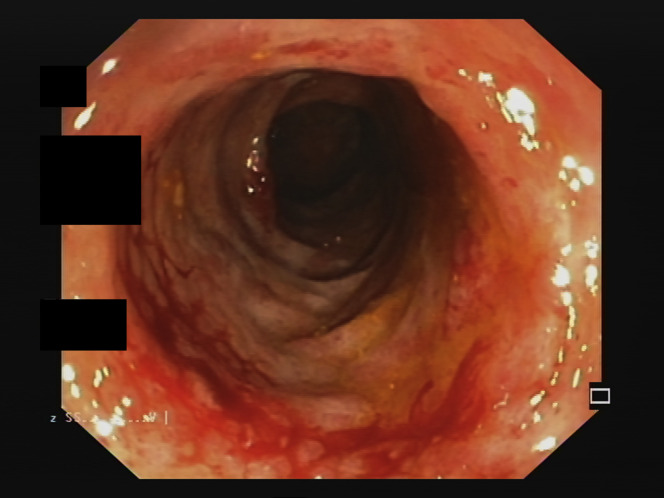
Supplementary Figure 2.
Endoscopic appearance of transverse colon of patient 2.
In the study period pre–COVID-19, we identified 3 patients who fulfilled the enrollment criteria of intestinal ischemia. This accounts for an increase of 133% in intestinal ischemia admitted to our hospital during the COVID-19 pandemic. One patient died from the sequelae of small bowel resection due to ischemia. The other 2 recovered from ischemic colitis. All 3 patients had important comorbidities, including chronic renal insufficiency on dialysis for the first patient who died, Hodgkin’s lymphoma in the second patient, and type 2 diabetes and hypertension in the third patient.
Mortality among patients with COVID-19–related intestinal ischemia was 1.7-fold higher (95% confidence interval, 0.3–9.6) than in the remnant cohort.
Discussion
This is, to our knowledge, the first report exploring clinical features and outcomes of patients with COVID-19 presenting with intestinal ischemia. Our study confirms a previous case report on the association of intestinal ischemia with COVID-193 and demonstrates an increased mortality rate in patients with intestinal ischemia in the COVID-19 period compared with SARS-CoV-2–negative patients.
GI symptoms have been described in 10% of patients with COVID-19.4 In most cases these symptoms are mild and self-limiting, even if they seem to increase the risk of complications.4 We found that GI symptoms at admission could also be the expression of an underlying intestinal ischemia.
A state of hypercoagulability has been found via thromboelastography in most of the COVID-19 patients admitted to intensive care units.5 Quantitative D-dimer has already been proposed as the best single marker of hypercoagulability in COVID-19 patients, as well as a negative prognostic marker.6
In addition, a recent publication has proposed down-regulation of angiotensin-converting-enzyme 2 as a possible pathogenetic hypothesis for acute vascular damage.7 The presence of angiotensin-converting-enzyme 2 protein, which has been proved to be a cell receptor for SARS-CoV-2, has been demonstrated by immunofluorescence in the gastrointestinal epithelium.8
The increase in mortality rate of this series accounts for part of the +568% mortality of Bergamo province reported in the first trimester of 2020 compared with the first trimester of the previous 4 years (2015–2019).9
No final conclusions can be drawn from such a small number of patients due to the ongoing characteristic of the pandemic. However, our experience suggests that a high level of suspicion for intestinal ischemia should be maintained in COVID-19 patients presenting with GI symptoms or with arising abdominal pain because this complication could account for an increase mortality risk. Nevertheless, in this subset, a D-dimer elevation should not only trigger prompt prophylactic use of anticoagulants, but also lead to consider an early abdominal computed tomography scan in patients with suggestive symptoms or biochemical markers of intestinal ischemia. Additional analysis will help to define the role of SARS-CoV-2 in the pathogenesis of such a detrimental manifestation.
CRediT Authorship Contributions
Lorenzo Norsa, MD, PhD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Writing – original draft: Lead). Bonaffini Pietro, MD (Data curation: Lead; Methodology: Lead; Writing – review & editing: Lead). Amedeo Indriolo, MD (Data curation: Lead; Writing – review & editing: Lead). Clarissa Valle, MD (Data curation: Lead; Methodology: Lead; Writing – review & editing: Lead). Sonzogni Aurelio, MD (Data curation: Lead; Supervision: Lead; Writing – review & editing: Lead). Sandro Sironi, MD (Supervision: Equal; Writing – review & editing: Equal).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dxdoi.org/10.1053/j.gastro.2020.06.041.
Supplementary Material
References
- 1.Guan W.-J. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhayana R. Radiology. 2020 May 11:201908. [Google Scholar]
- 3.Parry A.H. Acad Radiol. 2020;27:1190. doi: 10.1016/j.acra.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao R. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panigada M. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thachil J. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giardini V. Am J Hematol. 2020;95:E188–E191. doi: 10.1002/ajh.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao F. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ufficio Stampa Istat. https://www.istat.it/it/files/2020/05/Rapporto_Istat_ISS.pdf Available at: