Dear Sir
Patients with multiple sclerosis (pwMS) using disease-modifying treatments (DMT) can present a varying degree of immunodeficiency that can translate into an increased risk of infections (Luna et al., 2019). Whether pwMS are at increased risk of COVID-19 or at higher risk of developing severe complications is still unknown. Expert recommendations on the management of pwMS during the COVID-19 pandemic have rapidly emerged, with the Italian Society of Neurology and the Association of British Neurologists releasing the first examples. Overall, both groups consider safe to start or continue treatment with interferons β, glatiramer acetate, teriflunomide, dimethyl fumarate and non-lymphodepleting DMTs such as fingolimod and natalizumab. In relation with the lymphodepleting ocrelizumab and alemtuzumab, the recommendations are less straightforward, focusing on individual factors such as disease activity and lymphocyte count, but suggesting to temporarily delay the start or dosing of alemtuzumab. Nevertheless, such recommendations may be inadequate in pwMS with a highly active form of MS. Alemtuzumab is a humanized monoclonal antibody that depletes circulating lymphocytes by selectively targeting CD52, highly expressed on lymphocytes T and B, and effectively used to treat pwMS. Lymphocyte depletion is followed by a distinct pattern of T- and B-cell repopulation that begins within weeks, with B-cell counts returning to baseline levels within 6 months, whereas T-cell counts rise more slowly, generally approaching the lower limits of normal by 12 months (Li et al., 2018). Many neurologists have stopped prescribing alemtuzumab in pandemic times because of the strong acute post-infusion lymphodepletion.
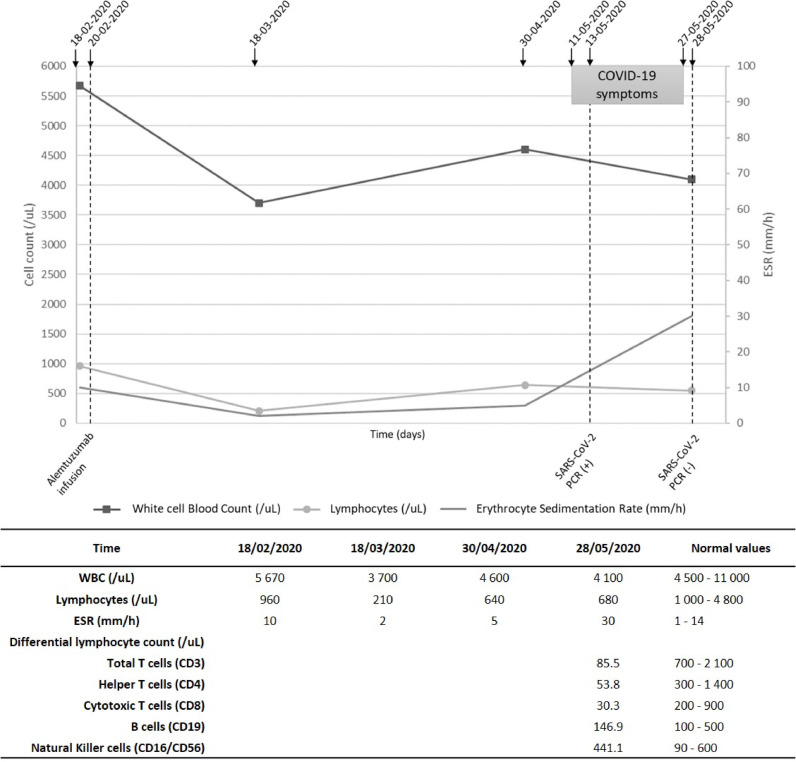
We present a pwMS with mild COVID-19 disease with severe lymphocyte depletion of the major circulating T lymphocytes (CD3+, CD4+ and CD8+) due to alemtuzumab. A 35-year-old man was diagnosed with relapsing remitting multiple sclerosis (RRMS) in November 2018 according to the Mc-Donald criteria. He did not have any other comorbidities. He developed progressive bilateral lower limb numbness and mild motor impairment, followed by diplopia. Magnetic resonance imaging (MRI) at time of diagnosis showed multiple demyelinating lesions in the brain and spinal cord, consistent with multiple sclerosis. In December 2018 he was infused with the first dose of alemtuzumab (12 mg daily IV for five days) and the second dose was infused (12 mg daily IV for three days) at the end of February 2020. Between both doses, he had not experienced clinical deterioration. Neurological examination revealed reduced muscle strength (4/5) on the right leg (Expanded Disability Status Scale 1). The last MRI, performed on March 2020, showed stability of the lesion burden in relation to the previous examinations of the brain and spinal cord, with absence of contrast-enhancing lesions (Fig. 1 ). Before the second infusion he had an absolute lymphocyte count (ALC) of 960/mm3, one month after the infusion ALC decreased to 210/mm3. He was asked to follow a voluntary quarantine, however, he had contact with his COVID-19 positive wife two and a half months after the infusion. He experienced dry cough and a nasopharyngeal swab was obtained for real-time PCR testing for SARS-CoV-2 and the test was positive. He evolved with mild fever, his respiratory rate and sounds and oxygen saturation were normal. A blood test revealed ALC of 680/mm3 with severely reduced major circulating T lymphocyte subsets (CD3+, CD4+ and CD8+). Mean CD3+ count was reduced by 88% from lower limit of normal (LLN) whereas CD4+ and CD8+were 82% and 85% below the LLN, respectively (Fig. 2 ). B lymphocytes and NK cells were between normal limits. Mild cold symptoms did not deteriorate. He stayed at home. Two weeks after the first swab, the second one was negative and the patient asymptomatic.
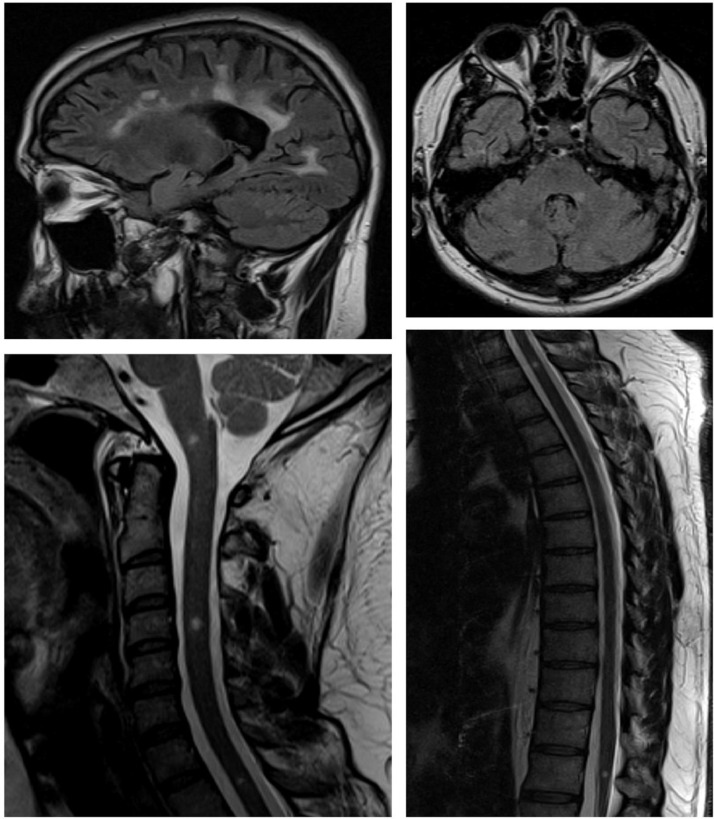
Fig. 1.
Last control MRI, performed in March 2020.The lesion burden remains stable in relation to the previous examinations, with multiple confluent supratentorial lesions (juxtacortical and periventricular), lesions in the posterior fossa (left middle cerebellar peduncle and right cerebellar hemisphere), one lesion at the bulbo-medullary junction and multiple spinal cord lesions (levels C4, D1 and at the conus medullaris). Lesions do not enhance with gadolinium.
Fig. 2.
Panel showing the timeline of the case report and laboratory data.
We described a pwMS with severely reduced major circulating T lymphocyte subsets (CD3+, CD4+ and CD8+) due to alemtuzumab that developed COVID-19 disease without complications. Carandini et al. have reported a mild uncomplicated infection in a 25-year old girl seven days after the second cycle of alemtuzumab. The patient did not have other co-morbidities and had ALC of 99/mm3 (Carandini et al., 2020). We speculate that the lack of lymphocytes may have played a favourable role during the COVID-19 infection by preventing an overly active immune response in these two patients. There is agreement that first-generation DMTs do not increase the risk of infection and could even be beneficial in the case of interferon β because of its antiviral characteristics, but second-generation DMTs have shown to augment patient risk of developing viral diseases. Currently, on the literature there are two pwMS treated with fingolimod diagnosed with severe COVID-19 disease with interstitial pneumonia and both had favourable in-hospital outcomes (Barzegar et al., 2020; Foerch et al., 2020). Another patient treated with interferon β developed COVID-19 disease with interstitial pneumonia and required seven days of admission (Gemcioglu et al., 2020). A patient on natalizumab developed mild bilateral interstitial pneumonia with resolution of symptoms within 10 days (Borriello and Ianniello, 2020). Moreover, a pharmacovigilance case series described 32 patients on ocrelizumab and COVID-19 disease with positive test results; five of them required intensive treatment units and no fatal outcomes were reported (Hughes et al., 2020). There have not been issues with absolute lymphocyte counts (ALC) in these patients.
It has been suggested that a group of patients with COVID-19 may have cytokine storm syndrome in which immunosuppression may be beneficial(Mehta et al., 2020), although other authors have described the possible risk of treating patients with immunosuppressors stating that this association established between disease severity and presence of inflammatory cytokines may not necessarily be one of causality (Russell et al., 2020).
The vast majority of patients treated with alemtuzumab during 2020 are facing this pandemic crisis with a low absolute lymphocyte count. A better understanding of the implications of COVID-19 in patients with immune-mediated diseases and the effects of immunosuppressive therapies is urgently needed to guide clinicians in the care of pwMS. The decision to start, continue or stop treatment with a given DMT should be strongly influenced by whether pwMS do follow recommendations or not to prevent exposure to the virus. In all cases any increased risk of infection and associated morbidity will need to be carefully balanced against the risks of stopping treatment and facing a rebound of disease activity.
Informed consent
Prior to inclusion, patient provided informed written consent for participation in this study and study FONIS SA1610026. The project was approved by the local research ethics committees of the University of Chile Hospital, Santiago, Chile.
CRediT authorship contribution statement
Carlos Guevara: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Supervision. Eduardo Villa: Validation, Formal analysis, Investigation, Writing - original draft. Marcela Cifuentes: Investigation. Rodrigo Naves: Investigation. José de Grazia: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Supervision.
Declaration of Competing Interest
Authors have no conflict of interest.
References
- Barzegar M., Mirmosayyeb O., Nehzat N., Sarrafi R., Khorvash F., Maghzi A.-.H., Shaygannejad V. COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurol. Neuroimmunol. neuroinflammation. 2020;7 doi: 10.1212/NXI.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriello G., Ianniello A. COVID-19 occurring during Natalizumab treatment: a case report in a patient with extended interval dosing approach. Mult. Scler. Relat. Disord. 2020 doi: 10.1016/j.msard.2020.102165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini T., Pietroboni A.M., Sacchi L., De Riz M.A., Pozzato M., Arighi A., Fumagalli G.G., Martinelli Boneschi F., Galimberti D., Scarpini E. Alemtuzumab in multiple sclerosis during the COVID-19 pandemic: a mild uncomplicated infection despite intense immunosuppression. Mult. Scler. 2020 doi: 10.1177/1352458520926459. [DOI] [PubMed] [Google Scholar]
- Foerch C., Friedauer L., Bauer B., Wolf T., Adam E.H. Severe COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Mult. Scler. Relat. Disord. 2020 doi: 10.1016/j.msard.2020.102180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemcioglu E., Davutoglu M., Ozdemir E.E., Erden A. Are Type 1 Interferons treatment in Multiple Sclerosis as a potential therapy against COVID-19? Mult. Scler. Relat. Disord. 2020 doi: 10.1016/j.msard.2020.102196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R., Pedotti R., Koendgen H. COVID-19 in persons with multiple sclerosis treated with ocrelizumab - a pharmacovigilance case series. Mult. Scler. Relat. Disord. 2020;102192 doi: 10.1016/j.msard.2020.102192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Richards S., Surks H.K., Jacobs A., Panzara M.A. Clinical pharmacology of alemtuzumab, an anti-CD52 immunomodulator, in multiple sclerosis. Clin. Exp. Immunol. 2018;194:295–314. doi: 10.1111/cei.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna G., Alping P., Burman J., Fink K., Fogdell-Hahn A., Gunnarsson M., Hillert J., Langer-Gould A., Lycke J., Nilsson P., Salzer J., Svenningsson A., Vrethem M., Olsson T., Piehl F., Frisell T. Infection Risks Among Patients With Multiple Sclerosis Treated With Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol. 2019;77 doi: 10.1001/jamaneurol.2019.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020 doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV Lung Injury. Lancet (London, England) 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]