Abstract
Background
The COVID-19 is a novel condition affecting all the world, so every manifestation of disease should be reported. Neurologic manifestations of the disease are increasingly identified and this will help clinician to improve their diagnostic and therapeutic skills in dealing with COVID-19 patients.
Case
In this article we report a 41-year-old male that developed ascending paresthesia and paralysis following infection with SARS-CoV-2 virus. Electrodiagnostic evaluation in patient revealed demyelinating type polyneuropathy and patient diagnosed as Guillain-Barré syndrome (AIDP type) and treated with IVIG which resulted in favorable response.
Conclusions
Considering this report and other reports that are mentioned in our short review, there is probably causal relationship between COVID-19 and development of Guillain-Barré syndrome.
Keywords: COVID-19, SARS-CoV-2, Guillain-Barré syndrome
Graphical abstract
Highlights
-
•
The COVID-19 affects both peripheral and central nervous system
-
•
Probably there is causal relationship between Guillain-Barré syndrome and SARS-CoV-2 infection
-
•
Both demyelinating and axonal type Guillain-Barré syndrome can occur in association with SARS-CoV-2 infection
1. Introduction
The outbreak of novel coronavirus infection emerged on 2019 December in Wuhan, China and soon spread to involve all the world.
Now the health care systems worldwide are facing with pandemic of severe acute respiratory coronavirus 2 (SARS-COV-2) and its associated disease, named coronavirus disease 19 (COVID-19). The identified symptoms of infection first thought to be confined to the respiratory system, but as the time passed extrarespiratory manifestations became increasingly identified.
At first it seemed that the COVED-19 spares the nervous system and neurologic complications were among the manifestations later to be identified. Now we know that both central and peripheral nervous system may be affected by COVID-19(Baig, 2020)(Mao et al., 2020). Involvement of central nervous system based on report by Helms et al. ranges from encephalopathy with agitation and confusion, and corticospinal tract signs to ischemic stroke(Helms et al., 2020). Peripheral nervous system is also affected by COVID-19, and several cases of acute polyneuropathy had recently reported.
Here, the authors report the case of an acute and severe peripheral polyneuropathy associated with SARS-COV-2 infection.
2. Case presentation
On April 15 (As of this date, 76,389 patients with COVID- 19 have been identified in Iran, of which 4777 deaths have been occurred by the virus, and according to statistics, Iran ranks sixth in the number of deaths due to COVID- 19) a 41-year-old male was referred to emergency department for acute onset of cough, dyspnea, and fever. Regarding symptom profile and chest CT scan findings (obtained same day) of multiple ground glass opacities and consolidations, typical of COVID-19 pneumonia (Fig. 1 ) the patient diagnosed as COVID-19 and was admitted to hospital. At the time of admission, the patient was febrile with an oral temperature of 39 °C, the blood pressure was 110/70 mmHg, the pulse was 110 beats per minute and the respiratory rate was 22 breaths per minute. O2 saturation was 88% on room air. His medical history included type 2 diabetes mellitus under treatment with metformin and gliclazide. Laboratory data revealed lymphopenia (WBC:5.9 × 109/L, PMN:85%, Lymphocyte:15%), elevated levels of C-reactive protein (CRP: ++) and erythrocyte sedimentation rate (ESR:69 mm/h), glucose (280 mg/dL) and HbA1c (8.7%).
Fig. 1.
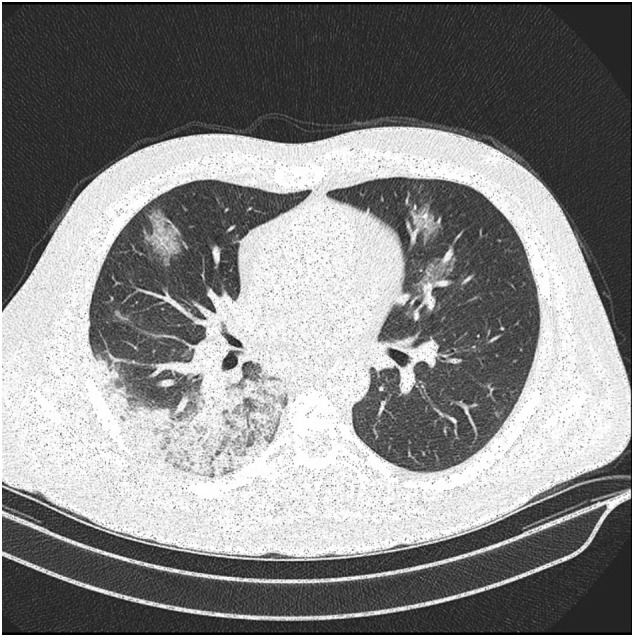
Chest CT scan revealed a peripheral ground-glass opacity.
The patient underwent treatment with antiviral agent lopinavir/ritonavir and hydroxychloroquine. Reverse transcription polymerase chain reaction (RT-PCR) assays of nasopharyngeal sample was positive for SARS-CoV-2.
Ten days after onset of pneumonia the patient experienced paresthesia in feet which later days progressed to involve more proximal parts and mild weakness added, but these symptoms unnoticed until patient discharged 14 days after the onset of COVID-19 symptoms, following improvement of respiratory symptoms (probably because of attributing these complaints by treating physician to diabetic neuropathy).
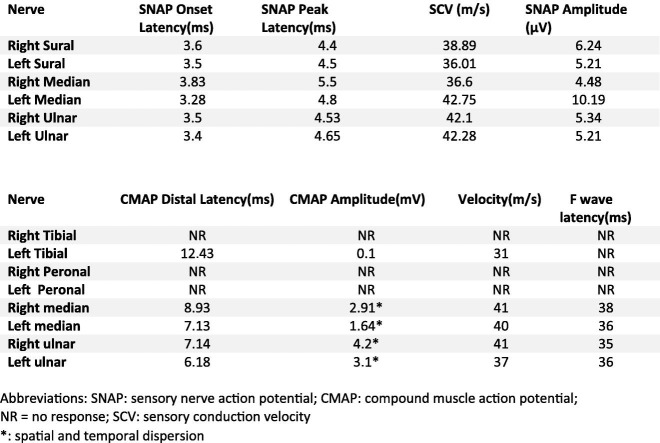
Seven days after onset of neurologic symptom regarding worsening of paresthesia and weakness the patient was referred for neurologic evaluation. In the first look at neurology office the patient was unable to stand and walk without assistance. The neurologic examination revealed normal mental status and speech. Motor examination showed absent muscle stretch reflexes in lower extremities and diminished reflexes in upper extremities. There was symmetric limb weakness (Medical Research Council score 4/5 at upper limbs and 3/5 at lower limbs), and symmetric and severe stocking-and-glove hypesthesia and reduced vibration and position sense at all 4 limbs (more pronounced at lower limbs). There was no cranial nerve deficit. Plantar responses were flexor. The patients complained of pain and an aching discomfort in the muscles, mainly those of the shoulder, hips, thighs, and back. There was no dyspnea, and cough if present was occasional. Electromyography and nerve conduction studies showed absence of right tibial and both peroneal nerves compound muscle action potentials (CMAP). F waves were unobtainable at lower limbs. Left tibial, Right and left median, and ulnar nerves distal latencies were markedly prolonged and velocities and amplitudes (with spatial and temporal dispersion) were decreased. All sensory nerve action potentials (SNAP) latencies were prolonged and amplitudes decreased (Table.1 ).
Table 1.
Nerve conduction study parameters.
| Nerve | SNAP Onset Latency(ms) | SNAP Peak Latency(ms) | SCV (m/s) | SNAP Amplitude (μV) |
|---|---|---|---|---|
| Right Sural | 3.6 | 4.4 | 38.89 | 6.24 |
| Left Sural | 3.5 | 4.5 | 36.01 | 5.21 |
| Right Median | 3.83 | 5.5 | 36.6 | 4.48 |
| Left Median | 3.28 | 4.8 | 42.75 | 10.19 |
| Right Ulnar | 3.5 | 4.53 | 42.1 | 5.34 |
| Left Ulnar | 3.4 | 4.65 | 42.28 | 5.21 |
|
| ||||
|---|---|---|---|---|
| Nerve | CMAP Distal Latency(ms) | CMAP Amplitude(mV) | Velocity(m/s) | F wave latency(ms) |
| Right Tibial | NR | NR | NR | NR |
| Left Tibial | 12.43 | 0.1 | 31 | NR |
| Right Peronal | NR | NR | NR | NR |
| Left Peronal | NR | NR | NR | NR |
| Right median | 8.93 | 2.91a | 41 | 38 |
| Left median | 7.13 | 1.64a | 40 | 36 |
| Right ulnar | 7.14 | 4.2a | 41 | 35 |
| Left ulnar | 6.18 | 3.1a | 37 | 36 |
Abbreviations: SNAP: sensory nerve action potential; CMAP: compound muscle action potential; NR = no response; SCV: sensory conduction velocity.
Spatial and temporal dispersion.
On needle EMG examination, no spontaneous activity was seen. Motor unit action potential (MUAP) morphology was normal, but with reduced recruitment in weak muscles including tibialis anterior, vastus lateralis, and iliacus.
These findings were consistent with demyelination pattern polyneuropathy, so the patient diagnosed as Guillain-Barré syndrome (GBS) associated with COVID-19, and at the same day admitted in hospital and treatment with IV immunoglobulins (0.4 g/kg per day for 5 consecutive days) was initiated. There was a favorable response to IVIG so the patient was able to stand and walk a short distance without assistance in day 3 of therapy. On discharge patient was ambulate but with some residual weakness in lower extremities, so was referred for rehabilitation clinic.
Personal protective equipment was used during first examination and subsequent visits.
3. Discussion
Guillain–Barré syndrome is considered an autoimmune process that affects peripheral nerves, and commonly manifests as demyelinating neuropathy with ascending paresthesia and weakness. Though the exact etiology and pathophysiology have poorly been identified, the syndrome is commonly preceded by a respiratory or gastrointestinal infection.
Although various microbial agents and antecedent events including various viral illnesses in adults and children have been associated with Guillain–Barré syndrome, Campylobacter jejuni, Epstein–Barr virus, cytomegalovirus, and Zika virus are among the most reported agents. There have been reports of an association between Guillain–Barré syndrome and Coronaviridae family viruses. For instance, during 2012 outbreak of Middle East respiratory syndrome (MERS) caused by MERS coronavirus (CoV) that shares many similar features with SARS-COV-2 a few cases of Guillain–Barré syndrome associated with infection had been reported(Kim et al., 2017).
The occurrence of autoimmune polyneuropathy in patients with aforementioned viral and bacterial infections indicates that these agents have the potential of autoimmune response induction. In the case of Campylobacter jejuni for instance molecular mimicry between GM1 gangliosides expressed on nerve fibers and lipooligosaccharides present on bacteria may account for their association with GBS.
The same pathophysiology has been proposed for Haemophilus influenzae, Cytomegalovirus, and Mycoplasma pneumoniae(Koga, 2018).
Considering these facts and based on prior knowledge of dealing with other viral epidemics we expected increasing number of reports on involvement of peripheral nervous system especially peripheral neuropathy in the era of COVID-19 pandemics.
The first case of Guillain-Barré syndrome associated with SARS-CoV-2 infection in the literature has probably been reported by Zhao et al. who reported the GBS as presenting syndrome in a 61 years old woman which later diagnosed as Covid-19(Zhao et al., 2020). Following this case other cases have been reported.
Remarkable fact in some reports including reports by Aberti(Alberti et al., 2020) and Sedaghat(Sedaghat and Karimi, 2020) and in our case is that these cases share a common comorbidity, the diabetes.
The interval of 10 days between the onset of viral illness respiratory symptoms and the first manifestations of Guillain–Barré syndrome in our case, is similar to cases reported by Galan(Galán et al., 2020) and Virani(Virani et al., 2020). This interval was 5–10 days in case series by Toscano that is quiet the same as occurs during or after other infections(Toscano et al., 2020). Considering the temporal association, we hypothesize that there is causal relationship between GBS and SARS-CoV-2 infection, but there is no evidence of direct invasion of nerves or nerve roots by virus, as the CSF was negative for SARS-CoV-2 in patient reported by Alberti(Alberti et al., 2020).
COVID-19 associated neuropathies are not confined to peripheral nerves as cranial nerve involvement as Miller Fisher syndrome have been reported(Gutiérrez-Ortiz et al., 2020).
The important clinical point about this patient is ignorance of patient's compliant in COVID-19 isolation ward. The treating physicians of patients affected with COVID-19 should be aware of neurological complications of infection, and pay attention to every single symptom and sing in patients; because the disease is a novel condition and not every feature of it identified.
We described this case as GBS but it should be kept in mind that our follow up period is short and acute-onset chronic inflammatory demyelinating polyneuropathy (A-CIDP) that accounts for 18% of CIDP cases(Bunschoten et al., 2019) should be in consideration as a differential diagnosis.
References
- Alberti P., Beretta S., Piatti M., Karantzoulis A., Piatti M.L., Santoro P., Viganò M., Giovannelli G., Pirro F., Montisano D.A., Appollonio I., Ferrarese C. Guillain-Barré syndrome related to COVID-19 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020 doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci. Ther. 2020;26:499–501. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunschoten C., Jacobs B.C., Van den Bergh P.Y.K., Cornblath D.R., van Doorn P.A. Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol. 2019 doi: 10.1016/S1474-4422(19)30144-9. [DOI] [PubMed] [Google Scholar]
- Galán A.V., del Saz Saucedo P., Postigo F.P., Paniagua E.B. Guillain-Barré syndrome associated with SARS-CoV-2 infection TT - Síndrome de Guillain-Barré asociado a infección por SARS-CoV-2. Neurol. 2020 doi: 10.1016/j.nrleng.2020.04.006. (English Ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Ortiz C., Méndez A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Mañas R., de Aragón-Gómez F., Benito-León J. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020 doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H., Ahn J.Y., Kim M.K., Choi J.P. Neurological complications during treatment of Middle East respiratory syndrome. J. Clin. Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga M. Experimental approach in research of Guillain–Barré syndrome: a range of pathogeneses mediated by molecular mimicry. Clin. Exp. Neuroimmunol. 2018;9:93–100. doi: 10.1111/cen3.12457. [DOI] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat Z., Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J. Clin. Neurosci. 2020 doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., Franciotta D., Baldanti F., Daturi R., Postorino P., Cavallini A., Micieli G. Guillain–Barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020 doi: 10.1056/nejmc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani A., Rabold E., Hanson T., Haag A., Elrufay R., Cheema T., Balaan M., Bhanot N. Guillain-Barré syndrome associated with SARS-CoV-2 infection. IDCases. 2020 doi: 10.1016/j.idcr.2020.e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]