Abstract
The 2019 coronavirus disease (COVID-19) presents with a large variety of clinical manifestations ranging from asymptomatic carrier state to severe respiratory distress, multiple organ dysfunction and death. While it was initially considered primarily a respiratory illness, rapidly accumulating data suggests that COVID-19 results in a unique, profoundly prothrombotic milieu leading to both arterial and venous thrombosis. Consistently, elevated D-dimer level has emerged as an independent risk factor for poor outcomes, including death. Several other laboratory markers and blood counts have also been associated with poor prognosis, possibly due to their connection to thrombosis. At present, the pathophysiology underlying the hypercoagulable state is poorly understood. However, a growing body of data suggests that the initial events occur in the lung. A severe inflammatory response, originating in the alveoli, triggers a dysfunctional cascade of inflammatory thrombosis in the pulmonary vasculature, leading to a state of local coagulopathy. This is followed, in patients with more severe disease, by a generalized hypercoagulable state that results in macro- and microvascular thrombosis. Of concern, is the observation that anticoagulation may be inadequate in many circumstances, highlighting the need for alternative or additional therapies. Numerous ongoing studies investigating the pathophysiology of the COVID-19 associated coagulopathy may provide mechanistic insights that can direct appropriate interventional strategies.
Keywords: COVID-19, SARS-CoV-2, coagulopathy, thrombosis, inflammation
Highlights
-
•
COVID-19 generates a significantly increased risk for thrombosis
-
•
Pulmonary inflammation and localized vasculopathy are central to the hypercoagulable state
-
•
Immune dysregulation is notable in severe illness
-
•
Rising D-dimer levels correlate with worse outcomes
-
•
Anticoagulant guidelines are rapidly evolving as we gather further insights
1. Introduction
The novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, China at the end of 2019 and is now a pandemic [1]. The disease it causes, coronavirus disease 2019 (COVID-19), has affected more than 7 million people worldwide and claimed more than 400,000 lives as of June 2020 [2,3]. The disease ranges from asymptomatic, or mild to severe illness with multi-organ failure and death [[4], [5], [6]]. Coagulopathy, in the form of venous and arterial thromboembolism, is emerging as one of the most severe sequela of the disease, and has been prognostic of poorer outcomes [[7], [8], [9], [10]]. Reports of high incidence of thrombosis despite prophylactic and therapeutic dose anticoagulation raise question about a pathophysiology unique to COVID-19 [11,12]. Proposed hypotheses include a severely heightened inflammatory response that leads to thrombo-inflammation, through mechanisms such as cytokine storm, complement activation, and endotheliitis[8,9,13,14]. It has also been suggested that the virus itself can possibly activate the coagulation cascade [15]. Although individual institutions have developed guidelines and protocols to institute prophylactic and therapeutic anticoagulation, the optimal management is rapidly evolving as we continue to gather new insights into the pathophysiology of this disease.
Retrospective studies have identified clinical parameters that predict poor prognosis. In addition to markers of coagulopathy such as D-dimer other hematologic parameters have been studied[9,10,[16], [17], [18], [19]]. Neutrophil count, lymphocyte count, neutrophil/lymphocyte ratio, and platelet count correlate with disease severity[8,[20], [21], [22]]. At present, it is clear that patients with COVID-19 infection have a significantly increased risk of thrombosis that prevails despite anticoagulation. A better understanding of the pathophysiology accompanied by identification of biomarkers predictive of disease outcomes are critical to develop appropriate interventional strategies for this devastating disease.
In this review, we summarize results of key studies, and discuss the current understanding of coagulopathy and hematological parameters in COVID-19 patients, as well as the pathophysiology and management of thrombosis.
2. The hypercoagulable state with COVID-19
Previous outbreaks of coronaviruses, including SARS-CoV-1 and Middle-Eastern respiratory syndrome (MERS-CoV) have been associated with increased risk of thrombosis [23]. Similarly, the novel SARS-CoV-2 appears to generate a profoundly prothrombotic milieu as evidenced by a surge in global reports of arterial, venous and catheter-related thrombosis [7,24,25]. We summarize the current literature on the incidence of venous and arterial thrombosis in Table 1 , as well as ongoing observational studies on the incidence of thrombotic outcomes in Table 2 .
Table 1.
Table summarizing global incidence of venous and arterial thromboembolic disease in COVID-19.
| Location (first author) | Type of study | Sample size | Use of thromboprophylaxis | Venous thromboembolism incidence | Arterial thrombosis incidence | Key characteristics of patient population/other salient features of the study |
|---|---|---|---|---|---|---|
| Wuhan, China (Cui et al) | Retrospective; hospitalized patients | 81 | No | VTE 25%; all lower extremity thrombi | None | 41% patients had other comorbidity (HTN, DM, CAD) and 43% were smokers |
| Netherlands (Klok et al) | Retrospective; multicenter; hospitalized patients | 184 | Yes (nadroparin at different doses) | VTE (n = 28) 27%; of those PE (n = 25) was most common finding in 81% | Ischemic strokes (n = 3) 3.7% | 76% were male, 2.7% had active cancer and 9.2% were on therapeutic anticoagulation from prior. Mean age was 64 and mean weight was 87 kg |
| Netherlands (Middeldorp et al) | Retrospective; single center; hospitalized patients | 198 | Yes (nadroparin 2850 units daily for <100 kg and 5700 units daily for >100 kg) | 7-day incidence of VTE (15%) and 14-day incidence of VTE (34%) | None | The 7-day and 14-day incidence of VTE was higher in the ICU (25% and 48% respectively) than the general wards (6.5% and 10% respectively) |
| Italy (Lodigiani et al) | Retrospective; single center; hospitalized patients | 388 | Yes (LMWH) Ward: 75% used (41% prophylactic dose, 21% intermediate dose; 23% therapeutic dose) ICU: 100% used |
VTE 21% (cumulative rate) ICU 27.6% and general ward 6.6% |
Ischemic stroke 2.5% and ACS/MI 1.1% | 68% were male, 24.1% had BMI ≥ 30, 47.2% had HTN, 22.7% with DM, 11.6% smokers, 6.4% with active cancer and 3.1% with history of prior VTE. |
| France (Llitjos et al) | Retrospective study; 2 ICUs | 26 | Yes (31% with prophylactic dose and 69% with therapeutic dose) | VTE 69% | None | 77% were male, 85% had HTN, 27% consumed tobacco, median BMI 30.2 kg/m2; median D-dimer was 1750 ng/mL. 56% of patients on therapeutic dose and 100% on prophylactic dose had VTE. |
| France (Helms et al) | Prospective study; COVID-19 ARDS patients at 4 ICUs in 2 centers | 150 | Yes (LMWH) | PE 16.7%; DVT 2% | Ischemic stroke 1.3%; limb ischemia 0.7%; mesenteric ischemia 0.7% | Patients with COVID-19 ARDS had significantly higher thrombotic events, especially PE (11.7% vs. 2.1%, OR 6.2, p = 0.008) |
| France (Poissy et al) | Retrospective case series; ICU | 107 | Yes | PE (20.6%) | None | 59.1% were male, median age was 57, median BMI was 30. Incidence of VTE was 2×- higher than a historical control period |
| Netherlands (Beun et al) | Retrospective; ICU | 75 | Unknown | PE (26.6%; 21.3% subsegmental and 5.3% central); DVT 4% | Ischemic stroke 2.7% | 4 patients had heparin resistance apparent by PTT based methods probably due to elevated factor VIII levels |
| New York, USA (Oxley et al) | Case series | 5 | No | None | Ischemic stroke 5 young patients in 2 week period | All patients were < 50 years of age. Historical incidence was 0.73 patients in a 2 week period |
| Beijing, China (Zhang et al) | Case series | 3 | Unknown | None | Ischemic strokes in 3 patients | Age 65-70, 2/3 were male, all with cardiovascular comorbidities including 2/3 with history of ischemic stroke. All with anti-phospholipid antibodies |
| Italy (Bellosta et al) | Observational cohort study | 20 | 25% were on anticoagulation at baseline due to atrial fibrillation | None | Acute limb ischemia in 20 patients (16.3%) | 90% patients were male, mean age was 75 years, 55% had HTN. Incidence increased at 16.3% compared with a baseline rate of 1.8% in this region |
DVT = deep venous thrombosis.
PE = pulmonary embolism.
LMWH = low molecular weight heparin.
Table 2.
Summary of ongoing observational trials on incidence of coagulopathic changes or thrombosis in patients with COVID-19.
| Clinical trial | Location | Status | Study description |
|---|---|---|---|
|
NCT04335162 (CovCardiovasc) |
France | Recruiting (100 patients) |
Screening of cardiovascular complications in COVID-19 |
| NCT04356950 | France | Not yet recruiting (175 patients) |
Analysis of coagulopathy developed in COVID-19 patients |
|
NCT04356144 (TGA-TM) |
Austria | Not yet recruiting (60 patients) |
Diagnostic TGA and TGA-thrombomodulin (TGA-TM) in critically ill patients |
| NCT04363528 | France | Not yet recruiting (50 patients) |
Incidence of DVT in COVID patients in the ICU |
| NCT04366778 | France | Not yet recruiting (330 patients) |
Thromboelastography with tPA to detect patients with high risk of thrombosis |
|
NCT04373486 (COVID-APE) |
France | Not yet recruiting (160 patients) |
Assessment of acute PE on CT angiography and relationship to D-dimer |
|
NCT04357847 (COVID-Thelium) |
France | Not yet recruiting (100 patients) |
Assessment of endothelial and hemostatic changes in Severe SARS-CoV-2 infection |
|
NCT04366752 (THROMBOCOVID) |
France | Recruiting (100 patients) |
Thrombembolic events in critical care patients with acute pneumopathy |
|
NCT04359212 (VTE-COVID) |
Italy | Not yet recruiting (90 patients) |
Thromboprophylaxis with LMWH or fondaparinux in patients recovered in ICU or medical ward |
Observational Clinical Trials presently listed on ClinicalTrials.gov.
LMWH = low-molecular-weight heparin; TGA = thrombin generation assay.
2.1. Venous thromboembolism
Pulmonary embolism is the most common thrombotic manifestation of COVID-19[26]. One of the first substantial datasets on risk of venous thromboembolism (VTE) in critically ill patients with COVID-19, reported a VTE incidence of 25%[16]. In a larger study in the Netherlands, 184 ICU patients with COVID-19 who were all on at least standard thromboprophylaxis had a 27% cumulative incidence of VTE, with pulmonary embolism (PE) being most frequent (81%) [24]. Middeldorp et al. reported a higher incidence of thrombotic complications in their ICU patient population (7-day and 14-day cumulative incidence of 25% and 48% respectively) compared to the patients admitted on the wards. All patients initially received standard of care thromboprophylaxis that was intensified later on[25]. Another data set from France that included 150 patients with COVID-19 associated acute respiratory distress syndrome (ARDS) showed a VTE rate of 18%, with PE being most common. When compared to a historical prospective cohort of non-COVID-19 ARDS after matching, patients with COVID-19 ARDS demonstrated a significantly higher rate of thrombotic events, mainly PEs (11.7% vs 2.1%, OR 6.2, p = 0.008) [27]. Current data is too scant to determine the demographic characteristics of patients with COVID-19 that are more likely to develop thrombosis. It has however been suggested that this hypercoagulability may be more pronounced in older age, male gender, Caucasian and African-American ethnicities[16,17,28].
2.2. Arterial thrombosis
In comparison to venous thrombosis, the incidence of arterial thrombosis in COVID-19 appears to be minor (Table 1). It is nevertheless of significant concern and merits further study.
2.2.1. Myocardial infarction
Myocardial infarction (MI) has not been commonly reported with COVID-19. In the Italian study by Lodigiani et al which included 388 patients with COVID-19, the incidence of MI or acute coronary syndromes was 1.1% [29]. Troponin levels have been noted to be significantly higher in the non-survivors, and may provide prognostic value [7,30]. While there are many explanations for elevated troponins (renal injury, myocarditis), ischemic injury as a result of plaque rupture and consequent infarction or secondary to demand ischemia has been reported, and is postulated as another cause of myocardial injury [14].
2.2.2. Stroke
Ischemic strokes were first reported by Dutch investigators[24]. Oxley et al have reported an alarming seven-fold increase in large vessel strokes in the <50-year-old age group in New York City, New York (5 patients in a 2-week period during COVID-19 pandemic compared with 0.7 patients pre-COVID) [31]. Another case series reports three patients with COVID-19 presenting with strokes and limb ischemia[32]. Clinical presentation with ischemic strokes was also noted by Klok et al (3.7%) and Lodigiani et al (2.5%) [24,29].
2.2.3. Microvascular thrombosis
Several clinical reports have demonstrated evidence of thrombotic microangiopathy (TMA) in patients with COVID-19, most notably in lung autopsies [[33], [34], [35]]. In a study by Menter et al, five out of eleven patients showed evidence of microthrombi in lung autopsies [34], and another case series by Ackermann et al. showed widespread thrombosis with microangiopathy in the lung autopsies of seven COVID-19 patients[35]. The authors compared these findings to those of severe influenza patients, and found that alveolar microthrombi were 9 times more prevalent in COVID-19 patients (p < 0.001) [35]. Interestingly, one report from China described the presence of extensive microvascular thrombosis in extrapulmonary organs where coronavirus was not detected, suggesting that a mechanism beyond viral infection is operative[36]. Tian et al provided evidence of microthrombi in pathological samples obtained from two asymptomatic patients who underwent lobectomies for lung adenocarcinoma, and were subsequently found to be positive for COVID-19 [37]. Pathological examinations revealed patchy inflammatory cellular infiltrate with focal areas of fibrin deposit, suggesting that a local hypercoagulable state in the pulmonary tissue may be an early occurrence. Furthermore, TMA may contribute to findings of unusual sites and presentations of thrombosis that have been described in several clinical reports (Table 3 ). COVID-19 induced chilblains eruption has been reported, where histopathology revealed microangiopathy thought to be induced by a robust interferon response to the virus [38]. Chilblain-like lesions have also been reported in otherwise asymptomatic adolescents[39]. Transient livedo reticularis has been reported in two patients and is believed to be secondary to coagulopathy[40]. Several reports have mentioned cases with bowel ischemia [14], limb or acral ischemia[32,41] and cutaneous ischemia[42]. Whether the renal injury seen in COVID-19 is related to direct viral toxicity or endothelial/microvascular injury has not been established. One review of 26 autopsy reports did not find evidence of fibrinous material in the renal vasculature[43], while this was found in 3 out of 18 patients in another autopsy review[34].
Table 3.
Demographic and clinical characteristics of reported cases of thrombi in unusual sites. An article written in Chinese could not be included in this table but has been cited in the main body of this review.
| Location (first author) | Age | Gender | Thromboembolic event | Pathologic findings | D-dimer (institution specific units) | Comorbidities | VTE prophylaxis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Switzerland (Varga et al) |
58 | F | Mesenteric ischemia | Endotheliitis in small intestine, lung, heart, kidney and liver | Unknown | Diabetes, hypertension, obesity | Unknown | Surgical removal of necrotic bowel, renal replacement therapy | Death |
| Switzerland (Varga et al) |
69 | M | Mesenteric ischemia | Endotheliitis of submucosal vessels in small intestine | Unknown | Hypertension | Unknown | Resection of small intestine, mechanical ventilation | Survived |
| New York (Magro et al) |
32 | M | Purpuric rash on buttocks | Thrombogenic vasculopathy with necrosis of epidermis and adnexa; complement deposits | D-dimer 1024 ng/mL that peaked at 2090 ng/mL on day 19 (normal 0-229 ng/mL); INR 1.6-1.9; normal PTT and platelets | Obstructive sleep apnea, anabolic steroid use | Unknown | Hydroxychloroquine, azithromycin, remedesvir, mechanical ventilation | Not mentioned |
| New York (Magro et al) |
66 | F | Purpuric rash on palms and soles | Superficial vascular ectasia with occlusive arterial thrombus; complement deposits | D-dimer 7030 ng/mL; low platelets at 128 × 109/L on day 10; normal INR and PTT | None | Yes | Hydroxychloroquine, prophylactic anticoagulation, renal replacement therapy and supportive care | Not mentioned |
| New York (Magro et al) |
40 | F | Livedo racemosa on chest, arms and legs | perivascular lymphocytic infiltrate in superficial dermis and deep seated small thrombi in rare venules; complement deposits | D-dimer 1187 ng/mL; INR 1.4; normal platelet and PTT | None | Unknown | Mechanical ventilation | Not mentioned |
| Beijing, China (Zhang et al) | 69 | M | Lower limb, digital ischemia in hand and stroke | None | D-dimer >21.0 mg/L; PT 17 s, PTT 43.7 s, fibrinogen 4.15 g/L, FDP 85.5 mg/L all on admission to ICU | Hypertension, diabetes and prior stroke | Unknown | Oseltamivir, intravenous immunoglobulin and mechanical ventilation | Not mentioned |
| Camden, New Jersey, USA | 84 | M | Renal infarct in addition to stroke and pulmonary embolus | None | D-dimer 21.6 μg/mL | Hypertension | No (Thromboembolism at presentation) | LMWH infusion, mechanical thrombectomy, mechanical ventilation | Death |
LMWH = low-molecular-weight heparin.
VTE = venous thromboembolism.
3. Pathophysiology of COVID-19 coagulopathy: inflammatory thrombosis
In addition to bedside evidence for a hypercoagulable state in COVID-19, laboratory tests have also been consistent with a prothrombotic milieu such as increased D-dimer, fibrinogen, factor VIII (FVIII), von Willebrand factor (vWF), decreased antithrombin, and TEG results [44]. While critical illness is known to cause a hypercoagulable state due to immobilization, mechanical ventilation, central venous access devices, and nutritional deficiencies, COVID-19 appears to cause a hypercoagulable state through mechanisms unique to SARS-CoV-2 and centers around the cross-talk between thrombosis and inflammation [45,46].
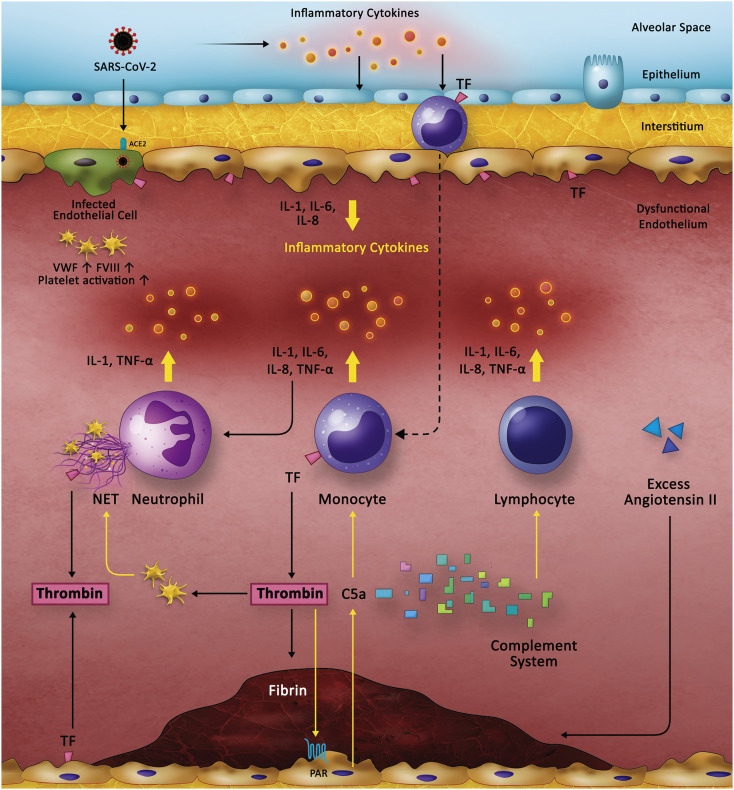
The causal, bi-directional relationship between inflammation and thrombosis is well established [47]. COVID-19 causes a profoundly pro-inflammatory state, as evident from multiple reports of high C-reactive protein, lactate dehydrogenase, ferritin, interleukin-6 and D-dimer levels [48]. IL-6 and fibrinogen levels are shown to correlate with each other in COVID-19 patients, providing credence to the idea of inflammatory thrombosis [46]. Researchers presently believe that the inciting event sparking the cycle of inflammation and thrombosis originates in the pulmonary alveoli, where SARS-CoV-2 enters the alveolar epithelium through the ACE2 receptor. Consequently, a severe inflammatory response is initiated that sets the stage for thrombosis through several mechanisms. We propose the following mechanisms as the central pathophysiological aspects of the inflammatory thrombosis caused by COVID-19, which we visually summarize in Fig. 1 .
Fig. 1.
Pathophysiology of the Hypercoagulable State in COVID-19. The current understanding of the pathophysiology of COVID-19 induced coagulopathy centers around the bidirectional cross-talk between inflammation (yellow arrows) and thrombosis (black arrows). COVID-19 leads to a severe inflammatory response that originates in the alveoli. Release of inflammatory cytokines leads to activation of epithelial cells, monocytes and macrophages. Direct infection of the endothelial cells through the ACE2 receptor also leads to endothelial activation and dysfunction, expression of TF, and platelet activation and increased levels of VWF and FVIII, all of which contribute to thrombin generation and fibrin clot formation. Thrombin, in turn, causes inflammation through its effect on platelets which promote NET formation in neutrophils. It also activates endothelium through the PAR receptor, which leads to release of C5A that further activates monocytes. These mechanisms are currently hypothetical based on existing findings in COVID-19 and previous understanding of the cross-talk between inflammation and thrombosis.
ACE2: Angiotensin-converting enzyme 2. FVIII: Factor VIII. IL: Interleukin. NET: Neutrophil extracellular trap. TF: Tissue factor. TNF: Tumor necrosis factor. VWF: von Willebrand factor.
3.1. Localized intravascular coagulopathy
A report by Tang et al. described a high rate (71.4%) of COVID-19 patients meeting ISTH disseminated intravascular coagulopathy (DIC) criteria[10]. However, clear evidence of overt clinical DIC in COVID-19 is lacking thus far. It is possible that the laboratory abnormalities noted are a reflection of a localized coagulopathy in the pulmonary vasculature, resulting from severe alveolar inflammation [17,49]. Progression of this process to the systemic circulation may explain microthrombotic complications and ensuing multi-organ failure. In fact, Ciceri et al propose to label this entire pathophysiology as microvascular COVID-19 lung vessels obstructive thrombo-inflammatory syndrome or MicroCLOTS.[50] The group postulates that, in individuals predisposed to severe outcomes, initial viral damage occurring in the alveoli generates inflammation and local microvascular pulmonary thrombosis. This is followed by more generalized endothelial dysfunction and thrombo-inflammation in the microvasculature of the brain, kidneys and other organs leading to a hypercoagulable state and multiple organ failure.
3.2. Inflammatory cytokines
Excessive cytokine release is postulated to cause the severe illness noted in younger patients without pre-existing conditions. Higher serum levels of several inflammatory cytokines and chemokines have been associated with severe illness and death in multiple studies [9,[51], [52], [53], [54]]. The cytokine profiles in patients with severe COVID-19 show increased production of IL-6, IL-7, TNF, and inflammatory chemokines such as CCL2, CCL3, and soluble IL-2 receptor, a profile similar to that seen in cytokine release syndromes, such as macrophage activation syndrome [55]. Excessive cytokine release contributes to thrombosis through multiple mechanisms, including activation of monocytes, neutrophils, and the endothelium, all of which generates a prothrombotic state.
3.3. Endothelial activation & dysfunction
Varga and colleagues first reported endothelial dysfunction in multiple vascular beds on post mortem specimens obtained from three patients [14]. In case series of 7 patients by Ackerman et al, lung autopsies of COVID-19 patients showed severe endothelial injury with presence of intracellular virus, as well as widespread thrombosis with microangiopathy[35]. Furthermore, significantly elevated levels of VWF and FVIII in COVID-19 patients are suggestive of endothelial activation in these patients[27,56]. Endothelial activation or dysfunction with COVID-19 may occur through multiple mechanisms. This includes inflammatory cytokines generated in the pulmonary interstitium, the activation of the complement components in blood, or possibly, as a direct result of SARS-CoV-2 infection of endothelial cells through the ACE2 receptor[57]. Endotheliitis, in turn, is a major forerunner to thrombosis. The observation that male sex, obesity, hypertension, and diabetes are poor prognostic factors for severe disease with COVID-19 further supports this theory due to the presence of endothelial dysregulation at baseline in these patients[14]. Whether anti-phospholipid (aPL) antibodies contribute to endothelial dysfunction and activation in COVID-19 is unclear. Anticardiolipin antibodies, β2 glycoprotein antibodies and positive lupus anticoagulant have all been reported in a few sudies [27,32,56,58]. The presence of aPL antibodies in the general population, especially in states of infection, is common[59]. In addition, the contribution of IgA aPL antibodies, reported by Zhang et al, to thrombosis is controversial. Many lupus anti-coagulant assays are sensitive to C-reactive protein (CRP), and lead to false positive results in states where CRP is markedly elevated such as COVID-19[59,60]. Thus, the clinical relevance of these findings is yet to be determined.
3.4. Mononuclear phagocytes (MNPs)
Monocytes and macrophages are theorized to play a crucial role in the inflammation and thrombosis seen in COVID-19. Liao et al demonstrated that MNPs account for 80% of the total bronchoalveolar fluid from patients with severe COVID-19 illness, compared to 60% and 40% in mild cases and healthy controls, respectively [61]. Furthermore, the composition of the cells was characterized by an abundance of inflammatory monocyte-derived macrophages in patients with severe disease. Bronchoalveolar fluid in severe patients is enriched with chemokines potently recruit monocytes [62]. COVID-19 patients requiring ICU hospitalization were noted to have a significant expansion of CD14+, CD16+ monocyte populations producing IL-6 in peripheral blood [55,[63], [64], [65]]. In another study, circulating monocytes were shown to have a sustained production of TNF-α and IL-6, a pattern that differs from bacterial or influenza sepsis [66]. Similarly, post-mortem analyses of COVID-19 positive patients revealed that lymphoid tissue macrophages infected with SARS-CoV-2 viral particles expressed IL-6. Further, the presence of IL-6+ macrophages was associated with severe depletion of lymphocytes from lymphoid tissue [67]. These findings suggest that COVID-19 is associated with a clinical and laboratory picture similar to that of macrophage-activation syndrome (MAS). However, there distinguishing features present in COVID-19, such as higher fibrinogen levels and a less pronounced elevation in ferritin and liver dysfunction in comparison to that seen in classical MAS[68]. Presently, the exact mechanisms through which COVID-19 leads to activation of monocytes and macrophages remains unclear. However accumulating data suggests a role for MNPs in the generation of severe illness, including possibly the prothrombotic sequelae. This is not surprising given the understanding that activated monocytes rapidly upregulate tissue factor (TF) expression. This triggers the coagulation cascade resulting in production of thrombin which in turn leads to thrombus generation, platelet activation, and amplification of pro-inflammatory pathways, primarily through PAR signaling[69].
3.5. Neutrophil extracellular traps (NETs)
While neutrophilia [70] and an elevated neutrophil-lymphocyte ratio (NLR) [71] have been reported by numerous studies now as predictive of worse disease outcomes, the contribution of neutrophil extracellular traps in the pathophysiology of COVID-19 was reported only recently[72]. NETs are implicated to portend pathogenicity in a wide variety of disorders including influenza-associated ARDS [73] and thrombo-inflammation [74,75]. Yu et al report elevated levels of serum NETs in hospitalized COVID-19 positive patients based on a finding of elevated cell-free DNA, myeloperoxidase-DNA and citrullinated histones in 50 patients with COVID-19. This was especially noted in hospitalized and mechanically ventilated patients [72]. Moreover, they showed that sera obtained from these patients stimulated NET generation in control neutrophils. Taken together with literature linking NETs to pulmonary diseases and thrombo-inflammation, these data begin to implicate NETs as causative in organ damage, widespread thrombosis and mortality that is noted in COVID-19 infection. Finally, a recent manuscript by Barnes et al describes an abundance of neutrophil infiltration in pulmonary capillaries of three patients who succumbed to COVID-19 and suggests that aberrant activation of neutrophils and NET generation may underlie the cytokine storm and severe disease outcomes noted in this disease[76].
3.6. Complement-mediated microangiopathy
Previous studies in animal models provide evidence for complement activation in serum and pulmonary tissue [77,78]. A growing body of evidence suggests a major role for dysregulated complement activation in severe COVID-19[79]. Several reports of post-mortem examinations have demonstrated evidence of TMA, including hyaline thrombi in the small vessels of the lungs and other organs [80,81]. One of those reports demonstrated lung and skin biopsy findings revealing a pauci-inflammatory thrombogenic vasculopathy with complement deposit. Researchers in China observed complement hyper-activation in COVID-19 patients, as well as significantly increased plasma C5a levels in severe cases[82]. Dysregulated complement system activation may be a major contributor to cytokine storm, particularly through the pro-inflammatory effects of anaphylatoxins C3a and C5a [83]. These effects are likely to become more detrimental in patients with a genetic predisposition for decreased complement regulation, and may contributed to findings of TMA and subsequent organ dysfunction.
3.7. Dysregulated renin angiotensin system (RAS)
Although dysfunction of RAS is known to play a significant role in ARDS in general [84,85], this system is specifically important in COVID-19 infections for several reasons. The SARS-CoV2 uses its Spike (S) protein and fuses with the enzyme Angiotensin-Converting Enzyme 2 (ACE2) located on the cell membrane of human cells to gain entry into cells. ACE2 is homologous to ACE, which cleaves angiotensin I (ANGI) to generate ANGII. ANGII binds to the Angiotensin Type I Receptor (AT1R) that leads to vasoconstriction and an increase in blood pressure. The inactivation of ANGII by ACE2 results in vasodilation. Conversely, ANGII also negatively regulates ACE2 [86], which is located on lung alveolar epithelial cells, renal tubular epithelial cells, enterocytes of the small intestine, endothelial cells, cardiomyocytes, fibroblasts and pericytes in the heart. SARS-CoV-2 has a high affinity for ACE2, and binding of the SARS-CoV-2 results in loss of ACE2 due to internalization of the virus and ACE2 shedding. This decrease in ACE2 leads to decreased degradation of ANGII resulting in excess ANGII binding to AT1R and increase lung injury [86]. Finally, studies suggest that ANGII binding to AT1R may stimulate IL-6 release, further contributing to the cytokine storm syndrome that is typical of severe COVID-19 infection. Supporting this hypothesis is the evidence showing increased risk of severe disease in patients infected with SARS-CoV or influenza H7N5 that had higher ANGII levels [87,88]. Further, in recent studies on COVID-19 positive patients, viral load and lung injury directly correlated with plasma ANGII levels[89]. In addition,COVID-19 appears to generate worse outcomes in patients with hypertension, cardiovascular disease and diabetes, all of which are associated with reduced baseline levels of ACE2 expression suggesting imbalance in ACE/ACE2 levels[8,90]. It has been shown that ANGII induces TF and plasminogen activator inhibitor 1 (PAI-1) expression by endothelial cells via AT1R, leading to a hypercoagulable state[91,92]. Thus, it is likely these derangements in the RAS pathways, in addition to the multiple pathways described above, likely contribute to the hypercoaguable state of COVID-19, and the ensuing mortality and morbidity of the disease. Presently, rapid investigation into the molecular mechanisms involved is essential in order to gain a better understanding of the pathophysiology of the disease and to direct appropriate, timely therapeutic interventions.
4. Impact of blood count abnormalities
COVID-19 is associated with a significant effect on the hematological and hemostatic system. In this section we will review the available data on the common hematologic parameters and their prognostic significance. See Table 4 for a summary of the hematologic parameters from various studies.
Table 4.
Summary of literature on the impact of common hematologic parameters on disease severity in COVID-19.
| Lab | Location (reference) | N (total, non-severe/severe) | Non-severe | Severe | P value |
|---|---|---|---|---|---|
| White blood cell count (×109/L) Median (IQR) or [SD] |
China (Guan, et.al NEJM) | 1099, 926/174 | 4.9 (3.8–6.0) | 3.7 (3.0–6.2) | NR |
| Wuhan, China (Qin, et al. Clin Inf Disease) | 452, 166/286 | 4.9 (3.7–6.1) | 5.6 (4.3–8.4) | <0.001 | |
| China (Wang et al. JAMA) | 138, 102/36 | 4.3 (3.3–5.4)a | 6.6 (3.6–9.8)a | 0.003 | |
| Shangai, China (Wu, et al. Jama) | 201, 117/84 | 5.02 (3.37 –7.18)b | 8.32 (5.07–11.20)b | <0.001 | |
| Wuhan, China (Chen, et al. BMJ) | 247, 161/113 | 5.0 (3.7–6.3)c | 10.2 (6.2–13.6)c | NR | |
| Wuhan, China (Zhou, et.al) | 191, 137/54 | 5.2 (4.3–7.7)c | 9.8 (6.9–13.9)c | <0.0001 | |
| Absolute neutrophil count (×109/L) Median (IQR) or [SD] |
Wuhan, China (Qin, et al. Clin Inf Disease) | 452, 166/286 | 3.2 (2.1–4.4) | 4.3 (2.9–7.0) | <0.001 |
| China (Wang et al. JAMA) | 138, 102/36 | 2.7 (1.9–3.9)a | 4.6 (2.6–7.9)a | <0.001 | |
| Shangai, China (Wu, et al. Jama) | 201, 117/84 | 3.06 (2.03–5.56)b | 7.04 (3.98–10.12)b | <0.001 | |
| Wuhan, China (Chen, et al. BMJ) | 247, 161/113 | 3.2 (2.4–4.5)c | 9.0 (5.4–12.7)c | NR | |
| Absolute lymphocyte count (×109/L) Median (IQR) or [SD] |
China (Guan, et.al NEJM) | 1099, 926/174 | 1.0 (0.8–1.4) | 0.8 (0.6–1.0) | NR |
| Wuhan, China (Qin, et al. Clin Inf Disease) | 452, 166/286 | 1.0 (0.7–1.3) | 0.8 (0.6–1.1) | <0.001 | |
| China (Wang et al. JAMA) | 138, 102/36 | 0.9 (0.6–1.2)a | 0.8 (0.5–0.9)a | 0.03 | |
| Shangai, China (Wu, et al. Jama) | 201, 117/84 | 1.08 (0.72–1.45)b | 0.67 (0.49–0.99)b | <0.001 | |
| Wuhan, China (Chen, et al. BMJ) | 247, 161/113 | 1.0 (0.7–1.4)c | 0.6 (0.4–0.7)c | NR | |
| Wuhan, China (Zhou, et.al) | 191, 137/54 | 1.1 (0.8–1.5)c | 0.6 (0.5–0.8)c | <0.0001 | |
| Neutrophil/lymphocyte ratio Median (IQR) or [SD] |
Beijing, China (Liu, et al. preprint) | 61, 44/17 | 2.2 (1.4–3.1) | 3.6 (2.5–5.4) | 0.003 |
| Wuhan, China (Qin, et al. Clin Inf Disease) | 452, 166/286 | 3.2 (1.8–4.9) | 5.5 (3.3–10.0) | <0.001 | |
| China (Yang, et al. Int Immun) | 93, 69/24 | 4.8 [± 3.5] | 20.7 [± 24.1] | <0.001 | |
| Wuhan, China (Ma. et al.) | 37, 17/20 | 2.6 (1.8–3.5)e | 5.5 (3.6–6.5)e | 0.022 | |
| Platelet count (×109/L) Median (IQR) or [SD] |
China (Guan, et.al NEJM) | 1099, 926/174 | 172 (139–212) | 137 (99–179.5) | NR |
| China (Wang et al. JAMA) | 138, 102/36 | 165 (125–188)a | 142 (119–202)a | 0.78 | |
| Shangai, China (Wu, et al. Jama) | 201, 117/84 | 178 (140.0–239.5)b | 187 (124.5–252.5)b | 0.73 | |
| Wuhan, China (Chen, et al. BMJ) | 247, 161/113 | 198 (160–256)c | 156 (111.8–219.3)c | NR | |
| Wuhan, China (Zhou, et.al) | 191, 137/54 | 220 (168–271)c | 165.5 (107–229)c | <0.0001 | |
| Hemoglobin (g/dL) Median (IQR) or [SD] |
China (Guan, et.al NEJM) | 1099, 926/174 | 13.5 (12.0–14.8) | 12.8 (11.2–14.1) | NR |
| Wuhan, China (Chen, et al. BMJ) | 247, 161/113 | 12.8 (11.8–13.8)c | 12.8 (11.4–14.5)c | NR | |
| Wuhan, China (Zhou, et.al) | 191, 137/54 | 12.8 (12.0–14.0)c | 12.6 (11.5–13.8)c | 0.3 | |
| New York, USA (Goyal et al. NEJM) | 393, 263/130 | 13.5 (12.4–14.8)d | 13.7 (12.3–15.3)d | NR |
NR = not reached.
ICU vs non-ICU.
Without ARDS vs with ARDS.
Survivors vs Non-Survivors.
Non-invasive vs invasive ventilation.
Cancer patients.
4.1. Neutrophil count
Although initial studies from Wuhan reported leukopenia in hospitalized COVID-19 positive patients, [9], [8], subsequent reports showed a trend of higher neutrophil count in patients who required ICU admission (ANC 4.2 vs 2.6 × 109/L, p = 0.17) [20]. Thus, patients requiring ICU care developed neutrophilia during the hospitalization, with a median peak Absolute Neutrophil Count (ANC) of 11.6 × 109/L, compared to 3.5 × 109/L in the non-ICU group (P value <0.001). Further, in a retrospective review of 25 patients who died with COVID-19 in Wuhan the neutrophil count trended up prior to death in 87.5% of patents with evaluable data [93].
4.2. Lymphocyte count
The lymphocyte count has gained much attention in COVID-19 patients and studies consistently report the presence of lymphopenia in this setting. In the report from China Medical Treatment Expert Group for COVID-19 83.2% had lymphocytopenia at hospital admission [8].
Importantly, the lymphopenia is a consistent marker of poor prognosis. Thus, when comparing 109 patients who died in Wuhan versus 116 patients who recovered, the patients who died presented with a decreased lymphocyte count (0.63 vs 1.0 × 109/L) and decreased lymphocyte percentage [94]. In a retrospective cohort study of 191 patients with 54 deaths, lymphocyte count was lowest at day 7 of illness onset in survivors and then improved, whereas severe lymphopenia was observed until death in non-survivors [7]. Wang et al. reported that non-survivors developed more severe lymphopenia over time [70]. Flow cytometry on peripheral blood lymphocytes of COVID-19 patients requiring ICU care showed significantly lower CD45+, CD3+, CD4+, CD8+, CD16+, and CD16/56+ counts, without an inversion of the CD4/CD8 ratio [20].
Interestingly, while the number of CD4+ and CD8+ T cells were reduced, both the proportion and number of B cells were not affected or even increased in most patients. Further, the producton of IFN-γ by CD4+ T cells and not CD8+ T cells or NK cells tended to be lower in severe cases. Finally, circulating CD8 + T cells contained high concentrations of cytotoxic granules including perforin and granulysin. All these data suggest a dysregulated immune system with overactivation of cytotoxic CD8 + T cells [95].
Potential mechanisms of lymphocytopenia may include direct infection of the lymphocytes by the virus, though the proportion of ACE2-positive lymphocytes is quite small. Lymphocytes express the coronavirus receptor Angiotensin converting enzyme (ACE2) and may be directly targeted [96,97]. In a retrospective comparison of 42 patients with COVID-19 and hypertension, the 17 patients treated with an ACE inhibitor/ARB had absolute numbers of CD3+ and CD8+ T cells that were significantly higher than that in the non-ACEI/ARB group, but no difference in the CD4+ T cells [98].
4.3. Neutrophil to lymphocyte ratio
NLR has been shown to have prognostic significance in septic shock, pancreatitis, pancreatic cancer, and bacteremia, amongst other diseases [99]. An increasing neutrophil count in the setting of a lymphopenia appears to be a sensitive marker of early inflammation and physiologic stress[[100], [101], [102]]. In a study of 413 healthy volunteers the mean NLR was 1.65 (±1.96 SD: 0.78–3.53) [102].
Several studies have shown a relationship between an elevated NLR and more severe COVID-19 infection. An increased NLR at presentation has a strong association with increased disease severity when compared to patients without severe disease at presentation. Moreover, when stratified by high NLR (>3.13) and age ≥ 50, 50% of the patients had severe illness. Similarly, in another analysis of 96 patients, Yang, et al. identified that 46.1% of non-severe patients with an NLR >3.3 and age >49.5 would transform into severe cases within a mean of 6.3 days.[103] In a study of 301 patients, an NLR of 2.973 (AUC 0.7338, sensitivity 75.8%, specificity 66.8%) was associated with progression of disease [104]. Finally, a meta-analysis of 5 studies from China with 828 patients, NLR was found to increase significantly in patients with severe disease (standardized mean difference = 2.404, 95% CI - 0.98-3.82) [21].
Increased NLR was also associated with VTE with a mean NLR of 9.5 (5.9-13) in 33 patients who developed VTE versus 5 (3.5-7.9) in 165 patients without VTE [25].
4.4. Platelets
Although a significant drop in platelet counts has not been a prominent feature of the disease, there are certain situations when the presence of severity thrombocytopenia is being recognized as a marker of worse outcomes. Thrombocytopenia was more pronounced in patients with severe infection with a mean platelet count of 137 × 109/L vs 172 × 109/L in non-severe patients. See Table 4 for the difference in platelet counts between patients with mild versus severe disease. In a retrospective review specifically investigating the relationship between thrombocytopenia and mortality of 1476 consecutive patients in Jinyintan Hospital, Wuhan, thrombocytopenia was reported in 20.7% of patients, using a cutoff of 125 × 109/l. 72.7% of non-survivors had <125 × 109/L platelets vs only 10.7% in survivors, p < 0.001[22]. 76 patients (5.1%) had a platelet nadir <50 × 109/L with a mortality rate of 92.1%. The mortality rate was 61.2% in the group of patients with a nadir platelet count between 50 and 100 × 109/L. Overall, the majority of patients appear to have a mild thrombocytopenia, which is more pronounced with severe infection.
Presently, it is unclear if a decreased platelet count reflects a more severe DIC and increased consumption or a direct platelet-viral interaction [105]. Multiple possible mechanisms are possible for viral infection induced thrombocytopenia. These include the development of autoantibodies and immune complexes mediating clearance; direct infection of hematopoietic stem/progenitor cells and the megakaryocytic lineage via CD13 or CD66a resulting in decreased production of platelets; and pathologic activation of the coagulation pathway and consumption of platelets[105,106].
4.5. Hemoglobin
No significant abnormalities have been described regarding red blood cells and anemia. There has been a trend of worse anemia in patients with more severe disease, [20] with a median hemoglobin (Hgb) of 13.2 g/dL in patients requiring ICU versus 14.2 g/dL in non ICU patients (p = 0.07), and the majority of patients presented with a normal Hgb count. Of 1099 patients with confirmed COVID-19 in China, the median Hgb was 13.5 g/dL in non-severe patients and 12.8 g/dL in severe patients[8]. However, this trend was not appreciated in a review of 393 patients who required or did not require invasive ventilation in New York City[107]. See Table 4 for a review of the median Hgb levels reported in the various studies.
5. Management
The first general rule in the management of coagulopathy is the treatment of the underlying cause. However, with COVID-19, treatments of the viral infection remain experimental at the current time. As such, until an effective treatment option is available, it is crucial to be able to appropriately manage the sequela of COVID-19-associated coagulopathy.
5.1. Monitoring of laboratory parameters
As a result of the crosstalk between inflammatory and thrombotic pathways, infections are almost always associated with a concomitant activation of the coagulation system, evidenced by elevation in the markers of an activated coagulation system. The D-dimer has been shown to be frequently elevated in patients with COVID-19 [7,16,19,28,108,109]. Increased fibrinogen, fibrin degradation products, prothrombin time (PT), activated partial thromboplastin time (aPTT), and shortened thrombin time (TT) have been described in patients with COVID-19 compared to healthy controls [26,110].
5.1.1. D-Dimer
Numerous studies in COVID-19 patients highlight the prognostic value of increased D-dimer (Table 5 ) [9,10,[16], [17], [18], [19]]. Accumulating data clearly suggests that an elevated D-dimer, and presence of coagulopathy, serve as prognostic indicators of worse morbidity and mortality in hospitalized patients with COVID-19. In general, the D-dimer is a marker of fibrin formation and degradation, and specifically of plasmin-catalyzed degradation of fibrin polymers and thus, in the case of COVID-19 infection, reflective of pathological activation of the hemostatic pathways. In this review, we describe D-dimer units as reported in the original studies. In the study by Lodigiani et al, the median D-dimer of survivors on admission was 353 ng/mL (μg/L), and trended to 529 ng/mL a week later, in comparison to 869 ng/mL and 1494 ng/mL for non-survivors, respectively [111]. Consistently, data by Tang et al shows that non-survivors of COVID-19 infection demonstrated higher D-dimer levels and platelet counts, lower fibrinogen and antithrombin levels, all highly suggestive of ongoing DIC [10]. Monitoring hemostatic parameters such as platelet counts, coagulation assays, D-dimer and fibrinogen is common practice in critical care, especially in DIC [[112], [113], [114], [115]], These data now provide evidence for the importance of these assessments especially in COVID-19 patients and offer crucial prognostic insights which will likely guide alterations in management.
Table 5.
Summary of current literature evidence on the prognostic value of elevated D-dimer in COVID-19 across the globe.
| Location (first author) | Sample size | Clinical setting | D-dimer assay (reference range) | D-dimer cut-off for risk assessment | Outcome of interest | Statistics (sensitivity/specificity/odds ratio with p-value) | Salient findings |
|---|---|---|---|---|---|---|---|
| Wuhan, China (Zhou et al) | 191 | Hospitalized | Unknown | >1 μg/mL | Mortality | OR 18.42, 95% CI: 2.64-128.55; p = 0.0033 | D-dimer>1 μg/mL indicative of higher odds of death |
| Wuhan, China (Yao et al) |
248 | Hospitalized | Immunoturbidimetric assay (0-0.50 mg/L) | >2.14 mg/L | Mortality | Se 88.2%/Sp 71.3% | D-dimer elevated in 74.6% of inpatients. Median D-dimer 6.21 mg/L and 1.02 mg/L in non-survivors and survivors respectively, p = 0.000 |
| Wuhan, China (Zhang et al) | 343 | Hospitalized | CS5100 automatic coagulation analyzer (0-0.5 μg/mL) | >2 μg/mL | Mortality | HR 51.5, p < 0.001; adjusted HR 22.4 (for age, gender and comorbidity), p = 0.003 | D-dimer>2.0 μg/mL had higher incidence of mortality when compared to <2 (12/67 vs 1/267, P < 0.001) |
| Wuhan, China (Tang et al) | 183 | Hospitalized | STA-R MAX coagulation analyzer | N/A (continuous variable) | Mortality | N/A | Median D-dimer values were 2.12 μg/mL vs 0.61 μg/mL in the non-survivors and survivors respectively, p < 0.001. 71.4% of non-curvivors had DIC per ISTH criteria. |
| Mainland China (Guan et al) | 1099 | Hospitalized | Not mentioned | N/A (continuous variable) |
Severe disease; Primary composite endpoint was admission to ICU/mechanical ventilation or death | N/A | 1) 59.6% of the severe cases presented with elevated D-dimer vs 43.2% of non-severe cases (p = 0.002). 2) 69.4% of patients with the composite primary endpoint had elevated D-dimer vs. 44.2% of those without (P = 0.001). |
| Wuhan, China (Huang et al) | 41 | Hospitalized | Not mentioned | N/A (continuous variable) |
ICU admission | N/A | Median D-dimer values were 2.4 vs 0.5 in the ICU patients and non-ICU patients respectively, p = 0.0042. |
| Wuhan, China (Wang et al) | 138 | Hospitalized | Not mentioned (0-500 mg/L) | N/A (continuous variable) |
ICU admission | N/A | Median D-dimer values were 414 mg/L vs 166 mg/L, p < 0.001 in ICU cases and non-ICU cases respectively. |
| Wuhan, China (Wu et al) | 201 | Hospitalized | Not mentioned | N/A (continuous variable) |
ARDS; mortality | ARDS HR = 1.03, p < 0.001; mortality HR = 1.02, p = 0.002 | Higher D-dimer associated with progress to ARDS and mortality |
| Milan, Italy (Lodigiani et al) | 388 | Hospitalized | Not mentioned | N/A (continuous variable) |
ICU; mortality | N/A | Table 2 in this published study highlights the higher D-dimer values in non-survivors vs survivors and also in ICU patients vs general ward patients. |
| Beijing, China (Cui et al) | 81 | ICU | Succeeder SF8200 automatic coagulation analyzer | >1.5 μg/mL | VTE | Se 85%/Sp 88.5%/NPV 94.7% | 20/81 (25%) patients had VTE. 8/20 patients with VTE died. D-dimer values were 5.2 ± 3.0 vs 0.8 ± 1.2 μg/ml in the VTE group and non-VTE group respectively, P < 0.001. |
| Strasbourg, France (Leonard-Lorant et al) | 106 | Hospitalized | Unknown | >2660 μg/L | Pulmonary embolism | Se 100%/Sp 67% | 32/106 (30%) patients had a PE. Median D-dimer values were IQR 6110 ± 4905 versus 1920 ± 3674 μg/L in the PE and non-PE group respectively, p < 0.001 |
The ASH expert panel recommends serial monitoring of platelet count, PT, aPTT, D-dimer, and fibrinogen in hospitalized COVID-19 patients. The panel suggests that since worsening of these parameters, specifically the D-dimer, indicates worsening illness, this predicts the need for aggressive critical care or experimental therapies. Likewise, improvement of these parameters along with stable or improving clinical condition may support the decision to step down on aggressive anticoagulation therapy [116]. The ISTH provides similar recommendations [117]. On the other hand, blood product support can be considered in bleeding scenarios as with bleeding in DIC, although clinically overt bleeding is reportedly uncommon in the setting of COVID-19 [112,118].
For therapeutic monitoring of patients who are on anticoagulation with heparin products, anti-Xa monitoring is recommended over aPTT since the latter may be elevated in COVID-19. Furthermore, this allows for less frequent visits to the patient room.
5.2. Anticoagulation
5.2.1. Use of prophylactic or therapeutic dose anticoagulants
As our understanding of the coagulopathy associated with COVID-19 evolves, the best approach to management continues to be explored. Given the paucity of data in the pathophysiology of this disorder, physicians globally are compelled to prepare guidelines for management of this hypercoagulable state based on the established understanding of crosstalk between inflammation and thrombosis. Thus, clinicians are using prophylactic, intermediate, or therapeutic doses of anticoagulation, based on coagulation parameters and the clinical scenario.
Although the optimal dosing remains unclear the benefit of anticoagulation with heparin products (mostly LMWH at prophylactic doses) in COVID-19 patients was demonstrated by a study in China. [119] Importantly, in the sub-group analysis, those with a sepsis-induced coagulopathy (SIC) score of greater than 3 (n = 97) had decreased 28-day mortality (40.0% vs 64.2%, P = 0.029), as did the 161 patients with D-dimer greater than 6 times the upper limited of normal (32.8% vs 52.4%, P = 0.017). Of concern, a Dutch study by Klok et al reported a 31% incidence of thrombotic complications that occurred despite the presence of at least prophylactic anticoagulation (with LMWH) in patients hospitalized for COVID-19 pneumonia [11]. Consistently, another study, albeit smaller, done in France demonstrated the significant hypercoagulability that exists in COVID-19 patients. Here, Llitjos et al showed that all patients with severe COVID-19 who received prophylactic anticoagulation (n = 8) developed VTE, as did 56% of those who received therapeutic dose anticoagulation (n = 18) [12].
These findings make a strong argument for considering higher doses (intermediate or therapeutic) of anticoagulation in the management of patients with severe COVID-19, especially in the absence of obvious contraindications such as ongoing bleeding. However, they also bring to mind, the concern that anticoagulation in itself may be insufficient to prevent thrombotic complications. While we battle with these dilemmas, hemostasis experts outline and revise guidelines based on rapidly accumulating data, bearing in mind that there may exist pathophysiologic differences in COVID-19 induced coagulopathy. Below, we summarize the current guidelines and recommendations from different societies, as of the date this article is written, on the role of prophylactic and therapeutic anticoagulation in Table 6 .
Table 6.
Current guidelines and recommendations on prophylactic and therapeutic anticoagulation from different societies and institutions.
| Recommending source | When to consider prophylactic dose anticoagulation | When to consider therapeutic dose anticoagulation |
|---|---|---|
| International Society of Thrombosis & Hemostasis | In all patients with COVID-19 who are hospitalized, including non-critically ill, in the absence of contraindications (active bleeding and platelet count <25 × 109/L). PT and PTT abnormalities are not considered a contraindication [117]. | |
| American Society of Hematology (Expert Panel) | All hospitalized patients with COVID-19. LMWH or fondaparinux (suggested over UFH to reduce contact) in the absence of increased bleeding risk[121]. |
|
| Thrombosis UK |
|
|
| National Institute for Public Health of the Netherlands | All patients with (suspected) COVID-19 admitted to the hospital, irrespective of risk scores. |
|
While all guidelines presently recommend intravenous or subcutaneous low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH) for anticoagulation, another proposed approach has been the use of nebulized heparin for an enhanced localized anticoagulant effect in the pulmonary vasculature. Prior data demonstrated that nebulized heparin significantly reduces coagulation activation in the lungs of critically ill patients [120]. However, as with so many other therapies, this approach is yet to be studied in the context of COVID-19.
5.2.2. Drug Interactions with anticoagulants and antiplatelets
Attention should be given to potential drug interactions between anticoagulants and experimental drugs for COVID-19. ISTH guidelines advice caution (although not avoidance) in patients on DOACs who are admitted with COVID-19 illness due to interactions with antiviral or any other investigational drugs. In a small study by Testa et al, DOAC patients treated with antiviral drugs showed an alarming increase in DOAC plasma levels [124]. The effect of direct oral anticoagulants appears to be potentiated by atazanavir, lopinavir/ritonavir, hydroxychloroquine, and decreased by tocilizumab. Furthermore, apixaban may confer increased risk for QT prolongation when used with hydroxychloroquine. Atazanavir and lopinavir/ritonavir may decrease the active metabolite of clopidogrel and prasugrel. Among atazanavir, lopinavir/ritonavir, remdesivir, hydroxychloroquine, tocilizumab, and interferon beta, there has not been shown to be interactions with heparin products, fondaparinux, or argatroban. A list of drug interactions (collated by the University of Liverpool) can be found at http://covid19-druginteractions.org [125].
5.2.3. Duration of Anticoagulation
Data on the optimal duration and method of anticoagulation for COVID-19 patients post-discharge is currently not available. Aspirin has been studied for extended VTE prophylaxis in low-risk orthopedic patients, and could be considered for COVID-19 VTE prophylaxis if the post-discharge criteria are met [126]. The ASH expert panel recommends that any decision to use extended post-discharge thromboprophylaxis with anticoagulation or aspirin should consider the individual patient's VTE risk factors, such as reduced mobility, coagulopathy, and bleeding risk [121]. The International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) VTE risk score has been used as a tool to identify patients who would benefit from extended-use prophylaxis with LMWH [127]. Protocols suggest that patients hospitalized with COVID-19, especially those with an IMPROVE VTE score of >3, an elevated D-dimer level (>2× upper limit of normal), and 2 or more of the following characteristics: age >60, previous VTE, known thrombophilia, current cancer, should be strongly considered for extended thromboprophylaxis up to 39-45 days post-discharge either with prophylactic dose LMWH or rivaroxaban [[127], [128], [129]]. For patients who have been empirically started on therapeutic anticoagulation for suspected PE, the ASH panel recommends that they should remain anticoagulated for at least 3 months, regardless of results of future investigation studies. Furthermore, cases of confirmed VTE should be considered as “provoked” and treated for 3-6 months duration [121].
5.3. Antifibrinolytics
There has been interest in the use of anti-fibrinolytics in the management of thrombosis and ARDS in the setting of COVID-19. Fibrin deposition in the alveolar spaces and lung parenchyma is a known observation in ARDS leading to worse respiratory outcomes [130]. Although inhibiting thrombin generation with heparin agents may prevent further fibrin deposition, unlike the use of anti-fibrinolytics, it is not effective against pre-existing fibrin deposits. The use of tPA to treat ARDS in COVID-19 has been proposed, following a case series of 3 COVID-19 patients in whom tPA was associated with temporary improvement in respiratory parameters [131,132]. However, bleeding complications remain a major concern, and given the paucity of data, the use of anti-fibrinolytics is not yet a strong recommendation. An alternative, safer approach that may confer benefit in COVID-19 induced ARDS is the use of nebulized fibrinolytics. In 2019, a study on 60 patients with ARDS showed that use of nebulized streptokinase in patients with severe ARDS resulted in improvements in oxygenation and lung mechanics more rapidly than nebulized heparin [133]. This approach would need further investigation in the COVID-19 setting.
Another agent with fibrinolytic properties that has been considered is Nafamostat. Nafamostat is a synthetic serine protease inhibitor that has been used in Japan for treatment of DIC in pancreatitis for decades. Nafamostat possesses both anti-fibrinolytic activity as well as anti-viral activity, and has thus generated interested in being repurposed as a potential therapeutic agent for COVID-19 in ongoing studies [134].
5.4. Future therapeutic targets and areas of research
As previously highlighted, SARS-CoV-2 presents unique mechanisms of inducing coagulopathy. The limited data revealing a high incidence of thrombosis despite anticoagulation underscores the necessity for novel therapeutic approaches to prevent thrombotic complications and mortality [11,12]. We enlist below the main areas of research that are either being pursued or warrant further study, and provide a summary of ongoing observation and interventional trials in Table 6 and Table 7 . These approaches remain theoretical or experimental, but offer insight into potential future strategies that bear promise.
Table 7.
Summary of ongoing interventional trials using different anti-thrombotic agents in COVID-19 patients.
| Clinical Trial | Location | Status | Intervention |
|---|---|---|---|
| NCT04345848 | Switzerland | Recruiting (200 patients) |
Low and high dose anticoagulation with UFH or LMWH |
|
NCT04354155 (COVAC-TP) |
USA | Not yet recruiting (38 patients) |
Thromboprophylaxis with enoxaparin |
|
NCT04344756 (CORIMUNO-COAG) |
France | Not yet recruiting (808 patients) |
Tinxaparin or UFH for 14 days |
| NCT 04357730 | USA | Not yet recruiting (60 patients) |
Fibrinolytic therapy to treat ARDS in COVID-19 patients |
| NCT04362085 | Canada | Not yet recruiting (462 patients) |
Therapeutic LMWH or UFH versus standard of care |
| NCT04360824 | USA | Not yet recruiting (170 patients) |
Standard versus intermediate dose enoxaparin |
|
NCT04367831 (IMPROVE) |
USA | Not yet recruiting (100 patients) |
Intermediate to prophylactic dose anticoagulation |
|
NCT04363840 (LEAD COVID-19) |
USA | Not yet recruiting (1080 patients) |
Aspirin 81 mg plus vitamin D |
|
NCT04373707 (COVI-DOSE) |
France | Not yet recruiting (602 patients) |
Weight adjusted versus fixed low dose LMWH for VTE |
|
NCT04354155 (COVAC-TP) |
USA | Not yet recruiting (38 patients) |
Thromboprophylaxis with LMWH in children |
Interventional Clinical Trials presently listed on ClinicalTrials.gov.
LMWH = low molecular weight heparin; UFH = unfractionated heparin.
5.4.1. Inflammatory thrombosis
As previously highlighted, the bidirectional cross-talk between inflammation and thrombosis, or “immuno-thrombosis”, is emerging as a major cornerstone of the prothrombotic complications of COVID-19. Experimental use of immunosuppressive agents can potentially set the brakes on this inflammatory process in COVID-19 patients and has been of interest [117].
5.4.1.1. Complement pathway
As previously mentioned, a growing body of evidence supports the role of complement hyper-activation contributing to severe COVID-19 [79]. These studies provide the basis for the suggestion that severe and fatal cases of COVID-19, that have findings consistent with excessive complement activation, may have an increased susceptibility to TMA due to a genetic predisposition to a pathogenic complement activation. Previous data showed that treatment with humanized anti-C5a antibody greatly ameliorated lung injury and inflammation in a monkey model of virus-induced acute lung injury (H7N9 virus) [135]. In fact, in the same study in China where elevated C5a levels were demonstrated in severe COVID-19, researchers also treated two severe cases with a recombinant C5a monoclonal antibody. Both patients showed significant improvement with resolved fever and increased oxygenation [82]. Thus, complement inhibition, with agents such as eculizumab, may also be a promising treatment for severe COVID-19 [13].
5.4.1.2. Neutrophil extracellular traps
Accumulating data show that neutrophils extracellular traps or NETs have the potential to propagate both inflammation and thrombosis. As highlighted above, NETs are postulated to play a role in COVID-19 associated immuno-thrombosis as well. This suggests a role for potentially targeting NETs to reduce the clinical severity of COVID-19. Barnes et al propose several mechanisms of either directly targeting the molecules that form NETs, or the process that leads to their formation[76]. Anakinra targets IL1β, which enhances NET formation, and is currently being investigated in COVID-19 trials (ClinicalTrials.gov identifiers: NCT04324021, NCT04330638, NCT02735707) [76].
5.4.1.3. Inflammatory cytokines
Tocilizumab, an interleukin-6 inhibitor, has been used in the setting of cytokine release syndrome in COVID-19, and recent pilot prospective data suggests a survival benefit when given early in the course of the disease [136]. While the impact of tocilizumab on thrombotic outcomes is unclear, it is possible that the survival benefit is secondary to reduced endothelial inflammation and microvascular thrombosis. Regardless, this warrants further investigation. In a small case series in China, the use of plasma exchange for the similar purpose of reducing cytokine storm was attempted with the suggestion of potential benefit [137]. Additionally, the use of non-anticoagulant heparin-like molecules has been proposed as a mechanism to attenuate the release of inflammatory cytokines. LMWH is known to possess anti-inflammatory properties, which could provide additional benefit in COVID19 patients subject to pro-inflammatory cytokines [8,9,138]. Specifically designed heparan sulfate oligosaccharides with neutralizing proinflammatory proteins and potential anti-viral effect are being investigated in managing COVID-19 [139].
5.4.2. Natural anticoagulant pathways
Since the pathogenesis of inflammation and sepsis-induced coagulopathy involves the generation of thrombin and the reduction of natural anticoagulant proteins, numerous studies have explored the benefit of using coagulation inhibitors such as antithrombin in these situations. Hayakawa et al demonstrated that AT treatment in sepsis-induced DIC may be associated with a reduced in-hospital all-cause mortality [140]. Similarly a study be Kato et al suggested possible mortality benefit in septic patients with coagulopathy treated with recombinant thrombomodulin [141]. Although none of the studies with anticoagulants provide data as robust as the PROWESS trial studying activated protein C (APC), the benefit of using APC is offset with the increased risk for bleeding [142]. In general, these data provide fuel for considering experimental therapies that address physiologic anticoagulant pathways that are most likely inhibited or inactivated in COVID-19. To this end, a group in Austria has already registered a study with Clinicaltrials.gov to examine the thrombin generation potential of COVID-19 positive patients, with and without the addition of thrombomodulin (NCT04356144). The results from such studies may provide the foundation for interventional studies exploring anticoagulants such as recombinant thrombomodulin or antithrombin for the management of COVID-19 coagulopathy.
5.5. COVID-19 in patients with bleeding disorders
While we still do not understand how COVID-19 infection may affect people with bleeding disorders, we are aware that severe illness is usually associated with a coagulopathy that resembles DIC. Since some of the treatments used presently for management of bleeding disorders may also interfere with clotting assays, this information is vital to the management of the disease in patients who may receive these therapies.
This is especially true of patients with hemophilia who are treated with emicizumab. The pharmaceutical company manufacturing emicizumab, Genetech, wasted no time in distributing guidelines and information to the physicians at hemophilia treatment centers and other healthcare professionals. Given in Table 8 is a summary of information they provided, which enlists the common coagulation assays that may be used to monitor patients with COVID-19 associated coagulopathy and if these assays are affected by emicizumab[143].
Table 8.
Coagulation assays used to monitor COVID-19 positive patients that may be affected by emicizumab (adapted from Genetech).
| Analyte/assay | Assay with interference with emicizumab? | Alternatives |
|---|---|---|
| aPTT | Yes (overestimate coagulation potential of emicizumab) | For heparin monitoring: anti Xa assay |
| PT | Yes (weak effect) | No mitigation required (small effect) |
| D-dimer | No | |
| Fibrinogen: Clauss method | No | |
| Fibrinogen: derived | Yes (weak effect) | No mitigation required (small effect); or use Clauss method |
| Protein C: chromogenic | No | |
| Protein C: aPTT-based | Yes (overestimate coagulation potential of emicizumab) | Chromogenic protein C assay |
| Antithrombin activity | No | |
| Anti-Xa activity | No | |
| FVIII activity: aPTT based | Yes (overestimate coagulation potential of emicizumab) | Chromogenic FVIII assay (see below guidance) |
| FVIII activity: chromogenic bovine reagents | No | Does not detect emicizumab, but allows measurement of endogenous or infused FVIII activity |
| FVIII activity: chromogenic human reagents | No | Responsive to emicizumab, but may overestimate clinical hemostatic potential of emicizumab |
For additional information on effects and interferences of emicizumab on coagulation assays, please refer to Adamkewicz et al. Thromb Haemost 2019;119:1084-1093.
6. Conclusion
The hypercoagulable state in COVID-19 is emerging as a major pathological occurrence with serious consequences in mortality and morbidity. It is clear that this derangement in the hemostatic pathways is unlike the one seen in other kinds of infection, sepsis, or ARDS. Strikingly, current reports indiciate clinical manifestations of both widespread microvascular as well as large vessel thrombosis. Of concern are the data suggesting that anticoagulation in itself may not be adequate in preventing these thrombotic events. Accumulating evidence supports the notion that the hypercoagulability of SARS-CoV-2 involves a unique mechanism of thrombo-inflammation triggered by viral infection, originating in the pulmonary vasculature. A better understanding of this pathophysiology will allow for the development of appropriate therapeutic modalities. Furthermore, the identification of biomarkers of thrombosis and severe illness can guide clinicians on early interventional strategies and focus healthcare resources towards the group of patients at risk for worse outcomes. The significant number of studies listed on ClinicalTrials.gov is very reassuring and physicians eagerly await the results of these investigations.
Declaration of compering interest
The authors report no conflicts of interest.
Author contributions
MA, AD, SK, YA, LN all contributed to the writing of the manuscript. MA illustrated the pathophysiology illustration. LN mentored and supervised the manuscript.
Acknowledgements
LN's work is supported by National Heart, Lung, and Blood Institute grants HL142647-01, HL121131-01, and U01 HL143402.
References
- 1.Zhu H., Wei L., Niu P. Glob Health Res Policy. 2020. The novel coronavirus outbreak in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Situation Reports. [Webpage] 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ Available from:
- 3.Coronavirus Disease (COVID-2019): Cases in the U.S. 2020 5/14/2020 5/14/2020; Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html.
- 4.Emami A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1):e35. doi: 10.22037/aaem.v8i1.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi Y. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klok, F., et al., Incidence of thrombotic complications in critically ill ICU patients with COVID-19, (in Thromb Res). [DOI] [PMC free article] [PubMed]
- 12.Llitjos J.F. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14869. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell C.M., Kahwash R. Will Complement Inhibition be the New Target in Treating COVID-19 Related Systemic Thrombosis? Circulation. 2020;141(22):1739–1741. doi: 10.1161/CIRCULATIONAHA.120.047419. [DOI] [PubMed] [Google Scholar]
- 14.Varga Z. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oudkerk, M., et al., Diagnosis, prevention, and treatment of thromboembolic complications in covid-19: report of the National Institute for Public Health of the Netherlands. Radiology. 0(0): p. 201629. [DOI] [PMC free article] [PubMed]
- 16.Cui S. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fogarty H. COVID-19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020;189(6):1044–1049. doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L. D-dimer levels on admission to predict in-hospital mortality in patients with covid-19. J. Thromb. Haemost. 2020;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W.J. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan B.E. Hematologic parameters in patients with COVID-19 infection. Am. J. Hematol. 2020;95(6):E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 21.Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25819. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. 2020;18(6):1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klok F.A. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middeldorp S. Incidence of venous thromboembolism in hospitalized patients with COVID-19. Journal of Thrombosis and Haemostasis. 2020 doi: 10.1111/jth.14888. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han H. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 27.Helms J. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonard-Lorant I. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020:201561. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lodigiani C. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo T. 2020. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oxley T.J. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wichmann D. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann. Intern. Med. 2020;M20-2003 doi: 10.7326/M20-2003. [DOI] [PubMed] [Google Scholar]
- 34.Menter T. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 doi: 10.1111/his.14134. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ackermann M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015432. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang T., Sun L.X., Feng R.E. Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0):E040. doi: 10.3760/cma.j.cn112147-20200311-00312. [DOI] [PubMed] [Google Scholar]
- 37.Tian S. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolivras A. JAAD Case Rep. 2020. Coronavirus (COVID-19) infection-induced chilblains: a case report with histopathologic findings. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guarneri C. Silent COVID-19: what your skin can reveal. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30402-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manalo I.F. A dermatologic manifestation of COVID-19: transient livedo reticularis. J. Am. Acad. Dermatol. 2020 doi: 10.1016/j.jaad.2020.04.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41(0):E006. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PubMed] [Google Scholar]
- 42.Magro C. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su H. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panigada M. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14850. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang T. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranucci M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14854. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson S.P., Darbousset R., Schoenwaelder S.M. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133(9):906–918. doi: 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 48.Esmon C.T. Inflammation and thrombosis. J. Thromb. Haemost. 2003;1(7):1343–1348. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 49.Marongiu F., Grandone E., Barcellona D. Pulmonary thrombosis in 2019-nCoV pneumonia? Journal of Thrombosis and Haemostasis. 2020;18(6):1511–1513. doi: 10.1111/jth.14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciceri F. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020 doi: 10.51893/2020.2.pov2. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen G. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin C. China; Clin Infect Dis: 2020. Dysregulation of immune response in patients with COVID-19 in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv. 2020 doi: 10.1101/2020.03.02.20029975. Submitted for publication. [DOI] [Google Scholar]
- 54.Gong J. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. medRxiv. 2020 doi: 10.1101/2020.02.25.20025643. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Escher R., Breakey N., Lammle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monteil V. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harzallah, I., A. Debliquis, and B. Drénou, Lupus anticoagulant is frequent in patients with covid-19, (in J Thromb Haemost). [DOI] [PMC free article] [PubMed]
- 59.Connell N.T., Battinelli E.M., Connors J.M. Coagulopathy of COVID-19 and antiphospholipid antibodies. Journal of Thrombosis and Haemostasis. 2020 doi: 10.1111/jth.14893. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schouwers S.M., Delanghe J.R., Devreese K.M. Lupus anticoagulant (LAC) testing in patients with inflammatory status: does C-reactive protein interfere with LAC test results? Thromb. Res. 2010;125(1):102–104. doi: 10.1016/j.thromres.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Liao M. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. p. 2020.02.23.20026690. [DOI] [PubMed] [Google Scholar]
- 62.Zhou, Z.A.R., Lili and Zhang, Li and Zhong, Jiaxin and Xiao, Yan and Jia, Zhilong and Guo, Li and Yang, Jing and Wang, Chun and Jiang, Shuai and Yang, Donghong and Zhang, Guoliang and Li, Hongru and Chen, Fuhui and Xu, Yu and Chen, Mingwei and Gao, Overly exuberant innate immune response to SARS-CoV-2 infection. Cell Host & Microbe-D-20-00205. [DOI] [PMC free article] [PubMed]
- 63.Zhou Y. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. National Science Review. 2020 doi: 10.1093/nsr/nwaa041. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang D. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv. 2020 doi: 10.1101/2020.03.24.20042655. Submitted for publication. [DOI] [Google Scholar]
- 65.Wen W. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6:31. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giamarellos-Bourboulis E.J. Complex Immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv. 2020 doi: 10.1101/2020.03.27.20045427. (Preprint) [DOI] [Google Scholar]
- 68.McGonagle D. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foley J.H., Conway E.M. Cross talk pathways between coagulation and inflammation. Circ. Res. 2016;118(9):1392–1408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- 70.Wang D. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv. 2020 doi: 10.1101/2020.02.10.20021584. (Preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zuo Y. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11):e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Porto B.N., Stein R.T. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front. Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frangou E. REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A) Ann. Rheum. Dis. 2019;78(2):238–248. doi: 10.1136/annrheumdis-2018-213181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fuchs T.A. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. U. S. A. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barnes B.J. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020:217(6). doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang Y. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect. 2018;7(1):77. doi: 10.1038/s41426-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun S. Inhibition of complement activation alleviates acute lung injury induced by highly pathogenic avian influenza H5N1 virus infection. Am. J. Respir. Cell Mol. Biol. 2013;49(2):221–230. doi: 10.1165/rcmb.2012-0428OC. [DOI] [PubMed] [Google Scholar]
- 79.Song W.-C., FitzGerald G.A. COVID-19, microangiopathy, hemostatic activation, and complement. The Journal of Clinical Investigation. 2020 doi: 10.1172/JCI140183. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yao X.H. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49(0):E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 81.Fox S.E. Pulmonary and cardiac pathology in covid-19: the first autopsy series from New Orleans. medRxiv. 2020 doi: 10.1101/2020.04.06.20050575. (Preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao T. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv. 2020 doi: 10.1101/2020.03.29.20041962. (Preprint) [DOI] [Google Scholar]
- 83.Zhang X. Regulation of Toll-like receptor–mediated inflammatory response by complement in vivo. Blood. 2007:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vrigkou E. The evolving role of the renin–angiotensin system in ARDS. Crit. Care. 2017;21(1):329. doi: 10.1186/s13054-017-1917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang H., Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit. Care. 2017;21(1):305. doi: 10.1186/s13054-017-1882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zores F., Rebeaud M.E. COVID and the Renin-Angiotensin System: Are Hypertension or Its Treatments Deleterious? Frontiers in Cardiovascular Medicine. 2020;7:71. doi: 10.3389/fcvm.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Itoyama S. ACE1 polymorphism and progression of SARS. Biochem Biophys Res Commun. 2004:1124–1129. doi: 10.1016/j.bbrc.2004.08.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang F. Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients. Nat Commun. 2014;5:3595. doi: 10.1038/ncomms4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tikellis C., Thomas M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012;2012 doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vaughan D.E., Lazos S.A., Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J. Clin. Invest. 1995;95(3):995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakamura S. Plasminogen activator inhibitor-1 expression is regulated by the angiotensin type 1 receptor in vivo. Kidney Int. 2000;58(1):251–259. doi: 10.1046/j.1523-1755.2000.00160.x. [DOI] [PubMed] [Google Scholar]
- 93.Li X. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deng Y. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin. Med. J. 2020;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu Z. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan L. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu H. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meng J. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou Y. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis containing 8252 patients. Clin. Chim. Acta. 2018;479:181–189. doi: 10.1016/j.cca.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 100.Benschop R.J., Rodriguez-Feuuerhahn M., Schedlowski M. Catecholamine-induced leukocytosis: Early observations, current research, and future directions. Brain Behav. Immun. 1996;10(2):77–91. doi: 10.1006/brbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- 101.Onsrud M., Thorsby E. Influence of in vivo hydrocortisone on some human blood lymphocyte subpopulations. I. Effect on natural killer cell activity. Scand. J. Immunol. 1981;13(6):573–579. doi: 10.1111/j.1365-3083.1981.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 102.Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 103.Yang A.-P. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Long L. Short-term Outcomes of Coronavirus Disease 2019 and Risk Factors for Progression. Eur. Respir. J. 2020;55(5):2000990. doi: 10.1183/13993003.00990-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Amgalan A., Othman M. Exploring possible mechanisms for COVID-19 induced thrombocytopenia: Unanswered questions. J. Thromb. Haemost. 2020;18(6):1514–1516. doi: 10.1111/jth.14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goeijenbier M. Review: viral infections and mechanisms of thrombosis and bleeding†. J Med Virol. 2012:1680–1696. doi: 10.1002/jmv.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goyal P. Clinical characteristics of covid-19 in New York City. N. Engl. J. Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9(1):687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen N. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Terpos E. Hematological findings and complications of COVID-19. American Journal of Hematology. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lodigiani C. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wada H. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J. Thromb. Haemost. 2013;11(4):761–767. doi: 10.1111/jth.12155. [DOI] [PubMed] [Google Scholar]
- 113.Taylor F.B., Jr. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 114.Levi M. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br. J. Haematol. 2009;145(1):24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 115.Di Nisio M. Diagnosis and treatment of disseminated intravascular coagulation: guidelines of the Italian Society for Haemostasis and Thrombosis (SISET) Thromb. Res. 2012;129(5):e177–e184. doi: 10.1016/j.thromres.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 116.Agnes Y.Y., Lee J.M.C., Kreuziger Lisa Baumann, Murphy Mike, Gernsheimer Terry, Lin Yulia. COVID-19 and Coagulopathy: Frequently Asked Questions. [Webpage] 2020. https://www.hematology.org/covid-19/covid-19-and-coagulopathy April 14th, 2020; Version 2.0:Available from:
- 117.Thachil, J., et al., ISTH interim guidance on recognition and management of coagulopathy in COVID-19. Journal of Thrombosis and Haemostasis. n/a(n/a). [DOI] [PMC free article] [PubMed]
- 118.Bikdeli B. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020:27284. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang N. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dixon B. Nebulized heparin reduces levels of pulmonary coagulation activation in acute lung injury. Crit Care. 2010:445. doi: 10.1186/cc9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kreuziger L.B. 2020. COVID-19 and VTE/Anticoagulation: Frequently Asked Questions. [Webpage] April 17, 2020 [cited 2020; Version 2.1] [Google Scholar]
- 122.Lee A. COVID-19 and Pulmonary Embolism: Frequently Asked Questions. [Webpage] 2020. https://www.hematology.org/covid-19/covid-19-and-pulmonary-embolism April 9, 2020; Version 1.0:Available from:
- 123.Hunt B., Retter A., McClintock C. Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19. Thrombosis UK. 20202020 https://b-s-h.org.uk/media/18171/th-and-covid-25-march-2020-final.pdf [Google Scholar]
- 124.Testa, S., et al., Direct oral anticoagulant plasma levels striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents. The Cremona experience. Journal of Thrombosis and Haemostasis. n/a(n/a). [DOI] [PMC free article] [PubMed]
- 125.Liverpool Drug Interactions Group Interactions With Experimental COVID-19 Therapies. 2020. https://www.covid19-druginteractions.org/ April 9th 2020; Available from:
- 126.Anderson D.R. Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N. Engl. J. Med. 2018;378(8):699–707. doi: 10.1056/NEJMoa1712746. [DOI] [PubMed] [Google Scholar]
- 127.Spyropoulos A.C. Modified IMPROVE VTE risk score and elevated D-dimer identify a high venous thromboembolism risk in acutely Ill medical population for extended thromboprophylaxis. TH Open. 2020;4(1):e59–e65. doi: 10.1055/s-0040-1705137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cohoon, K.P., et al., Emergence of institutional antithrombotic protocols for coronavirus 2019. Research and Practice in Thrombosis and Haemostasis. n/a(n/a). [DOI] [PMC free article] [PubMed]
- 129.Hull R.D. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann. Intern. Med. 2010;153(1):8–18. doi: 10.7326/0003-4819-153-1-201007060-00004. [DOI] [PubMed] [Google Scholar]
- 130.Whyte, C.S., et al., Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. Journal of Thrombosis and Haemostasis. n/a(n/a). [DOI] [PMC free article] [PubMed]
- 131.Moore H.B. Is there a role for tissue plasminogen activator (tPA) as a novel treatment for refractory COVID-19 associated acute respiratory distress syndrome (ARDS)? J Trauma Acute Care Surg. 2020;88(6):713–714. doi: 10.1097/TA.0000000000002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14828. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abdelaal Ahmed Mahmoud A. Streptokinase versus unfractionated heparin nebulization in patients with severe acute respiratory distress syndrome (ARDS): a randomized controlled trial with observational controls. J Cardiothorac Vasc Anesth. 2020;34(2):436–443. doi: 10.1053/j.jvca.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 134.Asakura H., Ogawa H. Potential of heparin and nafamostat combination therapy for COVID-19. J. Thromb. Haemost. 2020;18(6):1521–1522. doi: 10.1111/jth.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang R. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect. 2015;4(5) doi: 10.1038/emi.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sciascia S. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19. Clin. Exp. Rheumatol. 2020;38(3):529–532. [PubMed] [Google Scholar]
- 137.Ma J. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol. 2020:108408. doi: 10.1016/j.clim.2020.108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Poterucha T.J., Libby P., Goldhaber S.Z. More than an anticoagulant: do heparins have direct anti-inflammatory effects? Thromb. Haemost. 2017;117(3):437–444. doi: 10.1160/TH16-08-0620. [DOI] [PubMed] [Google Scholar]
- 139.Liu J. Using heparin molecules to manage COVID-2019. Research and Practice in Thrombosis and Haemostasis. 2020;4(4):518–523. doi: 10.1002/rth2.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hayakawa M. Antithrombin supplementation and mortality in sepsis-induced disseminated intravascular coagulation: a multicenter retrospective observational study. Shock. 2016;46(6):623–631. doi: 10.1097/SHK.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 141.Kato T., Matsuura K. Recombinant human soluble thrombomodulin improves mortality in patients with sepsis especially for severe coagulopathy: a retrospective study. Thromb. J. 2018;16(1):19. doi: 10.1186/s12959-018-0172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bernard G.R. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 143.Thachil J. DOACs and ‘newer’ haemophilia therapies in COVID-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14841. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]