Abstract
The evidence that fetal life and early infancy are “critical” or “sensitive” ages for later development of cardiometabolic disease is based on flawed methods for comparing different age periods. Moreover, most previous studies have limited their focus to weight gain, rather than growth in length/height or body mass index (weight (kg)/height (m)2). We undertook a secondary analysis of data from the Promotion of Breastfeeding Intervention Trial (1996–2010), a birth cohort study nested within a large cluster-randomized trial in the Republic of Belarus, that had repeated measurements of weight and length/height taken from birth to 11.5 years of age. We used mixed-effects linear models to analyze associations of changes in standardized weight, length/height, and body mass index during 5 age periods (conception to birth, birth to age 3 months, ages 3–12 months, ages 12 months–6.5 years, and ages 6.5–11.5 years) with fasting glucose, insulin, insulin resistance, β-cell function, and adiponectin at age 11.5 years. We observed strong associations between the metabolic markers and all 3 growth measures, with the largest magnitudes being observed during the latest age period (ages 6.5–11.5 years) and negligible associations during gestation and the first year of life. Later age periods appear more “sensitive” than earlier periods to the adverse metabolic association with rapid growth in childhood.
Keywords: developmental origins of health and disease, growth, metabolic risk, sensitive periods
Abbreviations
- BMI
body mass index
- ELISA
enzyme-linked immunosorbent assay
- HOMA-β
homeostasis model assessment of β-cell function
- HOMA-IR
homeostasis model assessment of insulin resistance
- IQR
interquartile range
- PROBIT
Promotion of Breastfeeding Intervention Trial
Investigators in observational studies have reported associations of environmental exposures during fetal life, infancy, and childhood (including physical growth, infection, socioeconomic factors, and lifestyle behaviors) with later-life chronic disease risk (1–3). Some investigators have attempted to identify “critical” and “sensitive” age periods during which these exposures may influence an outcome. A “critical” age period can be defined as one in which an exposure must occur to influence a later outcome, while a “sensitive” period is one in which an exposure has a larger effect than the same exposure during other periods.
Growth has been the most commonly studied exposure with respect to adult chronic cardiometabolic diseases. In a previous study (4), we examined various approaches to assessing sensitive age periods for the association between blood pressure at age 16 years and earlier weight gain. In that study, adolescence was a more sensitive period for relative weight gain than earlier periods. Other recent studies have obtained similar results (5, 6). We also found that use of relative weight gain standardized by age and sex, or regression-based standardized residuals based on conditional weight, yielded more valid estimates of association with later blood pressure than did absolute weight gain (4).
Most previous studies comparing associations with growth at different ages have focused on weight gain, rather than growth in length/height or changes in body mass index (BMI; weight (kg)/height (m)2). In this study, we examined all 3 growth measures in relation to blood-based biomarkers of metabolic risk measured in preadolescent children. Our aim was to assess which age periods were most strongly associated with metabolic risk and to examine evidence of critical or sensitive age periods.
METHODS
We undertook a secondary observational analysis of data from the Promotion of Breastfeeding Intervention Trial (PROBIT) (7), with repeated anthropometric measures during infancy and childhood. PROBIT is a multicenter, clustered-randomized controlled trial set in the Republic of Belarus (7). PROBIT randomized 31 maternity hospitals and 1 polyclinic (outpatient clinic) affiliated with each hospital to either 1) receive a breastfeeding promotion intervention based on the World Health Organization/United Nations Children’s Fund Baby-Friendly Hospital Initiative or 2) continue practices existing at the time of randomization. A total of 17,046 breastfed newborns and their mothers were recruited during their postpartum stay between June 1996 and December 1997. In addition to being breastfed, trial eligibility criteria for the infants were: being healthy, being a singleton, being born at ≥37 completed weeks of gestation, birth weight ≥2.5 kg, and 5-minute Apgar score ≥5.
Study sample
A total of 13,889 children (82% of the original cohort) attended the follow-up visit at age 6.5 years, and 13,879 (81%) attended the follow-up visit at age 11.5 years. A total of 12,973 (76%) children attended the follow-up visits at both 6.5 years of age (from 2002–2005) and 11.5 years of age (from 2008–2010), and 12,854 (75%) had valid weight and height measurements from all visits made during infancy, at age 6.5 years, and at age 11.5 years. Of the 13,879 children who attended the 11.5-year follow-up visit, 13,545 had blood samples collected. Our study sample therefore comprised 12,581 children (74% of the original cohort) with all valid weight and height measures (the first year and the 6.5- and 11.5-year follow-ups) and blood samples (at the 11.5-year follow-up).
Signed parental consent was obtained at enrollment and at each follow-up, and child assent was obtained at the 11.5-year follow-up. The institutional review board at Montreal Children’s Hospital (Montreal, Quebec, Canada) approved the consent and assent forms, as well as ethical approval for the initial study and all subsequent follow-up.
Anthropometric measurements
PROBIT infants were followed up by their polyclinic pediatricians at ages 1, 2, 3, 6, 9, and 12 months, when measurements of weight and length were obtained as part of routine follow-up clinical practice. At ages 6.5 years and 11.5 years, weight and height were measured at dedicated research clinics using identical equipment models across the polyclinics, with standardized training and quality assurance procedures (8–12). Data on standing height, measured with a wall-mounted stadiometer, and weight, measured on an electronic digital scale (Seca Bella 840 (Seca, Chino, California) for the 6.5-year follow-up visit; Tanita TBF 300GS body fat analyzer (Tanita Corporation, Tokyo, Japan) for the 11.5-year follow-up visit), were obtained in duplicate and averaged (8–12). After completion of these visits, audit visits were conducted in a randomly selected subsample to ensure interobserver reproducibility. Weight or height measurements were considered implausible if they were beyond 4 standard deviations above or below the sex- and age-specific mean, and were excluded (13).
Growth in weight, length/height, and BMI
We assessed child growth during 5 age periods with potential etiological relevance to metabolic risks at age 11.5 years: conception to birth, birth to age 3 months, ages 3–12 months, ages 12 months–6.5 years, and ages 6.5–11.5 years. The postnatal age periods were not arbitrarily chosen. The second and third were derived from a cubic spline-based analysis of the PROBIT cohort demonstrating knots at 3 and 12 months, with linear changes in weight gain between successive knots from birth to age 5 years (14); the last two were based on the timing of the PROBIT follow-ups at 6.5 and 11.5 years.
The follow-up visits at ages 6.5 and 11.5 years occurred within a window of ±3 months, as prescribed in the research protocol (8, 13). Weights and heights at ages 78 months (6.5 years) and 138 months (11.5 years) were then linearly extrapolated from the measurements obtained on the day of follow-up. Weight (height) at 78 months was linearly extrapolated from the weights (heights) measured at the 12-month and 6.5-year follow-up visits; weight (height) at 138 months was linearly extrapolated from the weights (heights) measured at the 6.5-year and 11.5-year follow-up visits.
Comparisons of absolute weight, length/height, or BMI gains in different periods of life will be affected by these underlying differences in growth rates, as well as duration, at different ages and may bias inferences about their relative importance for later outcomes. Standardization of relative changes in weight, length/height, and BMI is necessary to account for different growth velocities and durations of the studied age periods (4). Four different approaches for standardization were discussed and compared in our previous study; the results strongly suggested standardized residuals of conditional weight (or length/height) as the best approach (4). Weight gain was based on age- and sex-specific standardized residuals of conditional weight (4, 15, 16). At a given age (3 months, 12 months, 6.5 years (78 months), or 11.5 years (138 months)), conditional weights were calculated as residuals from sex-stratified linear regression of weight at that age on all prior weights; the regression model also included quadratic terms for prior weights to account for nonlinearity. The conditional weight residual is thus the deviation in a child’s weight from the child’s “expected” weight (i.e., the difference between the observed weight gain over the interval and the gain expected, given all prior weights) (16).
We standardized the conditional weight gain to allow comparisons across ages by dividing it by its standard deviation, estimated from the weight regression model. At birth, sex-specific weight-for-gestational-age z scores were used as a measure of fetal growth, based on an internal (PROBIT sample) standard. These standardized residuals of conditional weight at 3, 12, 78, and 138 months are independent of one another and thus uncorrelated with all prior weights (4, 15, 16), allowing for assessment of the unique contribution of weight gain in each age interval.
Length/height gain was quantified in a similar way to weight gain, that is, based on standardized residuals of conditional length (during infancy) or height (for later periods). At birth, sex-specific length-for-gestational-age z scores were used, again based on the internal (PROBIT sample) standard. Unlike weight and height, BMI does not increase monotonically with age (17). Changes in age- and sex-specific BMI-for-age z scores were therefore used to assess the impact of change in BMI on metabolic measures at age 11.5 years. Sex-specific BMI-for-age z scores were calculated on the basis of an internal (PROBIT sample) standard.
Metabolic risk biomarkers at age 11.5 years
At the 11.5-year follow-up visit, we measured 3 biomarkers reflecting metabolic risk: blood glucose, insulin, and adiponectin. Polyclinic pediatricians collected finger-prick whole-blood samples from the children after an overnight fast. The pediatricians directly measured glucose in 1 freshly expressed blood sample, using the Roche Diagnostics ACCU-CHEK Advantage meter system (Roche, Inc., Basel, Switzerland). The remaining samples were collected as 10 blood spots, each 10 mm in diameter, on specially manufactured filter paper. The filter-paper blood spots were left to air-dry and then stored in freezers at each of the 31 polyclinic sites at −20°C until transport to the laboratory at the National Mother and Child Centre in Minsk, Belarus, where they were stored at −80°C for a median of 18.6 (interquartile range (IQR), 13.8–22.2) months (9, 10). Insulin and adiponectin levels were assayed from the dried blood spots after a single thaw.
Standard operating laboratory procedures, training, and quality assurance ensured high-quality measurements from the dried blood spots, as reported previously in detail (9, 10). These included the use of whole blood standards for insulin, an enzyme-linked immunosorbent assay (ELISA) procedure for adiponectin that had been previously validated on dried blood spots, internal quality control samples, and verification of stability for 30 months at −80°C (10, 12).
Insulin was quantified from the dried blood spots using a commercial kit (Mercodia Insulin ELISA 10-1113-01; Mercodia AB, Uppsala, Sweden). We performed homeostasis model assessment of insulin resistance (HOMA-IR) and homeostasis model assessment of β-cell function (HOMA-β) using the following equations (18):
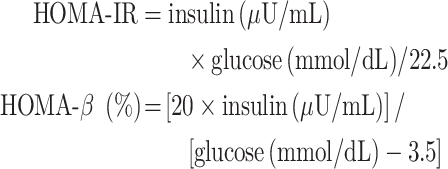 |
Adiponectin concentration, a marker of insulin sensitivity, was quantified using an assay kit (Human Adiponectin ELISA EIA-4177; DRG International, Inc., Springfield, New Jersey). Apolipoprotein A1 and apolipoprotein B measurements were also attempted as markers of cardiovascular risk, but, as previously reported (10), they showed unacceptable measurement error; results are therefore not reported here. We did not measure lipids directly, because their stability in dried blood spots has been questioned and measurement from dried blood spots has not been validated (10, 12).
Statistical analysis
Our analysis was based on mixed-effects linear regression models for assessment of the associations between child growth (standardized residuals of conditional weight, standardized residuals of conditional length/height, and changes in BMI z scores) during the studied age periods and 5 blood biomarkers of metabolic risk (fasting glucose, insulin, HOMA-IR, HOMA-β, and adiponectin) at age 11.5 years. All models accounted for clustering within polyclinics by including a random-effect term for polyclinic. All models also adjusted for trial arm and baseline covariates, including sex, maternal age, maternal and paternal height and BMI, maternal and paternal education, and geographical region. Maternal age was categorized as <20 years, 20–34 years, or ≥35 years; maternal and paternal height and weight were reported by the mother at the 6.5-year follow-up visit. Maternal and paternal education at baseline were categorized as “completed university,” “partial university,” “high school,” or “incomplete high school.” Geographical region/urbanicity of residence was defined by 4 categories based on east versus west and urban residence versus rural residence. Tanner pubic hair stage was measured at the 11.5-year follow-up visit. Although it is widely known that puberty stimulates growth in both height and weight, earlier weight gain has also been shown (including a recent report based partly on the PROBIT cohort (19)) to accelerate pubertal development. In other words, faster growth can be either a cause or a consequence of accelerated puberty. We therefore carried out a sensitivity analysis in which we also adjusted for Tanner pubic hair stage.
For weight (and length/height) gains, we included standardized residuals of conditional weight (standardized residuals of conditional length/height) for all ages in a single regression model, since all of the conditional measurements were uncorrelated, and compared the results among the studied age periods. For changes in BMI z scores, we used a series of sequential models: For each age period, the model adjusted for changes in BMI z scores in all preceding age periods, but not in subsequent age periods (4, 20, 21). Previous studies showed that children who did not attend either the 6.5-year follow-up or the 11.5-year follow-up were similar to those who did attend (4, 9, 10). Therefore, we did not impute missing data for those excluded. Data were analyzed using SAS, version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Baseline characteristics of the PROBIT children with complete data who were followed up at both age 6.5 years and age 11.5 years are summarized in Table 1. The median ages at the latter follow-up examinations were 6.5 (IQR, 6.4–6.6) years and 11.6 (IQR, 11.3–11.9) years, respectively. Mean weight, length/height, and BMI for the study sample at birth, age 3 months, age 12 months, age 78 months (6.5 years), and age 138 months (11.5 years), along with rates of weight gain and height gain, are shown in Table 2. As reported previously (4), infants grew fastest in the first 3 months after birth (0.89 kg/month in weight and 3 cm/month in length) and grew at half that rate from age 3 months to age 12 months (0.50 kg/month and 1.7 cm/month, respectively). Height gain rate decreased to 0.7 cm per month from age 12 months to age 6.5 years and further decreased to 0.5 cm per month from age 6.5 years to age 11.5 years. However, weight gain rate was lowest from age 12 months to age 6.5 years (0.18 kg/month) and nearly doubled from age 6.5 years to age 11.5 years (0.31 kg/month). BMI increased with age during the first year, from 12.7 at birth to 16.5 at age 3 months and 18.4 at age 12 months. It then decreased to 15.7 at age 6.5 years before increasing again to 18.3 at age 11.5 years.
Table 1.
Baseline Characteristics of Children Who Attended Both of the Follow-Up Visits at 6.5 and 11.5 Years of Age, Promotion of Breastfeeding Intervention Trial, Republic of Belarus, 1996–2010
| Characteristic | No. | % | Mean (SD) |
|---|---|---|---|
| Place of residence | |||
| East/urban | 4,056 | 32.2 | |
| East/rural | 2,009 | 16.0 | |
| West/urban | 2,998 | 23.8 | |
| West/rural | 3,518 | 28.0 | |
| Maternal age, years | |||
| <20 | 1,683 | 13.4 | |
| 20–34 | 10,368 | 82.4 | |
| ≥35 | 530 | 4.2 | |
| Maternal education | |||
| Completed university | 1,676 | 13.3 | |
| Partial university | 6,493 | 51.6 | |
| High school | 3,969 | 31.6 | |
| Incomplete high school | 443 | 3.5 | |
| Maternal heighta, cm | 164.4 (5.6) | ||
| Maternal BMIa,b | 24.5 (4.4) | ||
| Paternal heighta, cm | 176.1 (6.6) | ||
| Paternal BMIa | 25.7 (3.3) |
Abbreviations: BMI, body mass index; SD, standard deviation.
a Based on height and weight reported by the mother at the 6.5-year visit.
b Weight (kg)/height (m)2.
Table 2.
Anthropometric Characteristicsa of Study Participants at Selected Follow-Up Visits, Promotion of Breastfeeding Intervention Trial, Republic of Belarus, 1996–2010
|
Age,
months |
Anthropometric Characteristic | ||||
|---|---|---|---|---|---|
| Weight, kg | Rate of Weight Gain, kg/month | Length/Height, cm | Rate of Length/Height Gain, cm/month | BMI b | |
| Birth | 3.4 (0.4) | 52.0 (2.2) | 12.7 (1.1) | ||
| 3 | 6.1 (0.7) | 0.89 (0.19) | 61.0 (2.5) | 3.0 (0.8) | 16.5 (1.5) |
| 12 | 10.6 (1.0) | 0.50 (0.10) | 75.9 (2.6) | 1.7 (0.3) | 18.4 (1.5) |
| 78c | 22.6 (3.5) | 0.18 (0.05) | 119.8 (4.9) | 0.7 (0.1) | 15.7 (1.7) |
| 138c | 41.1 (9.3) | 0.31 (0.12) | 149.2 (7.2) | 0.5 (0.1) | 18.3 (3.2) |
Abbreviation: BMI, body mass index.
a Values are presented as mean (standard deviation) unless otherwise specified.
b Weight (kg)/height (m)2.
c Weights and lengths/heights at ages 78 months (6.5 years) and 138 months (11.5 years) were linearly extrapolated on the basis of weights and lengths/heights at the 12-month and 6.5-year follow-up examinations and the 6.5-year and 11.5-year follow-up examinations, respectively.
Parameter estimates for the associations of standardized residuals of conditional weight at birth, age 3 months, age 12 months, age 6.5 years, and age 11.5 years with glucose, insulin, and adiponectin concentrations at age 11.5 years are detailed in Table 3 and summarized visually in Figure 1. As shown in Table 3, similar patterns were observed for the other 2 growth measures, standardized residuals of conditional length/height and change in BMI z scores. For all 5 metabolic risk biomarkers, larger associations were observed for growth during childhood than during fetal life or infancy, with the largest being observed for the latest age period (ages 6.5–11.5 years). Negligible associations were observed at birth and during the first year (birth to age 3 months and ages 3–12 months), except for adiponectin, for which a small but statistically significant positive association was observed for birth weight and birth length (Table 3). All models accounted for clustering and adjusted for trial arm, child sex, maternal and paternal characteristics (maternal and paternal height, BMI, and education), and geographical region. The sensitivity analysis including further adjustment for Tanner pubic hair stage had almost no impact on the results (not shown; data available upon request).
Table 3.
Associationsa of Relative Growth Measures With 5 Metabolic Risk Biomarkers at Age 11.5 Years, Promotion of Breastfeeding Intervention Trial, Republic of Belarus, 1996–2010
| Standardized GrowthMeasurement | Metabolic Risk Biomarker | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose, mg/dL | Insulin, mU/L | HOMA-IR | HOMA-β | Adiponectin, μg/mL | ||||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Standardized residuals of conditional weight | ||||||||||
| Birth | 0.11 | −0.10, 0.32 | −0.07 | −0.18, 0.04 | −0.01 | −0.04, 0.03 | −0.74 | −2.07, 0.59 | 0.19 | 0.05, 0.34 |
| Age 3 months | 0.08 | −0.13, 0.28 | 0.23 | 0.11, 0.34 | 0.07 | 0.03, 0.10 | 2.14 | 0.81, 3.46 | −0.08 | −0.22, 0.07 |
| Age 12 months | 0.16 | −0.04, 0.37 | 0.19 | 0.08, 0.30 | 0.05 | 0.01, 0.09 | 2.13 | 0.81, 3.45 | 0.04 | −0.11, 0.18 |
| Age 6.5 years | 0.76 | 0.55, 0.97 | 1.09 | 0.97, 1.20 | 0.28 | 0.24, 0.32 | 10.91 | 9.56, 12.27 | −0.55 | −0.70, −0.40 |
| Age 11.5 years | 0.94 | 0.73, 1.14 | 1.31 | 1.20, 1.42 | 0.31 | 0.28, 0.35 | 13.24 | 11.93, 14.55 | −0.82 | −0.97, −0.68 |
| Standardized residuals of conditional length/height |
||||||||||
| Birth | −0.11 | −0.29, 0.08 | −0.11 | −0.21, 0.00 | −0.02 | −0.05, 0.01 | −0.68 | −1.89, 0.52 | 0.22 | 0.09, 0.35 |
| Age 3 months | 0.00 | −0.22, 0.21 | 0.13 | 0.01, 0.25 | 0.04 | 0.00, 0.08 | 1.38 | 0.01, 2.76 | −0.11 | −0.26, 0.04 |
| Age 12 months | 0.31 | 0.10, 0.52 | 0.26 | 0.15, 0.38 | 0.08 | 0.04, 0.11 | 2.96 | 1.61, 4.31 | −0.09 | −0.24, 0.06 |
| Age 6.5 years | 0.45 | 0.24, 0.67 | 0.65 | 0.53, 0.77 | 0.16 | 0.12, 0.20 | 6.58 | 5.17, 8.00 | −0.19 | −0.35, −0.04 |
| Age 11.5 years | 0.87 | 0.66, 1.07 | 0.88 | 0.77, 1.00 | 0.21 | 0.17, 0.25 | 8.73 | 7.40, 10.07 | −0.49 | −0.63, −0.34 |
| Change in BMIbz score | ||||||||||
| Birth | 0.16 | −0.05, 0.36 | −0.04 | −0.16, 0.08 | −0.01 | 0.04, 0.03 | −0.76 | −2.10, 0.57 | 0.03 | −0.12, 0.17 |
| Birth to age 3 months | 0.25 | −0.01, 0.51 | 0.12 | 0.00, 0.24 | 0.04 | 0.00, 0.08 | 0.98 | −0.41, 2.37 | 0.01 | −0.14, 0.17 |
| Ages 3–12 months | −0.06 | −0.28, 0.16 | 0.00 | −0.13, 0.12 | −0.01 | −0.05, 0.03 | −0.07 | −1.51, 1.36 | 0.15 | −0.01, 0.30 |
| Ages 1–6.5 years | 0.74 | 0.52, 0.96 | 0.99 | 0.86, 1.11 | 0.26 | 0.22, 0.30 | 9.71 | 8.28, 11.14 | −0.52 | −0.67, −0.36 |
| Ages 6.5–11.5 years | 1.03 | 0.76, 1.31 | 1.67 | 1.51, 1.82 | 0.40 | 0.35, 0.45 | 17.14 | 15.36, 18.91 | −1.04 | −1.23, −0.84 |
Abbreviations: BMI, body mass index; CI, confidence interval; HOMA-β, homeostasis model assessment of β-cell function; HOMA-IR, homeostasis model assessment of insulin resistance.
a Estimated associations per standard-deviation increase in conditional weight, conditional length/height, and change in BMI, with adjustment for trial arm, child sex, maternal and paternal characteristics (maternal and paternal height, BMI, and education), and geographical region.
b Weight (kg)/height (m)2.
Figure 1.
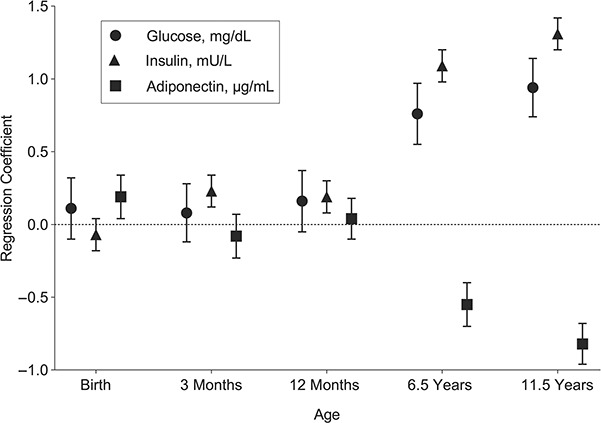
Estimated changes in glucose, insulin, and adiponectin concentrations per standard-deviation increase in standardized residuals of conditional weight at age 11.5 years, Promotion of Breastfeeding Intervention Trial, Belarus, 1996–2010. Results accounted for clustering and were adjusted for trial arm, child sex, maternal and paternal characteristics (maternal and paternal height, body mass index, and education), and geographical region. Bars, 95% confidence intervals.
DISCUSSION
For all 5 metabolic risk biomarkers (glucose, insulin, HOMA-IR, HOMA-β, and adiponectin), growth during childhood appears to be a more sensitive period for metabolic risk biomarkers measured in preadolescence, with magnitudes of association increasing with advancing age. The increase with age in the magnitude of positive associations of all 3 growth measures with glucose and insulin concentrations and both indices of insulin resistance, and the corresponding increase in the magnitude of negative associations with adiponectin concentration, all point in the same direction: worsening metabolic function with later increases in infant and child growth. These results are also consistent with those we previously reported for weight gain and systolic and diastolic blood pressures at age 16 years (4).
Most previous studies of critical or sensitive periods have focused on fetal life and infancy. Birth weight for gestational age and weight gain in early infancy have been previously associated with adiposity, obesity, blood pressure, and other metabolic outcomes later in childhood or adulthood (15, 16, 20, 22–24), yet investigators in several recent studies have reported that older childhood age periods may be more “sensitive” to weight gain (4–6). Few studies, however, have compared the magnitude of association during fetal life or infancy with that in older age periods. Moreover, few have studied growth in length/height or changes in BMI, and even fewer have obtained data on circulating biomarkers of metabolic risk (5, 6). It is important to point out that the association of faster length/height gain may not reflect a causal effect of growth in stature on metabolic risk. As we recently reported (25), faster length/height gain may itself be a consequence of faster weight gain, although final adult height may not be affected.
We observed that the age interval 6.5–11.5 years—that is, the period immediately preceding the age at which we measured metabolic risk biomarkers in blood—was more “sensitive” to growth in weight, length/height, and BMI than earlier age periods. The measurement of growth (in weight, height, and BMI) gain at each age is the deviation of each child’s measurement from his or her “expected” measurement, based on sex and size at the previous age, not the instantaneous weight velocity at that age. In our previous study (4), we demonstrated a pattern of increasing association with age between weight (length/height) gain and blood pressure measured at age 16 years. Our new finding in the present paper is consistent with those reported in several other studies (4–6) in observing that weight gain during later age periods is more strongly associated with later metabolic health. Moreover, we also confirmed recent findings from our own (4) and other (14–16) studies that standardization of relative changes in growth measures by age and sex provides a more valid demonstration of graded, “dose-response” associations with later metabolic outcomes than absolute change in weight, length, or BMI.
Strengths of our study include its multicenter, longitudinal (birth cohort) design, the large sample size, the high rate of follow-up, frequent measurements of weight and length/height at birth and throughout infancy, research-standard anthropometric measurements taken at ages 6.5 and 11.5 years, fasting biomarker studies at age 11.5 years, and control for a large number of potentially confounding factors (especially both mother’s and father’s height and BMI). One potential weakness is that, unlike the weights and heights obtained at ages 6.5 and 11.5 years, those obtained during infancy did not necessarily use identical measurement equipment and procedures across the 31 participating polyclinics, which probably increased random measurement error. Our large sample size should have compensated, at least in part, for that weakness. Another potential weakness is that we did not have access to serial fetal ultrasound measurements to model intrauterine growth trajectories. The birth-weight-for-gestational-age z score was used as a measure of overall fetal growth. While we have previously shown that fetal weight-gain trajectory does not add information to birth weight for gestational age in predicting later childhood size (25), the jury is still out with respect to predicting later cardiometabolic outcomes.
Earlier puberty is associated with faster growth during the age interval 6.5–11.5 years and potentially with metabolic risk at age 11.5 years. The differences reflected in the Tanner pubic hair stage we measured at age 11.5 years are likely to have begun months or even a year or two earlier, and thus we cannot rule out a mediating effect of puberty onset on the observed associations between growth between ages 6.5 and 11.5 years and the metabolic risk markers we measured at age 11.5 years. Nonetheless, the very similar results obtained with and without adjustment for Tanner pubic hair stage suggest that puberty is not a potent confounder or mediator.
Since we did not measure the metabolic risk biomarkers until age 11.5 years, we cannot exclude the possibility of reverse causality. In other words, it is possible that metabolic changes reflected by those biomarkers might have been the cause, rather than the consequence, of faster growth, especially in the interval from age 6.5 years to age 11.5 years.
Belarus is a nation of homogeneous ethnicity where childhood obesity is less prevalent than in most Western countries. Although our findings may not be generalizable to the latter settings, their similarity to those reported from other studies (5, 6) suggests that the pattern of associations we observed is robust.
Future follow-up into adulthood of our cohort and other cohorts should shed additional light on the relationships between growth in fetal life, infancy, and childhood and metabolic outcomes later in adult life. If those future studies confirm our findings about later sensitive periods, they have important implications for developing and testing preventive interventions to alter nutrition, physical activity, and other behaviors during later childhood and adulthood—a major challenge for future clinical care and public health.
ACKNOWLEDGMENTS
Author affiliations: Department of Pediatrics, Faculty of Medicine, McGill University, Montreal, Quebec, Canada (Xun Zhang, Michael S. Kramer); Department of Epidemiology, Biostatistics and Occupational Health, Faculty of Medicine, McGill University, Montreal, Quebec, Canada (Michael S. Kramer, Seungmi Yang); Department of Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, United Kingdom (Richard M. Martin); NIHR Bristol Biomedical Research Centre, Bristol, United Kingdom (Richard M. Martin); Division of Chronic Disease Research Across the Lifecourse, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts (Emily Oken); and Department of Obstetrics and Gynecology, School of Medicine, National University of Singapore, Singapore (Izzuddin M. Aris).
This work was supported by a grant from the Canadian Institutes of Health Research (grant 53155). The 11.5-year follow-up of PROBIT was also supported by grants from the European Union, the Early Nutrition Programming—Long-Term Efficacy and Safety Trials (EARNEST) program (grant FOOD-DT-2005-007036), and the US National Institutes of Health (grant R01 HD050758). The NIHR Bristol Biomedical Research Centre is funded by the United Kingdom National Institute for Health Research and is a partnership between the University Hospitals Bristol NHS Foundation Trust and the University of Bristol.
PROBIT is registered as International Standard Randomised Controlled Trial Number 37687716.
The views expressed in this article are those of the authors and not necessarily those of the United Kingdom’s National Health Service, National Institute for Health Research, or Department of Health.
Conflict of interest: none declared.
REFERENCES
- 1. Kuh D, Ben-Shlomo Y, eds. A Life Course Approach to Chronic Disease Epidemiology. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 2. Ben-Shlomo Y, Cooper R, Kuh D. The last two decades of life course epidemiology, and its relevance for research on ageing. Int J Epidemiol. 2016;45(4):973–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2005;26:1–35. [DOI] [PubMed] [Google Scholar]
- 4. Zhang X, Tilling K, Martin RM, et al. . Analysis of “sensitive” periods of fetal and child growth. Int J Epidemiol. 2019;4(1):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perng W, Rifas-Shiman SL, Kramer MS, et al. . Early weight gain, linear growth, and mid-childhood blood pressure: a prospective study in Project Viva. Hypertension. 2016;67(2):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aris IM, Bernard JY, Chen LW, et al. . Postnatal height and adiposity gain, childhood blood pressure and prehypertension risk in an Asian birth cohort. Int J Obes (Lond). 2017;41(7):1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kramer MS, Chalmers B, Hodnett ED, et al. . Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413–420. [DOI] [PubMed] [Google Scholar]
- 8. Kramer MS, Matush L, Vanilovich I, et al. . Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 years: new evidence from a large randomized trial. Am J Clin Nutr. 2007;86(6):1717–1721. [DOI] [PubMed] [Google Scholar]
- 9. Martin RM, Patel R, Kramer MS, et al. . Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years. JAMA. 2013;309(10):1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin RM, Patel R, Kramer MS, et al. . Effects of promoting longer term and exclusive breastfeeding on cardiometabolic risk factors at age 11.5 years: a cluster-randomized, controlled trial. Circulation. 2014;129(3):321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel R, Oken E, Bogdanovich N, et al. . Cohort profile: the Promotion of Breastfeeding Intervention Trial (PROBIT). Int J Epidemiol. 2014;43(3):679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin RM, Patel R, Zinovik A, et al. . Filter paper blood spot enzyme linked immunoassay for insulin and application in the evaluation of determinants of child insulin resistance. PloS ONE. 2012;7(10):e46752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kramer MS, Martin RM, Bogdanovich N, et al. . Is restricted fetal growth associated with later adiposity? Observational analysis of a randomized trial. Am J Clin Nutr. 2014;100(1):176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tilling K, Davies N, Windmeijer F, et al. . Is infant weight associated with childhood blood pressure? Analysis of the Promotion of Breastfeeding Intervention Trial (PROBIT) cohort. Int J Epidemiol. 2011;40(5):1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keijzer-Veen MG, Euser AM, van Montfoort N, et al. . A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol. 2005;58(12):1320–1324. [DOI] [PubMed] [Google Scholar]
- 16. Adair LS, Martorell R, Stein AD, et al. . Size at birth, weight gain in infancy and childhood, and adult blood pressure in 5 low- and middle-income-country cohorts: when does weight gain matter? Am J Clin Nutr. 2009;89(5):1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO Multicentre Growth Reference Study Group WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;475:76–85. [DOI] [PubMed] [Google Scholar]
- 18. Matthews DR, Hosker JP, Rudenski AS, et al. . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 19. Aris IM, Rifas-Shiman SL, Zhang X, et al. . Association of BMI with linear growth and pubertal development. Obesity. 2019;27(10):1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tilling K, Howe LD, Ben-Shlomo Y. Commentary: methods for analysing life course influences on health—untangling complex exposures. Int J Epidemiol. 2011;40(1):250–252. [DOI] [PubMed] [Google Scholar]
- 21. Gamborg M, Andersen PK, Baker JL, et al. . Life course path analysis of birth weight, childhood growth, and adult systolic blood pressure. Am J Epidemiol. 2009;169(10):1167–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Law CM, Shiell AW, Newsome CA, et al. . Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105(9):1088–1092. [DOI] [PubMed] [Google Scholar]
- 23. Barker DJP. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93(446):26–33. [DOI] [PubMed] [Google Scholar]
- 24. Singhal A, Cole TJ, Fewtrell M, et al. . Promotion of faster weight gain in infants born small for gestational age: is there an adverse effect on later blood pressure? Circulation. 2007;115(2):213–220. [DOI] [PubMed] [Google Scholar]
- 25. Hutcheon JA, Jacobsen GW, Kramer MS, et al. . Small size at birth or abnormal intrauterine growth trajectory: which matters more for child growth? Am J Epidemiol. 2016;183(12):1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]


