Abstract
Intestinal dysfunction, which may cause a series of metabolic diseases, has become a worldwide health problem. In the past few years, studies have shown that consumption of poultry eggs has the potential to prevent a variety of metabolic diseases, and increasing attention has been directed to the bioactive proteins and their peptides in poultry eggs. This review mainly focused on the biological activities of an important egg-derived protein named ovomucin. Ovomucin and its derivatives have good anti-inflammatory, antioxidant, immunity-regulating and other biological functions. These activities may affect the physical, biological and immune barriers associated with intestinal health. This paper reviewed the structure and the structure-activity relationship of ovomucin,the potential role of ovomucin and its derivatives in modulation of intestinal health are also summarized. Finally, the potential applications of ovomucin and its peptides as functional food components to prevent and assist in the pretreatment of intestinal health problems are prospected.
Keywords: Ovomucin, Hydrolytic peptide, Bioactivity, Intestinal health, Sialic acid
Graphical abstract
1. Introduction
The problem of intestinal health has become one of the most important causes of human death. The deterioration of intestinal barrier function and intestinal permeability disorders may increase the absorption of intestinal lumen antigens, promote the translocation of harmful substances and pathogens into the bloodstream, and cause ulcerative colitis, Crohn's disease and other diseases. Besides, intestinal disorders are early events of metabolic diseases (including cardiovascular diseases and cancer) [1], almost all chronic metabolic diseases are closely related to intestinal and/or intestinal microbiota [2,3]. Therefore, the intestine is an important target for early treatment and prevention of gastrointestinal inflammation and metabolic diseases.
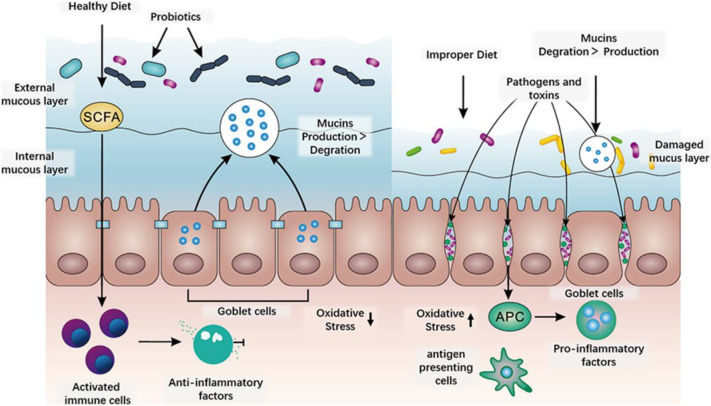
The integrity and function of intestinal structure are very important to intestinal health, and the imbalance dietary structure is a high risk factor for intestinal mucus damage. The intestinal mucus layer is a dynamic barrier that can be continuously replenished by goblet cell secretion, but if the bacteria degrade mucus faster than the cell secretion supplement, the integrity of this critical barrier will be compromised, giving opportunities for intestinal bacteria to contact the inner mucosa layer [4]. Antigen-presenting cells (APC) recognize intestinal bacteria as pathogens and stimulate the production of excess reactive oxygen species and free radicals. At the same time, they also produce a large number of pro-inflammatory cytokines and activate immune responses, leading to intestinal inflammation damage [5,6]. Food ingredients or environmental changes can induce imbalances in microbiota and mucus degrade, thereby promoting the infection of pathogens and harmful substances, and further inducing chronic inflammation and metabolic diseases [[7], [8], [9]]. For example, the short-chain fatty acids (SCFAs) can be released from the dietary residues using the digestive enzymes of the intestinal microbiota. By regulating immune and gene expression, the SCFAs maintain the integrity of the mucus barrier and inhibit inflammation. At the same time, protein residues and fat-stimulated bile acids may also be metabolized by microbiota into inflammation and/or carcinogenic metabolites [9]. The above processes can be summarized in Fig. 1 . Therefore, potential intervention strategies to combat intestinal damage should include maintaining the integrity of the intestinal mucus layer, reducing the abundance or viability of mucus-degrading bacteria (regulating the microbiota balance), inhibiting oxidative stress and inflammatory factors in the intestinal mucosa secretion, or a combination of the three above.
Fig. 1.
The effect of dietary structure on the intestinal mucosal barrier [4,5,9,10].
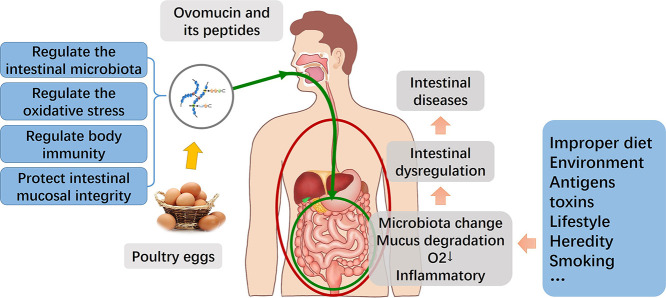
In the past few years, increasing interest has been directed to food-derived bioactive proteins and their peptides, for their excellent potential for maintaining immune homeostasis [11]. For example, milk casein, rice albumin and egg white protein have been proved to have immunomodulatory activity [12]. Biopeptides with anti-inflammatory and anti-oxidant activities can help prevent chronic diseases [13,14]. Egg-derived proteins, including egg transferrin, lysozyme, and phosphoprotein are showing excellent anti-inflammatory, antioxidant, immunomodulatory [15] and other biological activities. As an important structural protein in poultry eggs, ovomucin is an important linear high molecular sulfated glycoprotein, which belongs to the mucin family [16]. It is mainly found in the thick and thin proteins of egg whites, accounting for 2%–4% of the total protein in egg whites, as well as in the frenulum and the outer layer of the yolk membrane. The functions of ovomucin, such as anti-virus [[17], [18], [19], [20], [21], [22], [23]], anti-tumor [[24], [25], [26], [27]], anti-inflammation [28], anti-oxidation [29,30] and immune regulation [31], have been confirmed one after another in the past decades. Based on the existing studies, this paper summarizes the structure, biological activity of ovomucin and its peptides in the prevention and treatment of chronic metabolic diseases, and looks forward to its further research. It is expected to provide a new strategy for the protection of intestinal mucosal barrier. The potential application of ovomucin and its peptides as functional food ingredients for preventing and auxiliary pretreating intestinal health problems are also highlighted.
2. Composition and structure of ovomucin
Previous interest in this protein aimed to understand its role in egg white thinning, and it has been found that ovomucin is the main reason for maintaining the gel properties and foam stability of egg white [32]. Recently, a variety of biological activities of ovomucin have been reported. For example, ovomucin derivatives have superior antitumor activities through inhibiting the formation of tumor blood vessel [33]. It has potential to inhibit the growth and reproduction of some microorganisms, preventing cells from being adhered by influenza viruses [22]. However, as a novel functional food ingredient, ovomucin has not been fully studied and utilized, probably due to its complex molecular architecture and little direct evidence for the structure-activity relationship.
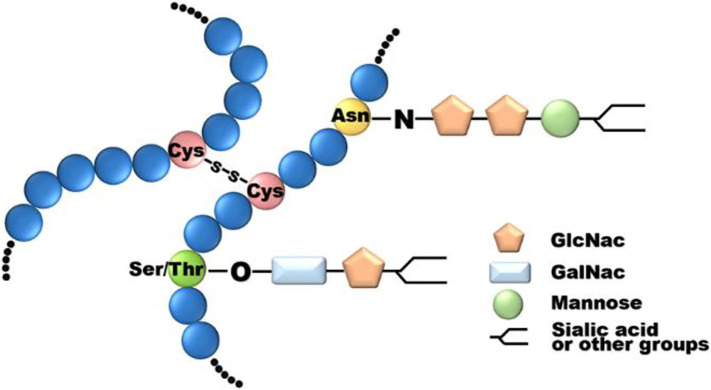
Ovomucin is a glycoprotein with high molecular weight. The linear molecule of ovomucin forms a randomly curled structure at the end, which is considered to have the same highly polymerized macromolecular structure as mammalian mucin. The structural model of ovomucin is depicted in Fig. 2 . Briefly, ovomucin is composed of a carbohydrate-rich β-subunit (Molecular weight is about 400 kDa) and a carbohydrate-poor α-subunit with approximately 60% and 15% carbohydrates, respectively [34]. Carbohydrates presenting in ovomucin mainly are mannose, galactose, N-acetyl-D-galactosamine, N-acetyl-D-glucosamine, N-acetylneuraminic acid and fructose, and sulfated saccharides [35]. These carbohydrate groups bind to long-chain molecule in the form of N-glycosylated and O-glycosylated at specific amino acid sites, and it has been confirmed that these carbohydrate groups act as an important part to resist microorganism invasion [36]. The electrophoretic study of α-ovomucin is further divided into α1-ovomucin and α2-ovomucin, relative molecular weight is about 150 kDa and 220 kDa, respectively. These three subunits are polymerized into ovomucin macromolecule by disulfide bond [34], and the corresponding three peaks could be eluted by gel filtration [37]. In particular, the ɑ-subunit mainly contains acidic amino acids such as glutamic acid and aspartic acid; while the β-subunit mainly contains hydroxyl amino acids such as threonine and serine [34].
Fig. 2.
Linear structure of ovomucin with multiple glycosylation sites [34,38,39].
Our previous findings had demonstrated that, ovomucin consists of 60% of carbohydrate content [40]. Kato et al. [41] had elucidated the composition of three carbohydrates side chains of ovomucin, one chain is composed of galactose, galactosamine, sialic acid and sulfate in an equal molar ratio, another is composed of galactose and galactosamine in equal molar ratio, and the last is composed of mannose and galactosamine in equal molar ratio. Sialic acid, also known as neuraminic acid, is a 9-carbon pyranose which was first extracted and isolated from salivary gland mucin. Sialic acid is widely found in various biological tissues and is an important component of glycoproteins, oligosaccharides and glycolipids, which usually located at the ends of glycoproteins and glycolipids [42]. Neu5Ac (N-acetylneuraminic acid) and Neu5Gc (N-Glycolylneuraminic acid) are two types of sialic acid and ovomucin only contains Neu5Ac. The sialic acid group on the glycoside glycoprotein terminal in ovomucin is thought to play an important role in its functional properties because its strong negative electrical charge may affect the diffusion of molecules and the interaction with other molecules [43]. Sialic acid promotes the structural diversity of complex carbohydrates and can directly participate in various recognition processes [44]. It is the most common ligand for pathogenic and non-pathogenic viruses, bacteria and protozoa. Sialic acid can participate in various recognition processes in the body, and has anti-infective activity against a variety of viruses or bacteria, such as influenza virus, cholera, coronavirus, Escherichia coli, and Helicobacter pylori [45,46]. In addition, sialic acid can also remove toxic hydrogen peroxide in vitro and inhibit H2O2–induced cell death [47]. Therefore, the existence of sialic acid groups may be one of the important reasons why ovomucin has shown many biological activities in various studies. As a kind of high molecular glycoprotein with multiple activities, ovomucin has profound research value in revealing the relationship between carbohydrate side chains and protein functional properties.
3. Bioactivities of ovomucin and its peptides
Serial studies have shown that ovomucin and its peptides possess good anti-inflammatory and anti-oxidant activities in vitro [[28], [29], [30]]. They can participate in and mediate the regulation of various cell functions, stimulate the proliferation of macrophages and lymphocytes [31], promote the synthesis of cytokines such as interleukin, tumor necrosis factor, and interferon, thereby affecting the immune system and inhibit tumorigenesis [25,26,33]. Furthermore, ovomucin can also reduce the serum cholesterol level [48]. These important bioactivities reported from ovomucin and its derived components using cell experiments and animal models are given in Table 1 .
Table 1.
Bioactivities of ovomucin and its derivatives.
| Bioctivity | Active substance | Model | Effect | References |
|---|---|---|---|---|
| Anti-inflammatory activity | Low-molecular-weight ovomucin hydrolysate treated with alkaline protease | TNF-α-induced human dermal fibroblasts | Inhibit the activation of NF-κB pathway | [28] |
| Antibacterial | Ovomucin glycopeptide | Binding to enterohemorrhagic Escherichia coli O157: H7 | [50] | |
| Ovomucin | Helicobacter pylori colonized mice | Production and masking urease to inhibit Helicobacter pylori infection | [51] | |
| Ovomucin-Protex 26 L hydrolysate | Porcine small intestinal epithelial cells | Preventing the adhesion of ETEC K88ac | [60] | |
| Terminal β-linked galactose of α-ovomucin | Porcine red blood cells | Preventing the adhesion of ETEC K8 resistant 8 ac | [59] | |
| Antitumor | β-ovomucin | Meth-A sarcoma cells | Heal proximal tumors and inhibit distal tumor growth | [24] |
| β-ovomucin | Xenograft sarcoma-180 cells in mice | Inhibits cell growth and heals tumors | [25,26] | |
| Highly glycosylated fragment of β-ovomucin | SR-180 tumor cells | Binding to cytokine growth factor receptor bFGFR inhibits tumors | [27] | |
| Immune activation | O-linked sulfated carbohydrate chain | Mouse peritoneal membrane culture | Macrophage activation | [31] |
| Antiviral | β-ovomucin | High affinity for bovine rotavirus, human influenza virus and Newcastle disease virus | [[17], [18], [19]] | |
| Ovomucin | Chicken red blood cells and Newcastle disease virus (NDV) of La Sota strain | Inhibition of NDV hemagglutination, and high affinity for NDV | [20,21] | |
| Sialic acid group in ovomucin | Anti-virus and bacterial infection | [22] | ||
| Ovomucin | Chicken red blood cells | Inhibition of influenza virus hemagglutination | [23] | |
| Antioxidant activity | LDEPDPL and NIQTDDFRT sequences isolated from α-ovomucin | In vitro | Free radical ABTS scavenging power | [29] |
| Ovomucin hydrolysate | In vitro | Free radical scavenging ability, ACE inhibitory activity, metal ion chelating ability | [30] | |
| Hypocholesterolemic action | Ovomucin | Mouse Caco-2 cells | Attenuates hypercholesterolemia in rats and inhibits cholesterol absorption in Caco-2 cells | [48] |
3.1. Bioactivities of ovomucin and its subunits
3.1.1. Anti-adhesion activity
In the 1940s, Gottschalk and Lind [23] have demonstrated the anti-hemagglutination activity of ovomucin against influenza virus, and found that the interaction between viral enzymes and the viral hemagglutinin inhibitory components of ovomucin results in carbohydrate peptide complex release. Through hemagglutination inhibition and ELISA test, Tsuge et al. [[17], [18], [19]] found that ovomucin has a high affinity for bovine rotavirus (RV), Newcastle disease virus (NDV) and human influenza virus (IV). N-acetylneuraminic acid (NeuNAc) residues in the β-subunit are the key to the binding of ovomucin to NDV, while the disulfide bond between and within the subunit promoted the binding of ovomucin to antibodies. The decrease of binding force to NDV in heated samples depends on the destruction of NeuAc residues in ovomucin [19].
Many studies have reported the anti-infective activity of sialic acid against bacteria such as Escherichia coli, Streptococcus, and Helicobacter pylori [45,49], and ovomucin also showed similar characteristics. Kobayashi et al. [50] proved that the sialic acid groups of pronase-treated ovomucin have the ability of binding to Escherichia coli O157: H7, and this activity is lost after the ovomucin glycopeptide is treated with sialidase. Ovomucin can also inhibit the adhesion of urease produced by Helicobacter pylori to gastric mucosa. Urease is located on the surface of Helicobacter pylori cells, and ovomucin inhibits the adhesion of Helicobacter pylori to gastric mucosa receptors by predominantly binding urease [51]. Sialic acid at the end of the carbohydrate chain can not only be used as sites in “molecule-cell” and “cell-cell” recognition, but also can mask recognition sites. Glycosides linked to sialic acid at the end of glycoconjugates can effectively prevent some important antigen sites and recognition markers on the cell surface, thus protecting cells from enzymatic hydrolysis and immune attacks. [45,52]. Therefore, the anti-adhesion activity of ovomucin and its derivatives may be caused by the sialic acid group, for they can occupy the recognition site of pathogenic microorganisms.
3.1.2. Antitumor activity
Ohami [53] observed the cytotoxic effects of β-ovomucin on SEKI (human melanoma) and 3LL (Lewis lung cancer cells) through scanning electron microscopy, and the dose and time effects of toxicological to sarcoma-180 (SR-180) cells were further studied [25,26]. The β-subunit-treated tumor cells have a reduced number of microvilli in the cell membrane, vesicle formation, irregular chromatin accumulation, irregular nuclear shapes, swollen cytoplasmic organs, accompanied by degradation and necrosis of cell deformation. Meantime, a large number of neutrophils, macrophages and lymphocytes were found in the marginal area of degenerate and necrotic tumor tissue. These findings indicate that β-subunits and its fragments may exert direct cytotoxicity on tumor cells by activating the immune system, and may also produce indirect cytotoxic effects through the host's immune system, leading to tumor regression [31].
Watanabe et al. [24] also found pronase treated ovomucin (220 and 120 kDa, highly glycosylated peptides) completely cured the proximal tumor, directly and indirectly inhibit the growth of tumor surrounding tissue. Asialization experiments showed that sialic acid residues in the 120 kDa fragment had an indirect effect on distant tumors. Oguro et al. [33] later demonstrated that massive neutrophils, macrophages and lymphocytes were found to accumulate, and angiogenesis (the formation of new capillaries) is inhibited in tumor tissues of mice injected with highly glycosylated fragment OVMa70F in the α-subunit of streptavidin-treated ovomucin. Yokota et al. [27] found that highly glycosylated fragment of β-ovomucin can bind with basic fibroblast growth factor receptor (bFGFR) to competitively block the interaction between basic fibroblast growth factor (bFGF) and bFGFR, which promote tumor angiogenesis, thereby indirectly inhibiting the growth of SR-180 tumor cells.
3.1.3. Immune activation
Sulfated glycopeptides in ovomucin show strong macrophage-like cells stimulating activity, and the active ingredient involved in macrophage activation is O-linked sulfated carbohydrate chain [31]. The in vitro culture assay with macrophages showed that the protease E hydrolysates of ovomucin induced cell morphologic changes and increased the generation of H2O2 and IL-1 at a low concentration (100 μg/mL). In the immune system, macrophages play an important role in phagocytosis, antigen presentation, tumor cell toxicity and antimicrobial activity. Activated macrophages secrete TNF, interleukin-1 (IL-1), and accelerate the immune response through activating T cells. Properly activated macrophages can enhance the immune system's ability to eliminate foreign pathogens and even cancer cells. In addition, ovomucin can also be used as an immune booster to promote the proliferation of mouse spleen lymphocytes stimulated by lipopolysaccharide [54].
3.1.4. Hypocholesterolemic action
Metabolic disorders of cholesterol at cellular and systemic levels have been shown to induce cytotoxicity and inflammation, disorders of cholesterol metabolism and inflammation have been involved in the pathophysiology of many chronic diseases, and disorders of metabolic and neurological tissues [55]. Ovomucin also showed a cholesterol-lowering effect compared to casein: ovomucin inhibited cholesterol uptake in Caco-2 cells which could significantly reduce serum cholesterol in rats. Most peptides with high bile acid binding ability can inhibit the reabsorption of bile acid in the ileum and reduce the level of blood cholesterol (ileal effect), which may also be the mechanism of ovomucin [46]. The decrease of solubility of cholesterol micelles may inhibit cholesterol absorption through the direct interaction between cholesterol mixed micelles and ovomucin in jejunal epithelium, which is also part of the mechanism of cholesterol lowering induced by ovomucin (jejunal effect). Therefore, the cholesterol-lowering effect of ovomucin may involve both jejunal and ileal effects. Many previous studies have revealed the relationship between the sialic acid group of ovomucin and its physiological activity, but whether the cholesterol-lowering effect induced by ovomucin in vivo is related to N-acetylneuraminic acid is currently being studied [48].
3.2. Bioactivities of ovomucin-derived peptides
3.2.1. Antibacterial activity
Existing reports have verified the in vitro bacteriostatic effect of the ovomucin derivatives on common spoilage bacteria such as Escherichia coli, Staphylococcus aureus, and Salmonella, and it has a certain degree of thermal stability and acid-base stability [56]. The enzymatic hydrolysates (2.0 kDa–70.0 kDa average molecular weight) obtained by the reaction of ovomucin and serine protease, papain, metalloprotease, trypsin, and pepsin, etc. Fu, et al. [57] analyzed the antibacterial mechanism of ovomucin by flow cytometry and scanning electron microscopy. The result showed a strong inactivation effect on food poisoning bacteria such as enterotoxigenic Escherichia coli, enteroinvasive Escherichia coli, pathogenic Vibrio bacterium, Bacillus dysentericus, and Pseudomonas aeruginosa [58]. Ovomucin can cause cell membrane damage or apoptosis in Staphylococcus aureus, Staphylococcus saprophyticus and Streptococcus mutans, revealing that the bacteriostatic mechanism of ovomucin derivatives is by affecting the permeability of the cell membrane, leading to the cell wall and membrane eventually ruptured.
3.2.2. Anti-adhesion activity
Sun et al. [59] found that ovomucin hydrolysates prepared by acid protease II, rather than intact ovomucin, can interfere with the adhesion of K88 (Ac) enterotoxigenic Escherichia coli to porcine red cells and have the potential to be used as an anti-adhesion agent for infectious diseases. The glycopeptides of α-ovomucin consists of the pentosaccharide core of GlcNAc2man3 and a bisecting GlcNAc, which may contain terminal β-linked galactose, are involved in this anti-agglutinating activity. In a further study, ovomucin-Protex 26L hydrolysates can act as the decoy receptors to prevent the binding of K88 (Ac) strain to porcine intestinal epithelial cell line (IPEC-J2) [60]. Four high affinity fractions were identified from α-ovomucin hydrolysate, which significantly increased (about 90%) adhesion of strain ECL 13998 to IPEC-J2 cells.
3.2.3. Antioxidant activity
Hydrolysates and mock digests of ovomucin have shown varying degrees of antioxidant activity. Chang et al. [29] evaluated the free radical scavenging activities of oval transferrin, ovalbumin, lysozyme and ovomucin, and found that under the same conditions, ovomucin had the strongest scavenging efficiency. The antioxidant activity of ovomucin hydrolyzed peptides was 6 times higher than that of untreated ovomucin. Two peptide sequences, LDEPDPL and NIQTDDFRT, which mainly exert antioxidant activity, were isolated from α-ovomucin. It is suggested that the antioxidant activity of ovomucin may depend on their amino acid composition. Abeyrathne et al. [30] found that the ovomucin hydrolyzed in alkaline condition showed the best metal ion binding activity and antioxidant activity, and hydrolysates treated by papain and alkaline protease showed good ACE inhibitory activity. The LC-MS result also proposed that the number and size of peptides are closely related to the biological activity of ovomucin.
3.2.4. Anti-inflammatory activity
Sun et al. [28] tested the anti-inflammatory activities of different ovomucin hydrolysates and their components in human skin fibroblasts and found that desalted alkaline protease hydrolysates could significantly reduce the expression of ICAM-1 (intercellular cell adhesion molecule-1) induced by TNF. The desalted alkaline protease hydrolysate is rich in low molecular weight peptides, while the free part only contains high molecular weight peptides. Only desalted components attenuated the activation of NF-κB mediated by TNF, which further proved that the specific anti-inflammatory potential of ovomucin peptides may be mediated by its low molecular weight peptides. The anti-inflammatory activity of these small molecular peptides is regulated by inhibiting the activity of B cells activated by tumor necrosis factor-mediated nuclear factor-κ light chain enhancer. This result further demonstrates the key role of desalting and reducing the molecular weight of peptides in enhancing potentially beneficial activities such as reducing tissue inflammation.
4. Potential role of ovomucin in intervention of intestinal health
Based on the above studies, ovomucin and its derivatives may act on intestinal physical, immune and biological barriers, inhibit the incidence of intestinal mucosal disorders and chronic inflammation by the following ways.
4.1. Ovomucin and its peptides maintain the structure of healthy intestinal microbiota
The intestinal microbiota, which consists of millions of microorganisms that reside in the gastrointestinal tract and consistently interact with the host, is the most important factor affecting intestinal inflammation [8]. Host factors such as diet and disease status affect the composition of the microbiota, meantime the microbiota itself produces metabolites that can further manipulate host physiology. The reduction of beneficial bacteria and their metabolites, as well as the increasement of harmful bacteria and their toxic metabolites have jointly changed the micro-ecology in the host. Intestinal microbiota is an important characteristic of patients with metabolic diseases, some of which are involved in disrupting the host's intestinal epithelial barrier and altering the immune system [61]. The pathogenic effect of dysfunctional intestinal microbiota is far greater than the contribution of genetic defects to pathogenesis, and the contribution of dietary structure to the change of intestinal microbiota is much higher than the effect of genotype [62].
Most researches on preventing or treating intestinal diseases focus on regulating the intestinal microbiota. The homeostasis of intestinal biological barrier is realized by inhibiting the colonization of pathogenic bacteria and promoting the metabolism of beneficial bacteria. A promising strategy for the prevention of pathogenic infectious is to use anti-adhesive agents to interfere with bacterial adhesion and colonization on host tissues in the early stages of infection, or to isolate bacteria from tissues [59]. Adhesion of many infectious bacteria is mediated by surface lectins in the form of submicroscopic multisubunit organelles designated as pili or fimbriae, with distinct carbohydrate specificities. The glycosyl groups on the target cells will be recognized by lectins, further lead to adhesion and infection. Anti-adhesion agents do not directly kill bacteria, so they can effectively reduce the occurrence of drug-resistant strains [63]. Ovomucin or its hydrolysate in vitro has a significant inhibitory effect on the growth of environment-dependent pathogens such as Escherichia coli [50,56]. And ovomucin has good adhesion activity to Escherichia coli, Salmonella, and Staphylococcus aureus [21] as we mentioned in the previous section, which can suppress the colonization of these bacteria on intestinal epithelial cells [59]. Adhered microbial antigens such as Escherichia coli are considered to be the activating factor for intestinal mucosal damage [5]. By activating the TLR4/NF-κB pathway, the secretion of inflammatory cytokines is triggered and exacerbates the inflammatory injury response [64,65]. Sun et al. [66] also proved the potential of ovomucin hydrolyzed peptides as prebiotics, suggesting that they may be degraded in the infant gastrointestinal tract by different Bifidobacterium or other members of the intestinal microbiota and form SCFAs. SCFAs can regulate immunity, resist pathogenic microorganisms and anti-inflammatory, regulate intestinal microbiota balance, and protect intestinal mucosal function [67]. SCFAs can reduce the expression of leukocyte adhesion molecules and IL-6 and play an anti-inflammatory effect [68]. Therefore, ovomucin or its metabolites are likely to prevent the intestinal mucus barrier damage by interfering with the structure of the intestinal microbiota, affecting the colonization of intestinal Escherichia coli, then affecting the NF-κB signaling pathway and secretion of inflammatory factors.
4.2. Ovomucin and its peptides regulate the interaction between reactive oxygen species and intestinal barrier
The medical significance of oxidative stress has become increasingly recognized. It is now considered to be a component of virtually every disease process. Intestinal metabolic diseases are closely related to changes in oxidative stress and redox status caused by excess reactive oxygen species (ROS) [69]. Due to the over activation of phagocytes in the mucosa of intestinal diseases, a large amount of oxidative substances are generated, resulting in continuous exposure of the intestinal epithelium to endogenous or exogenous ROS. Excessive ROS can lead to oxidative stress, inflammatory response, lipid and protein modification, DNA damage, apoptosis or cancer cell transformation [70], and simultaneously activates inflammatory cells and induces proinflammatory cytokines (IL-1β, IL-6, IL-8) secretion through the NF-κB signaling pathway to induce inflammatory damage. The activated inflammatory cells in the intestine will in turn produce more ROS and aggravate the inflammatory damage of the intestinal mucosal barrier. The cellular antioxidant system against ROS-induced oxidative stress has three antioxidant mechanisms [71]. Except for eliminating or reducing the production of ROS through endogenous antioxidant enzymes and endogenous non-enzymatic antioxidants, the third aspect of antioxidation is exogenous antioxidants, including vitamin E, ascorbic acid and bioactive peptides. The strategy of using ovomucin to scavenge free radicals and reduce ROS obviously belongs to the third kind [72].The antioxidative effects of ovomucin and the anti-intestinal inflammation of egg white peptides have been confirmed in vitro [29,30,73], it can be speculated that ovomucin may have the potential to strengthen the intestinal antioxidant defense system when chronic intestinal mucosal injury occurs. Past research has confirmed that the cleavage of peptide bonds can lead to the opening and exposure of some active amino acids, increasing hydrogen donors and protons, and enhancing antioxidant activity. Ovomucin-derived peptides have different acid-base amino acid composition and hydrophobicity from the peptide molecules in other egg protein active substances, which may be the main reason for their stronger antioxidant activity [74]. However, whether ovomucin can still retain its antioxidant and anti-inflammatory activity in the intestine after dietary intake and maintain the intestinal mucosal homeostasis has not been reported yet, which is worthy of further study.
4.3. Ovomucin and its peptides regulate the interaction between intestinal immunity and intestinal health
The intestine is one of the tissues in which the animal's body is in close contact with the external environment, and is the body's largest immune organ. Intestinal epithelial cells and intestinal dendritic cells sense different sources of infection and produce different effector response factors. By acting on immune pathways, controlling cytokine expression is an important way for active substances to suppress inflammatory responses. Clinical trials have shown that disorders of the intestinal epithelial barrier, dysfunction of the innate immune system and T cell-mediated adaptive immune system are closely related to suppressing inflammation and the immune cascade of tumors. Ovomucin hydrolysis can produce a large number of active low-molecular peptides. These active fragments may inhibit the activation of NF-κB pathway mediated by various inflammatory factors to achieve immune protection effects. Ovomucin sulfated glycopeptides can activate artificially cultured macrophages and increase the production of interleukin-1 (IL-1). Small amount of IL-1 expression induces an appropriate inflammatory response that activates specific immunity. In contrast, excess IL-1 causes a widespread inflammatory response. Besides, Ovomucin contains a large number of sialic acid residues that have been shown to have immunomodulatory activity. In mouse models of respiratory and intestinal tract inflammation, sialic acid significantly reduces the level of IL-17 secreted by Th cells and supports regulatory T cells differentiation [75]. Th17 cells can secrete IL-17A, IL-17F, IL-6 and TNF-a. These cytokines can collectively mobilize, recruit and activate neutrophils, thus effectively mediating tissue inflammation [76]. Therefore, we believe that the presence of sialic acids make ovomucin hydrolysates take part in signal-recognizing process in the immune system, regulating the differentiation of T helper cells and the expression of NF-κ B-related immune factors in the inflammatory pathway. Moreover, ovomucin may also be used as an immune enhancer, acting on spleen lymphocytes and macrophages, properly activating the immune system, maintaining the normal secretion of inflammatory factors and adjusting the level of H2O2 in the body, so that it can protect the body from intestinal damage and cause inflammation.
4.4. Ovomucin and its peptides protects intestinal mucosal integrity
Intestinal injury always accompanied by the disturbance of intestinal physical barrier function, such as the increase of permeability [77], the decrease of goblet cells, the decrease of mucus layer thickness and the changes of mucin, phosphatidylcholine and other components. Goblet cells are secretory cells in the intestinal epithelium. The gel secreted by goblet cells forms mucin MUC2, to prevent large granular matter and bacteria from invading the epithelial cell layer [78]. Mucin is critical to the integrity of the intestinal mucosa [4]. Studies have shown that MUC2-knockout mice will spontaneously form colitis [79]. Egg white ovomucin is an important member of the mucin family. It has a high degree of genetic homology with MUC2 in human intestinal mucus, it is interesting that β-ovomucin was reported to be a homologous protein of human MUC6; while α-ovomucin is highly homologous to MUC2 protein, lacks only a PTS domain [16]. We have reason to believe that ovomucin and intestinal mucin have theoretical similarities in function. Therefore, we are very interested in whether ovomucin can enter the intestinal tissue in the form of exogenous protein in the case of intestinal mucus damage, so that can replace the damaged intestinal mucin to play the corresponding function. Some experiments have confirmed that ovomucin can resist the effects of digestive enzymes such as pepsin and trypsin to a certain extent [30,80]. This resistance may be related to its highly glycosylated molecular structure. Therefore, it can be speculated that ovomucin has the potential to enter the gastrointestinal tract after dietary intake. When various stimuli cause intestinal inflammation, one of the most common phenomena is that the degradation of mucus increases and the thickness of the mucus layer decreases [81]. Ovomucin is highly homologous with biological mucin MUC2, and has great similarity in glycosylation structure (O-link glycosylation, N-link glycosylation and terminal sialic acid group). The latest study of Takada et al. [82] have found that ovomucin oligosaccharides are also a high-quality source of carbohydrates for some bacteria, including certain intestinal microbes. These intestinal microorganisms that degrade mucin in humans include Akkermansia muciniphila [83,84] which is associated with the reduction of obesity and type 2 diabetes, and probiotic Bifidobacterium bifidum. The sugars, produced by the degradation of mucins by extracellular glycosidase in these species can not only be assimilated by themselves, but also be shared within the bacterial community and consumed by specific microorganisms, which can potentially affect the structure of intestinal microbiota. When the abundance of mucus-degrading bacteria, such as Akkermansia muciniphila and Bacteroides caccae abundance is too high [85], it will result in a sharp decrease in the thickness of the intestinal mucus layer, and the thinned mucus layer enables exogenous antigens to pass through the mucus layer and reach the intestinal epithelial cells without hindrance. Therefore, when ovomucin and intestinal mucin coexist in intestinal tissue, microorganisms using mucin as carbohydrate source may decompose ovomucin at the same time, thus reducing the amount of decomposition of intestinal mucin, helping to maintain the integrity of the mucus layer of the intestine. However, whether ovomucin hydrolyzed peptide can retain its structure and function when it reaches the intestinal tract after dietary intake, and what its metabolites under the action of digestive enzymes of the body in cooperation with the intestinal microbiota need to be further studied.
5. Future perspective and challenges
Compared with other proteins which are widely studied in egg white, the research on the biological activities of ovomucin and its derivatives are still not comprehensive. Ovomucin treated by different enzymes have been reported to have free radical scavenging, antiviral and bacterial activities, but most of the studies are still limited in vitro. In the more complex microbiota structure in vivo, how ovomucin will adjust the composition of microbiota and affect the metabolism of microorganisms remain unclear. Further intervention of inflammation-related metabolic pathway NF-κB, intestinal oxidative stress pathway Nrf2, intestinal epithelial cell apoptosis pathway JNK and other key pathways to regulate the occurrence of acute and chronic intestinal diseases will be the focus of our attention. Although sialic acid has been confirmed to be the main active group in ovomucin, there are still many activities that have not been confirmed to be related to sialic acid groups. Besides, on the existing basis, it is of great significance to isolate or identify more specific anti-tumor, antioxidant or immune-activating peptides by desalination, enzymolysis and purification to reveal the structure-activity relationship of these protein peptide groups. Because of the special similarity between ovomucin and human mucin, the possible substitution or synergistic effect between ovomucin and endogenous mucin in the process of maintaining intestinal mucosal barrier is a new direction in research of ovomucin properties. To explore the bioactivities of ovomucin and its peptides in different stages of digestion will be the basis of this study. By using the methods of modern nutrition, immunology and molecular biology, the effects of ovomucin on microecology, immune regulation and gene expression mechanism will open a new window for the further utilization of this important glycoprotein.
Declaration of competing interest
The authors have declared that there is no conflict of interest.
Acknowledgments
The authors gratefully acknowledge financial support provided by China Postdoctoral Science Foundation (Grant No. 2019M663834), the National Key Research and Development Program of China (2018YFD0400303) and the National Natural Science Foundation of China (Grant No. 31501506).
References
- 1.Tu H.-K., Wen C.-P., Tsai S.-P., Chow W.-H., Wen C., Ye Y.-Q., Zhao H., Tsai M.-K., Huang M.-S., Dinney C.P., Tsao C.-K., Wu X.-F. Cancer risk associated with chronic diseases and disease markers: prospective cohort study. BMJ Clinical Research. 2018;360 doi: 10.1136/bmj.k134. k134.10.1136/bmj.k134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchesi J.R., Adams D.H., Fava F., Hermes G.D.A., Hirschfield G.M., Hold G., Quraishi M.N., Kinross J., Smidt H., Tuohy K.M., Thomas L.V., Zoetendal E.G., Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nowak A., Paliwoda A., Błasiak J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2019;59(21):3456–3467. doi: 10.1080/10408398.2018.1494539. [DOI] [PubMed] [Google Scholar]
- 4.McGuckin M.A., Lindén S.K., Sutton P., Florin T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011;9(4):265. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 5.Mirsepasi-Lauridsen H.C., Vallance B.A., Krogfelt K.A., Petersen A.M. Escherichia coli pathobionts associated with inflammatory bowel disease. Clin. Microbiol. Rev. 2019;32(2) doi: 10.1128/CMR.00060-18. e00060-18.10.1128/CMR.00060-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alzoghaibi M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J Gastroenterol: WJG. 2013;19(39):6540. doi: 10.3748/wjg.v19.i39.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai M.S., Seekatz A.M., Koropatkin N.M., Kamada N., Hickey C.A., Wolter M., Pudlo N.A., Kitamoto S., Terrapon N., Muller A. A dietary fiber-deprived gut microbiota degrades the intestinal mucus barrier and enhances pathogen susceptibility. Cell Host Microbe. 2016;167(5):1339–1353. doi: 10.1016/j.cell.2016.10.043. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine A., Boneh R.S., Wine E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut. 2018;67(9):1726–1738. doi: 10.1136/gutjnl-2017-315866. [DOI] [PubMed] [Google Scholar]
- 9.Makki K., Deehan E.C., Walter J., Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff S.C., Barbara G., Buurman W., Ockhuizen T., Schulzke J.-D., Serino M., Tilg H., Watson A., Wells J.M. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14(1):189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cicero A.F., Fogacci F., Colletti A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: a narrative review. Br. J. Pharmacol. 2017;174(11):1378–1394. doi: 10.1111/bph.13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalamaiah M., Yu W., Wu J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018;245:205–222. doi: 10.1016/j.foodchem.2017.10.087. [DOI] [PubMed] [Google Scholar]
- 13.Leo E.E.M., Fernández J.J.A., Campos M.R.S. Biopeptides with antioxidant and anti-inflammatory potential in the prevention and treatment of diabesity disease. Biomed. Pharmacother. 2016;83:816–826. doi: 10.1016/j.biopha.2016.07.051. [DOI] [PubMed] [Google Scholar]
- 14.Cicero A.F., Fogacci F., Colletti A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: a narrative review. Br. J. Pharmacol. 2016 doi: 10.1111/bph.13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel Lozano-Ojalvo E.M., López-Fandiño R. Hydrolysates of egg white proteins modulate T- and B-cell responses in mitogen-stimulated murine cells. Food Funct. 2016;7(2) doi: 10.1039/c5fo00614g. 10.1039.C5FO00614G.10.1039/C5FO00614G. [DOI] [PubMed] [Google Scholar]
- 16.Lang T.-G., Hansson G.C., Samuelsson T. An inventory of mucin genes in the chicken genome shows that the mucin domain of Muc13 is encoded by multiple exons and that ovomucin is part of a locus of related gel-forming mucins. BMC Genomics. 2006;7(1):197. doi: 10.1186/1471-2164-7-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuge Y., Shimoyamada M., Watanabe K. Binding of egg white proteins to viruses. Biosci. Biotechnol. Biochem. 1996;60(9):1503–1504. doi: 10.1271/bbb.60.1503. [DOI] [PubMed] [Google Scholar]
- 18.Tsuge Y., Shimoyamada M., Watanabe K. Differences in hemagglutination inhibition activity against bovine rotavirus and hen Newcastle disease virus based on the subunits in hen egg white ovomucin. Biosci. Biotechnol. Biochem. 1996;60(9):1505–1506. doi: 10.1271/bbb.60.1505. [DOI] [PubMed] [Google Scholar]
- 19.Tsuge Y., Shimoyamada M., Watanabe K. Bindings of ovomucin to Newcastle disease virus and anti-ovomucin antibodies and its heat stability based on binding abilities. J. Agric. Food Chem. 1997;45(12):4629–4634. doi: 10.1021/jf970445f. [DOI] [Google Scholar]
- 20.Shan Y.-Y., Huang X., Ma M.-H., Miao F.-L. Role of magnesium chloride on the purity and activity of ovomucin during the isolation process. Int. J. Biol. Macromol. 2012;50(2):421–427. doi: 10.1016/j.ijbiomac.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Shan Y.-Y., Xu Q., Ma M.-H. Mg2+ binding affects the structure and activity of ovomucin. Int. J. Biol. Macromol. 2014;70:230–235. doi: 10.1016/j.ijbiomac.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 22.Xu Q., Shan Y.-Y., Wang N., Liu Y.-P., Zhang M.-J., Ma M.-H. Sialic acid involves in the interaction between ovomucin and hemagglutinin and influences the antiviral activity of ovomucin. Int. J. Biol. Macromol. 2018;119:533–539. doi: 10.1016/j.ijbiomac.2018.07.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A. Gottschalk, P.E. Lind, Product of interaction between influenza virus enzyme and ovomucin, Nature 164(4162) (1949) 232. 10.1111/j.1365-2389.2006.00834.x. [DOI] [PubMed]
- 24.Watanabe K., Tsuge Y., Shimoyamada M., Ogama N., Ebina T. Antitumor effects of pronase-treated fragments, glycopeptides, from ovomucin in hen egg white in a double grafted tumor system. J. Agric. Food Chem. 1998;46(8):3033–3038. doi: 10.1021/jf9800615. [DOI] [Google Scholar]
- 25.Yokota T., Ohishi H., Watanabe K. Antitumor effects of β-subunit from egg white ovomucin on xenografted sarcoma-180 cells in mice. Food Sci. Technol. Res. 1999;5(3):279–283. doi: 10.3136/fstr.5.279. [DOI] [Google Scholar]
- 26.Yokota T., Ohishi H., Watanabe K. In vitro studies of cytotoxic effect on sarcoma-180 cells of β-subunit from egg white ovomucin. Food Sci. Technol. Res. 1999;5(3):273–278. doi: 10.3136/fstr.5.273. [DOI] [Google Scholar]
- 27.Yokota T., Tani H., Ohishi H., Oguro T., Watanabe K. Interaction between 120 kDa fragment in β-subunit from egg white ovomucin and sarcoma-180 cells through basic fibroblast growth factor receptor. Food Sci. Technol. Res. 2000;6(4):275–279. 10.3136/fstr.6.275. [Google Scholar]
- 28.Sun X.-H., Chakrabarti S., Fang J., Yin Y.-L., Wu J.-P. Low-molecular-weight fractions of Alcalase hydrolyzed egg ovomucin extract exert anti-inflammatory activity in human dermal fibroblasts through the inhibition of tumor necrosis factor-mediated nuclear factor κB pathway. Nutr. Res. 2016;36(7):648–657. doi: 10.1016/j.nutres.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Chang O.-K., Ha G.-E., Han G.-S., Seol K.-H., Kim H.-W., Jeong S.-G., Oh M.-H., Park B.-Y., Ham J.-S. Novel antioxidant peptide derived from the ultrafiltrate of ovomucin hydrolysate. J. Agric. Food Chem. 2013;61(30):7294–7300. doi: 10.1021/jf4013778. [DOI] [PubMed] [Google Scholar]
- 30.Abeyrathne E., Lee H., Jo C., Suh J., Ahn D. Enzymatic hydrolysis of ovomucin and the functional and structural characteristics of peptides in the hydrolysates. Food Chem. 2016;192:107–113. doi: 10.1016/j.foodchem.2015.06.055. [DOI] [PubMed] [Google Scholar]
- 31.Tanizaki H., Tanaka H., Iwata H., Kato A. Activation of macrophages by sulfated glycopeptides in ovomucin, yolk membrane, and chalazae in chicken eggs. Journal of the Agricultural Chemical Society of Japan. 1997;61(11):1883–1889. doi: 10.1271/bbb.61.1883. [DOI] [PubMed] [Google Scholar]
- 32.Shan Y.-Y., Tang D.-Y., Wang R., Tu A.-B., Yi Y.-L., Wang X., Liu B.-F., Zhou Y., Huang Q., Lü X. Rheological and structural properties of ovomucin from chicken eggs with different interior quality. Food Hydrocoll. 2020;100:105393. doi: 10.1016/j.foodhyd.2019.105393. [DOI] [Google Scholar]
- 33.Oguro T., Watanabe K., Tani H., Ohishi H., Ebina T. Morphological observations on antitumor activities of 70 kDa fragment in α-subunit from pronase-treated ovomucin in a double grafted tumor system. Food Science and Technology. 2000;6(3):179–185. doi: 10.3136/fstr.6.179. [DOI] [Google Scholar]
- 34.Itoh T., Miyazaki J., Sugawara H., Adachi S. Studies on the characterization of ovomucin and chalaza of the hen’s egg. J. Food Sci. 1987;52(6):1518–1521. doi: 10.1111/j.1365-2621.1987.tb05868.x. [DOI] [Google Scholar]
- 35.Omana D.A., Wang J., Wu J. Ovomucin - a glycoprotein with promising potential. Trends Food Sci. Technol. 2010;21(9):455–463. doi: 10.1016/j.tifs.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geng F., Wang J.-Q., Liu D.-Y., Jin Y.-G., Ma M.-H. Identification of N-glycosites in chicken egg white proteins using an omics strategy. J. Agric. Food Chem. 2017;65(26):5357–5364. doi: 10.1021/acs.jafc.7b01706. [DOI] [PubMed] [Google Scholar]
- 37.Hiidenhovi J., Mäkinen J., Huopalahti R., Ryhänen E.-L. Comparison of different egg albumen fractions as sources of ovomucin. J. Agric. Food Chem. 2002;50(10):2840–2845. doi: 10.1021/jf011333y. [DOI] [PubMed] [Google Scholar]
- 38.Strecker G., Wieruszeski J., Cuvillier O., Michalski J., Montreuil J. 1H and 13C-NMR assignments for sialylated oligosaccharide-alditols related to mucins. Study of thirteen components from hen ovomucin and swallow nest mucin. Biochimie. 1992;74(1):39–51. doi: 10.1016/0300-9084(92)90182-E. [DOI] [PubMed] [Google Scholar]
- 39.Strecker G., Wieruszeski J.-M., Martel C., Montreuil J. Complete 1H-and 13C-NMR assignments for two sulphated oligosaccharide alditols of hen ovomucin. Carbohydr. Res. 1989;185(1):1–13. doi: 10.1016/0008-6215(89)84016-9. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z.-H., Tu A.-B., Tang D.-Y., Shan Y.-Y. Effectively preparing soluble ovomucin with high antiviral activity from egg white. Int. J. Biol. Macromol. 2018;118:504–510. doi: 10.1016/j.ijbiomac.2018.06.101. [DOI] [PubMed] [Google Scholar]
- 41.Kato A., Fujinaga K., Yagishita K. Nature of the carbohydrate side chains and their linkage to the protein in chicken egg white ovomucin. Agric. Biol. Chem. 1973;37(11):2479–2485. doi: 10.1271/bbb1961.37.2479. [DOI] [Google Scholar]
- 42.Ye L., Mu L., Li G., Bao Y. Assessing sialic acid content in food by hydrophilic chromatography-high performance liquid chromatography. J. Food Compost. Anal. 2020;87(C) doi: 10.1016/j.jfca.2019.103393. [DOI] [Google Scholar]
- 43.Kato A., Miyachi N., Matsudomi N., Kobayashi K. The role of sialic acid in the functional properties of ovomucin. Agric. Biol. Chem. 1987;51(3):641–645. doi: 10.1271/bbb1961.51.641. [DOI] [Google Scholar]
- 44.Zhou X.-M., Yang G.-L., Guan F. Biological functions and analytical strategies of sialic acids in tumor. Cells. 2020;9(2) doi: 10.3390/cells9020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelm S., Schauer R. Sialic acids in molecular and cellular interactions. Int. Rev. Cytol. 1997:137–240. doi: 10.1016/S0074-7696(08)62127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schauer R. Sialic acids: fascinating sugars in higher animals and man. Zoology. 2004;107(1):49–64. doi: 10.1016/j.zool.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Iijima R., Takahashi H., Namme R., Ikegami S., Yamazaki M. Novel biological function of sialic acid (N-acetylneuraminic acid) as a hydrogen peroxide scavenger. FEBS Lett. 2004;561(1):163–166. doi: 10.1016/S0014-5793(04)00164-4. [DOI] [PubMed] [Google Scholar]
- 48.Nagaoka S., Masaoka M., Zhang Q., Hasegawa M., Watanabe K. Egg ovomucin attenuates hypercholesterolemia in rats and inhibits cholesterol absorption in Caco-2 cells. Lipids. 2002;37(3):267–272. doi: 10.1007/s11745-002-0890-6. [DOI] [PubMed] [Google Scholar]
- 49.Schauer R. Victor Ginsburg's influence on my research of the role of sialic acids in biological recognition. Arch. Biochem. Biophys. 2004;426(2):132–141. doi: 10.1016/j.abb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi K., Hattori M., Hara-Kudo Y., Okubo T., Yamamoto S., Takita T., Sugita-Konishi Y. Glycopeptide derived from hen egg ovomucin has the ability to bind enterohemorrhagic Escherichia coli O157: H7. J. Agric. Food Chem. 2004;52(18):5740–5746. doi: 10.1021/jf0353335. [DOI] [PubMed] [Google Scholar]
- 51.Kodama Y., Kimura N. 2001. Inhibitor of Helicobacter Pylori Colonization, US Patent 6,235,709. [Google Scholar]
- 52.Spichtig V., Michaud J., Austin S. Determination of sialic acids in milks and milk-based products. Anal. Biochem. 2010;405(1):28–40. doi: 10.1016/j.ab.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Ohami H. Cytotoxic effect of sialoglycoprotein derived from avian egg white ovomucin on the cultured tumor cell. Med. Biol. 1993;126:19–23. [Google Scholar]
- 54.Otani H., Maenishi K. Effects of hen egg proteins on proliferative responses of mouse spleen lymphocytes. LWT-Food Science Technology. 1994;27(1):42–47. doi: 10.1006/fstl.1994.1010. [DOI] [Google Scholar]
- 55.Andersen C., Doerr A., Murphy K., McNeely T. Handbook of Cholesterol. Wageningen Academic Publishers; 2016. The relationship between cholesterol metabolism and inflammation in chronic disease; pp. 34019–34024. [Google Scholar]
- 56.Shan Y.-Y., Ma M.-H., Yuan Z.-W., Zhu H.-Y., Huang X., Zuo S.-M. Antibacterial activity of ovomucin and its stability to pH and temeperature. Food Sci. 2013;34:1–4. 10.7506/spkx1002-6630-201305001. [Google Scholar]
- 57.Fu D., Jin Y.-G., Han M.-Q., Huang X., Ma M.-H. Studies on antibacterial activity and antibacterial mechanism of ovomucin. Journal of Chinese Institute of Food Science and Technology. 2016;16(10):28–35. doi: 10.16429/j.1009-7848.2016.10.004. [DOI] [Google Scholar]
- 58.Ryoko K., Makoto H., Yukiko K., Kazuo K., Noriyuki I., Tsutomu O., Juneja R. 2004. Inactivation Composition of Food Poisoning Bacteria, Patent JP2004269420. [Google Scholar]
- 59.Sun X.-H., Gaenzle M., Wu J.-P. Identification and characterization of glycopeptides from egg protein ovomucin with anti-agglutinating activity against porcine K88 enterotoxigenic Escherichia coli strains. J. Agric. Food Chem. 2017;65(4):777–783. doi: 10.1021/acs.jafc.6b04299. [DOI] [PubMed] [Google Scholar]
- 60.Sun X.-H., Ganzle M., Wu J.-P. Glycopeptides from egg white ovomucin inhibit K88(ac) enterotoxigenic Escherichia coli adhesion to porcine small intestinal epithelial cell-line. J. Funct. Foods. 2019;54:320–328. doi: 10.1016/j.jff.2019.01.033. [DOI] [Google Scholar]
- 61.Sittipo P., Lobionda S., Lee Y.K., Maynard C.L. Intestinal microbiota and the immune system in metabolic diseases. J. Microbiol. 2018;56(3):154–162. doi: 10.1007/s12275-018-7548-y. [DOI] [PubMed] [Google Scholar]
- 62.Zhang C.-H., Zhang M.-H., Wang S.-Y., Han R.-J., Cao Y.-F., Hua W.-Y., Mao Y.-Y., Zhang X.-J., Pang X.-Y., Wei C.-C. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. The ISME journal. 2010;4(2):232. doi: 10.1038/ismej.2009.144. [DOI] [PubMed] [Google Scholar]
- 63.Ofek I., Sharon N. A bright future for anti-adhesion therapy of infectious diseases. Infection Immunity. 2002;59(10):1666. doi: 10.1007/PL00012494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu J.-B., Dong B.-Y., Chen A.-L., He F., Peng B.-F., Wu Z.-X., Cao J., Li W.-L. Escherichia coli promotes DSS-induced murine colitis recovery through activation of the TLR4/NF-κB signaling pathway. Mol. Med. Report. 2019;19(3):2021–2028. doi: 10.3892/mmr.2019.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nell S., Suerbaum S., Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat. Rev. Microbiol. 2010;8(8):564. doi: 10.1038/nrmicro2403. [DOI] [PubMed] [Google Scholar]
- 66.Sun X.-H., Gaenzle M., Field C.J., Wu J.-P. Effect of proteolysis on the sialic acid content and bifidogenic activity of ovomucin hydrolysates. Food Chem. 2016;212:78–86. doi: 10.1016/j.foodchem.2016.05.153. [DOI] [PubMed] [Google Scholar]
- 67.Jukes C., Ricci L., von Coburg E., Nasr M., Walker A.W., Duncan S.H., Hegazy A.N. P021 the influence of short-chain fatty acids produced by commensal bacteria on macrophage phenotype. J. Crohn’s Colitis. 2020;14(Supplement_1):S141–S142. doi: 10.1093/ecco-jcc/jjz203.150. [DOI] [Google Scholar]
- 68.Tedelind S., Westberg F., Kjerrulf M., Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol: WJG. 2007;13(20):2826. doi: 10.1186/1471-230X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian T., Wang Z., Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxidative medicine cellular longevity. 2017;2017 doi: 10.1155/2017/4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Postić S. 2012. Free Radicals and Antioxidants in Normal Physiological Functions and Human Diseases. [DOI] [PubMed] [Google Scholar]
- 71.Sorg O. Oxidative stress: a theoretical model or a biological reality? C. R. Biol. 2004;327(7):0–662. doi: 10.1016/j.crvi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Birben Esra, Sahiner Umit Murat, Sackesen Cansin. Oxidative stress and antioxidant defense. World Allergy Organization Journal. 2012 doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee M., Kovacs-Nolan J., Archbold T., Fan M.Z., Juneja L.R., Okubo T., Mine Y. Therapeutic potential of hen egg white peptides for the treatment of intestinal inflammation. J. Funct. Foods. 2009;1(2):161–169. doi: 10.1016/j.jff.2009.01.005. [DOI] [Google Scholar]
- 74.Sarmadi B.H., Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31(10):1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 75.Frei R., Ferstl R., Roduit C., Ziegler M., Schiavi E., Barcik W., Rodriguez-Perez N., Wirz O.F., Wawrzyniak M., Pugin B., Nehrbass D., Jutel M., Smolinska S., Konieczna P., Bieli C., Loeliger S., Waser M., Pershagen G., Riedler J., Depner M., Schaub B., Genuneit J., Renz H., Pekkanen J., Karvonen A.M., Dalphin J.-C., van Hage M., Doekes G., Akdis M., Braun-Fahrlander C., Akdis C.A., von Mutius E., O'Mahony L., Lauener R.P., S. Prevention Allergy Risk Factors, R. Protection Against Allergy Study Exposure to nonmicrobial N-glycolylneuraminic acid protects farmers' children against airway inflammation and colitis. J. Allergy Clin. Immunol. 2018;141(1):382. doi: 10.1016/j.jaci.2017.04.051. [DOI] [PubMed] [Google Scholar]
- 76.Simone V. De, Franzè E., Ronchetti G., Colantoni A., Fantini M.C., Fusco D.D., Sica G.S., Sileri P., Macdonald T.T., Pallone F. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene Res. 2015;34(27):3493–3503. doi: 10.1038/onc.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vivinus-Nébot M., Frin-Mathy G., Bzioueche H., Dainese R., Bernard G., Anty R., Filippi J., Saint-Paul M.C., Tulic M.K., Verhasselt V., Hébuterne X., Piche T. Vol. 63. 2014. Functional Bowel Symptoms in Quiescent Inflammatory Bowel Diseases: Role of Epithelial Barrier Disruption and Low-Grade Inflammation; pp. 744–752. (5). (Gut) [DOI] [PubMed] [Google Scholar]
- 78.Tawiah A., Cornick S., Moreau F., Gorman H., Kumar M., Tiwari S., Chadee K. High MUC2 mucin expression and misfolding induce cellular stress, reactive oxygen production, and apoptosis in goblet cells. Am. J. Pathol. 2018;188(6):1354–1373. doi: 10.1016/j.ajpath.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 79.Maloy K.J., Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 80.Akbari A., Wu J. Ovomucin nanoparticles: promising carriers for mucosal delivery of drugs and bioactive compounds. Drug Delivery and Translational Research. 2017;7(4):598–607. doi: 10.1007/s13346-017-0406-3. [DOI] [PubMed] [Google Scholar]
- 81.Strugala V., Dettmar P.W., Pearson J.P. Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn's disease. Int. J. Clin. Pract. 2008;62(5):762–769. doi: 10.1111/j.1742-1241.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- 82.Takada H., Katayama T., Katoh T. Sialylated O-glycans from hen egg white ovomucin are decomposed by mucin-degrading gut microbes. J. Appl. Glycosci. 2020;67(2):31–39. doi: 10.5458/jag.jag.JAG-2019_0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Cani P.D. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U. S. A. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.D.C., E.A., D.C., P.H., V.H. M, V.-S. S, F.G., R.J., M.D., D. NM Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 2019;25(7):1096. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Desai M.S., Seekatz A.M., Koropatkin N.M., Kamada N., Hickey C.A., Wolter M., Pudlo N.A., Kitamoto S., Terrapon N., Muller A. 2016. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. [DOI] [PMC free article] [PubMed] [Google Scholar]