Abstract
Currently, there are no licensed human vaccines or antivirals for treatment of or prevention from infection with encephalitic alphaviruses. Because epidemics are sporadic and unpredictable, and endemic disease is common but rarely diagnosed, it is difficult to identify all populations requiring vaccination; thus, an effective post-exposure treatment method is needed to interrupt ongoing outbreaks. To address this public health need, we have continued development of ML336 to deliver a molecule with prophylactic and therapeutic potential that could be relevant for use in natural epidemics or deliberate release scenario for Venezuelan equine encephalitis virus (VEEV). We report findings from in vitro assessments of four analogs of ML336, and in vivo screening of three of these new derivatives, BDGR-4, BDGR-69 and BDGR-70. The optimal dosing for maximal protection was observed at 12.5 mg/kg/day, twice daily for 8 days. BDGR-4 was tested further for prophylactic and therapeutic efficacy in mice challenged with VEEV Trinidad Donkey (TrD). Mice challenged with VEEV TrD showed 100% and 90% protection from lethal disease when treated at 24 and 48 hours post-infection, respectively. We also measured 90% protection for BDGR-4 in mice challenged with Eastern equine encephalitis virus. In additional assessments of BDGR-4 in mice alone, we observed no appreciable toxicity as evaluated by clinical chemistry indicators up to a dose of 25 mg/kg/day over 4 days. In these same mice, we observed no induction of interferon. Lastly, the resistance of VEEV to BDGR-4 was evaluated by next-generation sequencing which revealed specific mutations in nsP4, the viral polymerase.
Keywords: New World alphaviruses, antiviral efficacy, ML336, Venezuelan Equine Encephalitis Virus, Eastern Equine Encephalitis Virus, drug resistance
1. Introduction
Viruses in the family, Togaviridae, genus, Alphavirus, cause illness that may progress to encephalomyelitis in humans (Calisher, 1994; Knipe and Howley, 2013; Weaver et al., 2012). These viruses include Venezuelan (VEEV), Eastern (EEEV) and Western equine encephalitis viruses (WEEV). These viruses have been recognized since the 1930’s when they were associated with outbreaks in the North and South America in horses and humans. The recognition of disease in humans often follows an epizootic course in domestic or wild animals. VEEV has been known to affect hundreds of thousands of people in the Americas over the past century. An estimated 70,000 – 100,000 humans, and similar numbers of equines, infected with VEEV were reported during the last outbreak in 1995, including hundreds of fatalities and still-births along with thousands of survivors with neurologic sequelae (Rivas et al., 1997). Serious human disease associated with EEEV is primarily associated with isolates circulating in North America although more recently cases have been reported in Latin America (Carrera et al., 2013; Sabattini et al., 1991). CDC estimates that only 4–5% of human EEEV infections lead to clinical disease resulting in approximately seven human cases of EEEV each year of which approximately 41% are fatal (Lindsey et al., 2018). Most of the cases show neuroinvasive disease and occur in the eastern part of the US. Physical and mental sequelae are prominent in EEE survivors and range from minimal brain dysfunction to severe intellectual impairment, personality disorders, seizures, paralysis, and cranial nerve dysfunction.
Currently, there are no licensed vaccines or antivirals for treatment or prevention of V/E/WEEV infection and disease. Because epidemics of V/E/WEEV are sporadic and unpredictable, and endemic disease is common (estimated 10,000 cases annually in the Americas) but rarely diagnosed (Aguilar et al., 2011), it is difficult to identify all populations requiring vaccination; thus, an effective post-exposure treatment method is needed to interrupt ongoing outbreaks. To address this public health need, we have been active in the development of novel small molecule antivirals that could be relevant for use both in natural epidemics of New World alphaviruses as well as a deliberate release scenario (Chung et al., 2010; Chung et al., 2016; Chung et al., 2014).
ML336 is a benzamidine that emerged as part of a medicinal chemistry campaign focused on VEEV antivirals (Schroeder et al., 2014). ML336 analogs were prepared to better understand the structure-activity and structure-property relationships associated with the chemical series. Herein we report the evaluation of BDGR-4 and other new derivatives of ML336 (Schroeder et al., 2014) for inhibitory activity against wild type strains of VEEV and EEEV in vitro and in vivo. Of the new derivatives advanced for animal studies, BDGR-4 shows excellent efficacy for therapeutic and prophylactic treatment of VEEV and prophylactic treatment of EEEV in mice.
2. Material and Methods
2.1. Cells and viruses
EEEV (strain FL93–939) was obtained from WRCEVA (Galveston, TX), VEEV strain Trinidad Donkey (TrD) came from BEI (item #: NR-332) and VEEV TC-83 (lyophilized vaccine) was a gift from Dr. Connie Schmaljohn (United States Army Medical Research Institute of Infectious Diseases, MD). WEEV (strain California) was obtained from ATCC (VR-70). Virus seed stocks were amplified in Vero76 cells or BHK-21 in Dulbecco’s modified essential media (DMEM) or Minimum Essential Media with Earle’s modification (MEM-E) with 10% fetal bovine serum (FBS) and clarified by low speed centrifugation. For studies in mice, seed stock virus as diluted to a concentration of 3.33×108 pfu/ml (1×107 pfu/0.03 ml) in Phosphate Buffered Saline PBS (HyClone).
2.2. Chemistry
Syntheses of ML336 and BDGR-4 have been previously described (Schroeder et al., 2014). Previously undescribed analogs used in this work include BDGR-5, BDGR-69 and BDGR-70, the syntheses and characterization of which are included in the supplementary information. All final compounds were purified after synthesis, characterized by 1H and 13C NMR, high-resolution mass spectroscopy, and analyzed for purity by UV-LCMS (> 95%). Chiral compounds were analyzed by chiral HPLC to determine enantiomeric excess.
2.3. In vitro antiviral assays
The 50% effective concentration (EC50), titer-reduction activity, and cytotoxicity were measured as described previously (Chung et al., 2014). Briefly, measurement of the EC50 used Vero 76 cells that were seeded and cultured in white, solid, 96-well plates overnight. The compounds were diluted in cell culture media (2-fold, 8 points, serially diluted) was added to each well. Two hours later, the cells were infected with virus at an MOI of 0.05. The infected plates were incubated for 48 h and the cell viability, protected from virus-induced cytopathic effect, was measured with Cell Titer-Glo (Promega). All experiments were replicated in three plates and EC50 were calculated from normalized cell viability values from the replicate data using XLfit software (IBDS) with Module 204, 4-parameter logistic model. Cytotoxicity was conducted in parallel with cell culture media in place of virus.
For titer reduction assays, Vero76 cells were grown in 12-well plates overnight and then infected with virus at a MOI of 0.05 diluted in MEM-E, 10% FBS, l-glutamine, and 12.5 mM HEPES. After one hour of adsorption on ice, cells were washed with PBS twice and media containing 5 μM of compound was added to each well. After an 18 hour of incubation in an incubator at 37 °C with 5% CO2, the cell culture supernatant was removed and stored at −80 °C. Virus titer was determined by with a virus infection center assay as previously described (Adcock et al., 2017). Briefly, we made serial dilutions of supernatant taken from treatments and infected confluent Vero76 cells grown in 24-well plates. We added a semisolid overlay media (cell culture media with 0.75% methylcellulose). Virus infection centers were visualized with 0.2% crystal violet in 4% paraformaldehyde and 10% ethanol.
Virus titers in brain were measured by plaque assay or 50% tissue culture infectious dose (TCID50) assay. Briefly, brain was homogenized with tissue homogenizer (Omni International) on ice in the virus culture media (EMEM supplemented with 2% of heat-inactivated FBS, 2mM L-glutamine, and 1% penicillin/streptomycin) to be 10% w/v, centrifuged, and then kept clarified supernatant at −80C freezer until use. For plaque assay, upon reaching more than 90% confluence of Vero 76 cells grown overnight on 6 well tissue culture plates, growth media was removed and 100 μl of 10-fold virus dilutions from 101 to 108 were added in a duplicate format and incubated for 1 h, with rocking every 15 min to allow virus adsorption. After 1h adsorption, cells were washed twice with DPBS, and 2 ml overlay consisting 1.2 % Avicel in virus culture media was added, and the plates were incubated at 37 °C for 72 h. After incubation, cells were fixed for 15 minutes by adding 1 ml of 10% buffered formalin and then removed overlay in a waste container. Fixed cells were stained with 0.2% crystal violet for 15 minutes. For the TCID50 assay, cells were grown to more than 90% confluence of Vero 76 cells in 96 well tissue culture plates, growth media was removed and 100 μl of 10-fold virus dilutions from 101 to 108 in the virus culture media were added in a quadruplicate format. After 72 h incubation, infected cells were observed for CPE under the 40X microscope followed by performing MTS based cell viability assay (Promega) according to manufacturer’s guidance. TCID50 titers were decided by the method of Reed and Muench (Reed and Muench, 1938).
EEEV titers in tissues or plasma were assayed using an infectious cell culture assay where a specific volume of either tissue homogenate or serum was added to the first tube of a series of dilution tubes. Serial dilutions were made and added to Vero cells. Three days later cytopathic effect (CPE) was used to identify the end-point of infection. Four replicates were used to calculate the 50% cell culture infectious doses (CCID50) per mL of plasma or gram of tissues.
2.4. Mouse efficacy studies
All groups of mice were randomly assigned to experimental groups and marked with ear tags. In all studies, mice were monitored for mortality and disease signs until 21 days post-virus inoculation (dpi). Individual weights were recorded daily 0–7 dpi. The VEEV studies conducted at the University of Louisville were conducted in accordance with the approval of the Institutional Animal Care and Use Committee (IACUC) protocol #16644. The EEEV study conducted at Utah State University (USU) was conducted in accordance with the approval of the IACUC of USU (Protocol #10081). The work was done in the AAALAC-accredited USTAR Laboratory facility of USU. The National Institutes of Health approval for USU and UofL are PHS Assurance no. D16–00468 and PHS Assurance no. A3586–01 in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Revision; 2015).
TC-83 Screening.
The in vivo efficacy of BDGR-4, 69 and 70 compounds were screened at 25 mg/kg/day in approximately 5–6-week-old C3H/HeN mice (Charles River) by challenge with 1 × 107 pfu VEEV TC-83. Compounds were formulated in 21.4% PEG400/ 8.6% Kolliphor RH40/ 70% Water. On day 0, mice were given the first dose via intraperitoneal (IP) injection approximately 2 h before intranasal (IN) virus challenge. A second dose was administered approximately 6 hours post-challenge. Dosing continued twice daily (BID, 12 h) for five days. Virus titers in mouse brains were measured using TCID50 or plaque assay as described above.
TrD Screening.
The in vivo efficacy of BDGR-4 and ML336 compounds were screened with 25 mg/kg/day in 5–6-week-old BALB/c mice (Charles River) by challenge with VEEV TrD administered by the subcutaneous route. Dosing by IP occurred every 12 h for 8 days starting on Day 0. The first dose was administered at 2 h prior to virus challenge. On Day 4 following virus challenge, a subset of mice from each group were humanely euthanized via CO2 asphyxiation followed by cervical dislocation. The brain from each mouse was collected, placed into individual pre-labeled tubes, and snap frozen in liquid nitrogen. Virus titers in mouse brains were measured using TCID50 or plaque assay as described above.
EEEV screening.
C57BL/6 mice, supplied by Jackson Laboratories, were used. Mice (n=15) were administered initial doses of BDGR-4 at 2 h prior to virus challenge via i.p. injection in a volume of 0.1 mL. Mice were challenged with 104.3 CCID50/0.2 mL EEEV strain FL93–939, which was administered via bilateral s.c. injection (0.1 mL per injection) in the inguinal fold on each side of the abdomen. Placebo, sham, and untreated and uninfected normal control groups were included in this study. Compounds were provided in powder form and were diluted to the appropriate concentration in 25% Kollisolv PEG400, 10% Kolliphor RH40 and 65% water with intermediate vortexing. Mice were treated with BDGR-4 twice daily for 8 days. On 6 dpi, 5 mice from groups treated with the highest doses of BDGR-4 or placebo, as well as from the placebo-treated, sham-infected group were necropsied and brain tissues were collected for virus titration via infectious cell culture assay. Serum was collected from all surviving mice on 21 dpi for assessment of neutralizing antibodies by PRNT assay.
2.5. Quantification of neutralizing antibody in EEEV-infected mice
Neutralizing antibody was quantified using a 50% plaque reduction neutralization titer (PRNT50) assay. Serum samples were heat inactivated at 56°C for 30 minutes in a water bath. One half serial dilutions, starting at a 1/10 dilution, of test sera were made. Dilutions were then mixed 1:1 with an appropriate titer of EEEV in MEM containing 2% fetal bovine serum (FBS) and incubated at 4°C overnight. The virus-serum mixture was then added to individual wells of a 12-well tissue culture plate with Vero76 cells (4 × 105 cells/well). Viral adsorption proceeded for one hour at 37°C and 5% CO2, followed by addition of 1.7% (4000cps) methylcellulose overlay medium containing 10% FBS to each well. Plates were incubated for 4 days, then stained with crystal violet (with 1% (wt/vol) crystal violet in 10% (vol/vol) ethanol) for 20 min. The reciprocal of the dilution of test serum that resulted in ≥50% reduction in average plaques from virus control was recorded as the PRNT50 value.
2.5. Drug resistance profiling
BDGR-4-resistant viruses were selected by blind passaging VEEV TC-83 in the presence of the compound 50 nM (Passage 0) and ending at 3200 nM (Passage 7) in six-well plates. Drug concentration was increased by two-fold at each new passage. Cells were infected at an initial MOI of 1 in Eagle’s Minimum Essential Medium supplemented with 2% FBS and 5 mM L-glutamine (Corning CellGro). At each passage, three replicates were made for each drug concentration. VEEV TC-83 was also passaged without compound in triplicate. To sequence viruses at each passage, the supernatant from each passage was harvested and RNA was isolated using Trizol LS (Invitrogen). A tiling approach was used to amplify 1 kb fragments of the entire genome which overlapped each other by 500 bp. cDNA was synthesized from purified RNA using random hexamer primers and Superscript IV (Thermofisher). cDNA was amplified through 30 rounds of PCR, using the Phusion High-Fidelity Kit (Thermofisher F553L) and a set of 25 primer pairs to doubly cover the whole genome, making PCR fragments either 500 bp or 1 kb in length. PCR products were purified using the Wizard SV Gel and PCR Clean-Up System (Promega A9282). Libraries for next-generation sequencing were prepared using the Nextera XT kit (Illumina), then sequenced on either the Illumina MiSeq or NextSeq 500. Data was analyzed using CLC Genomics Workbench v.10 (Qiagen). We filtered reads with a Phred score < 20, mapped reads to the seed stock consensus sequence, and identified variants that appeared above 1% in all reads at positions with a coverage of at least 400.
2.6. Mouse studies for Type I IFNs and clinical chemistry profiles
BALB/c mice were injected IP with a 25 mg/kg of BDGR-4 BID for 4 days and the expression of Type I IFNs in the brain and serum and a clinical chemistry panel were measured. To measure IFN, we used the ProcartaPlex™ multiplexed immunoassay (Affymetrix eBioscience, CA) according to manufacturer’s instructions. Cytokine standards were prepared to determine the concentration of cytokine in the samples and the samples were analyzed on a MagPix (Luminex Corp.). Data analysis was performed using the xPONENT software. A curve fit was applied to the standards and the sample concentrations extrapolated from the standard curve using five-parameter logistic method. Clinical chemistry analysis was performed on the serum using a DiaSys respons® 910 Vet analyzer (DiaSys Diagnostic Systems, USA, Wixom, MI). Machine was calibrated, and controls run prior to sample analysis.
2.7. Statistical analyses
P values were generated from comparisons of survival data using the Log-Rank (Mantel-Cox) test using Prism 6 (Graph Pad Software, Inc) and compared using the Bonferroni method. P values will be calculated for each group (K=8). Analyses of each P value generated by the Mantel-Cox test were evaluated by comparison to a Bonferroni corrected threshold of 0.0125 (p=0.05) to determine significance.
3. Results and Discussion
3.1. Chemical synthesis and in vitro characterization of ML336 analogues, BDGR-4, BDGR-5, BDGR-69 and BDGR-70.
BDGR-4, BDGR-5, BDGR-69, and BDGR-70 (Figure 1) were synthesized as described in detail in the Supplemental Information. The inhibitory activity of each compound was tested in vitro using a cytopathic effect (CPE) assay and titer reduction assay. Both assays used Vero 76 cells that were infected with 0.05 MOI of VEEV TC-83. The endpoint of the CPE assay was taken at 2 days post-infection and the EC50 values represent the mean ± standard deviation from more than three independent experiments of which each experiment was performed with more than 8-concentrations prepared by two-fold serial dilution. The titer reduction assays were determined after 18 hours of incubation (Table 1). The standard deviations reflect the testing completed for several independent syntheses. With ML336 and BDGR-4 in hand, we regionally diversified the parent structure to assess structure-activity and structure-property relationships, yielding 30 amidine analogs. These compounds were prepared to survey structural differences on the phenyl ring core, the N-amide substituent, and augmentations of the amidine ring itself. Most of the compounds showed at least a 10-fold loss in VEEV CPE potency and/or a lackluster demonstration of titer reduction (< log 5), compared to BDGR-4. Resultantly, BDGR-4, -5, -69 and -70 showed the best overall CPE potency and titer reduction, and these were further evaluated for initial physiochemical and ADME determination (Table 1). While ML336 and BDGR-4 were evenly matched in terms of no discernable mammalian cytotoxicity, potent CPE inhibition, robust viral titer reduction, reasonable mouse plasma stability and mouse plasma protein binding, BDGR-4 was >2.5-fold more soluble and showed an 11% improvement in microsomal stability solubility compared to ML336 (Table 1). Given these advancements, BDGR-4, which featured a 4-methoxyphenyl moiety in place of the ML336 N-phenyl ring on the amide group, was used as the benzamidine prototype against which newer benzamidine analogs and other scaffolds were compared. The 4-methoxyphenyl amide moiety that was deemed beneficial in BDGR-4 was incorporated in BDGR-5; however, a methyl group was integrated at the C3 position of the 5-nitrophenyl core. Potent antiviral activity in the in vitro viral assays was observed, but BDGR-5 showed inferior microsomal stability with only 14.6% of the parent remaining after 1 h of exposure. Enantiomers BDGR-69 and BDGR-70, prepared to assess the effect of introducing a chiral center at the alpha-position of the amidine moiety, also offered the possibility of integrating a potentially beneficial methyl group that might occupy a similar region of space as the methyl group of BDGR-5. The structural change was well tolerated in terms of antiviral activity, affording comparable protection from VEEV CPE with EC50 values = 25–28 nM, robust reduction in viral titer (log > 7 for both), and gains in microsomal stability compared to BDGR-5; however, BDGR-4 outperformed these analogs in terms of at least a 26% improvement in mouse microsomal stability. Ultimately, the overall profile of BDGR-4 was shown to be superior to ML336 and the analogs described here.
Figure 1.
Structures of ML336 and related analogs, BDGR-4, BDGR-5, BDGR-69 and BDGR-70.
Table 1.
In vitro ADME and anti-VEEV (TC-83 strain) activity of ML336, BDGR-4, BDGR-5, BDGR-69, and BDGR-70.1
| compound | VEEV EC502 (nM) |
VEEV log titer reduction3 |
PBS solubility4 (mg/mL) |
Microsomal stability5, % parent after 1h |
Plasma stability6, % parent after 3h |
plasma protein binding6, % bound at 1 μM / 10 μM |
|---|---|---|---|---|---|---|
| ML336 | 32 ± 5 | 7.0 ± 0.4 | 40.4 | 42.9 | 65.4 | 85.0 / 77.0 |
| BDGR-4 | 47 ± 21 | > 6.2* | 105.6 | 54.5 | 66.4 | 84.3 / 73.7 |
| BDGR-5 | 29 ± 6 | 5.0 ± 1.8 | > 41 | 14.6 | ND | ND |
| BDGR-69 | 28 ± 7 | 7.2 ± 0.7 | > 41 | 27.7 | ND | ND |
| BDGR-70 | 25 ± 8 | 7.0 ± 0.6 | > 41 | 25.1 | ND | ND |
None of these compounds showed mammalian cytotoxicity (CC50 > 50 μM, VERO76 cells);
50 cytopathic effect (CPE) assay in VERO76 cells;
done at 18 h post infection using 5 μM compound concentration in VERO76 cells;
kinetic solubility in 1xPBS buffer;
mouse microsomes;
mouse plasma;
No progeny virus plaques were detected. ND = not determined.
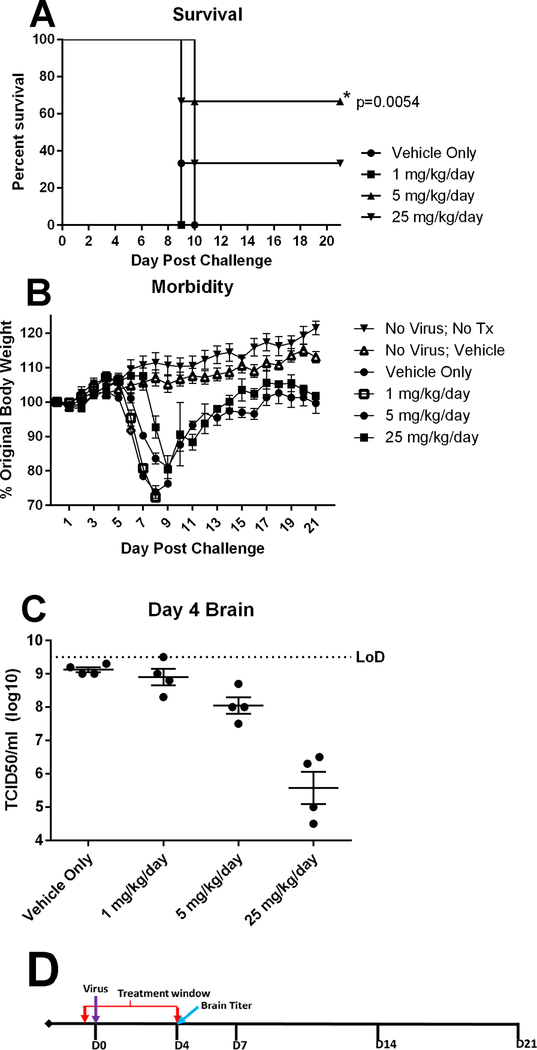
3.2. Prophylactic efficacy of BDGR-4 at several doses BID for 5 days in C3H/HeN mice infected with VEEV TC-83
Based on the in vitro characterization data of the BDGR compounds (Table 1), BDGR-4 was the first compound chosen to examine efficacy over a 21-day period. Based on our prior antiviral efficacy studies of ML336, we chose a range of doses that were higher and lower than the initial assessment at 5 mg/kg/day (Schroeder et al., 2014). The antiviral efficacy was measured for BDGR-4 at 1, 5 and 25 mg/kg/day; compound was administered twice daily (BID) at 12 hours apart. Groups of C3H/HeN mice were treated with BDGR-4 or with placebo beginning 2 hours prior to VEEV TC-83 challenge and continuing for 5 days. Mice were monitored for survival, weight loss and viral titer in brain (Figure 2). The 5 mg/kg/day group protected 4/6 mice while the 25 mg/kg/day protected only 2/6 mice (Figure 2A). This suggested that the dosing and/or formulation was not optimal. Qualitatively, our ability to formulate BDGR-4 was better than ML336 as determined by visual inspection suggesting greater solubility. The recovery of mice by body weight was similar at 5 and 25 mg/kg/day (Figure 2B). Interestingly, the 25 mg/kg/day reduced viral titers in the brain by approximately two logs better that then 5 mg/kg/day dose (Figure 2C). Based on the results of this study, two additional derivatives were chosen for additional testing and evaluation in mice.
Figure 2. Prophylactic efficacy of BDGR-4 administered for 5 days in C3H/HeN mice challenged with intranasal VEEV TC-83.
Groups of six C3H/HeN mice per treatment, vehicle, BDGR-4 at 1, 5 or 25 mg/kg/day, were assessed for (A) survival and (B) morbidity over 21 days following an i.n. challenge with VEEV TC-83. (C) Brains (n = 4) were taken for VEEV TC-83 titer by TCID50 on day 4. (D) Treatments were administered i.p. in a volume of 0.1 ml in 25%PEG400/10%RH40/65%water starting at 2 hours prior to virus infection (D0) and were given BID for 5 days (D0-D4).
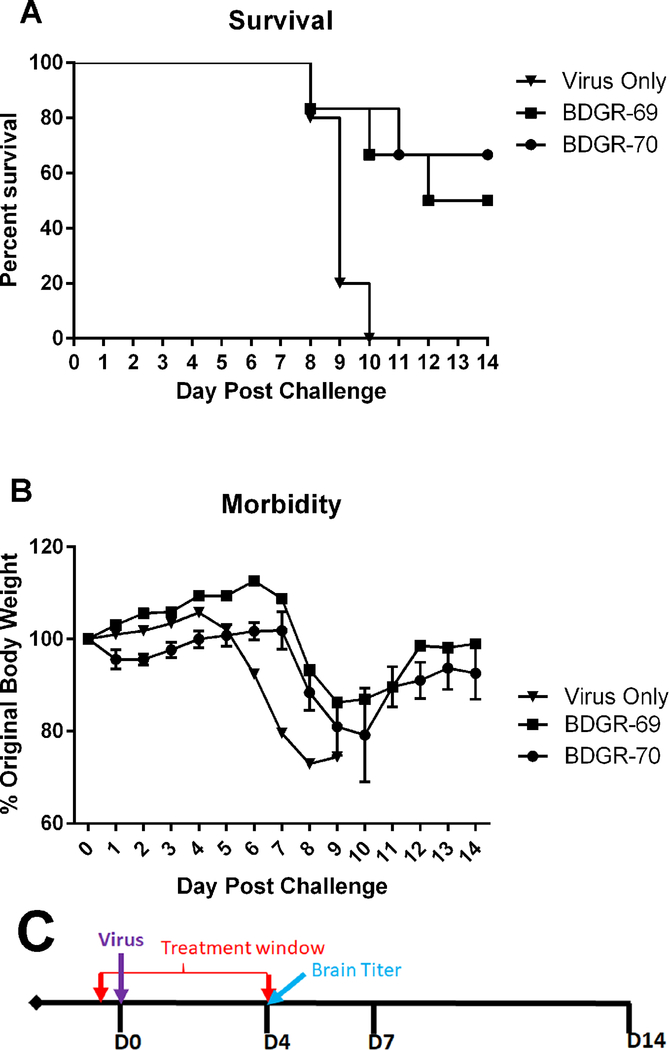
3.3. Prophylactic efficacy of BDGR-69 and BDGR-70 at 50 mg/kg/day BID for 5 days in C3H/HeN mice infected with VEEV TC-83
We screened two additional derivatives, BDGR-69 and BDGR-70, in a mouse efficacy study. Based on the brain titer results observed for BDGR-4 at 25 mg/kg/day (Figure 2C), we selected a higher dose for screening (50 mg/kg/day) and we also adjusted the formulation slightly from 21.4%PEG400/8.6%RH40/70%Water to 25%PEG400/10%RH40/65%Water. This formulation was used in all subsequent animal experiments. Groups of C3H/HeN mice were treated with 25 mg/kg/day BID with BDGR-69 or BDGR-70 or with placebo beginning 2 hours prior to VEEV TC-83 challenge and continuing for 5 days as above. Mice were monitored for survival and weight loss (Figure 3). BDGR-69 and 70 protected 3/6 and 4/6 mice respectively (Figure 3A). The recovery of mice that survived challenge was similar for both compounds as determined by body weight (Figure 3B).
Figure 3. Prophylactic efficacy of BDGR-69 and -70 tested in C3H/HeN mice and challenged intranasally with VEEV TC-83.
Groups of six C3H/HeN mice per treatment, vehicle, BDGR-69 or BDGR-70 at 50 mg/kg/day, were assessed for (A) survival and (B) morbidity over 14 days following a s.c. challenge with VEEV TC-83. (C) Treatments were administered i.p. in a volume of 0.1 ml in 21.4% PEG400/8.6% RH40/70% water starting at 2 hours prior to virus infection (D0) and were given BID for 5 days (D0-D4).
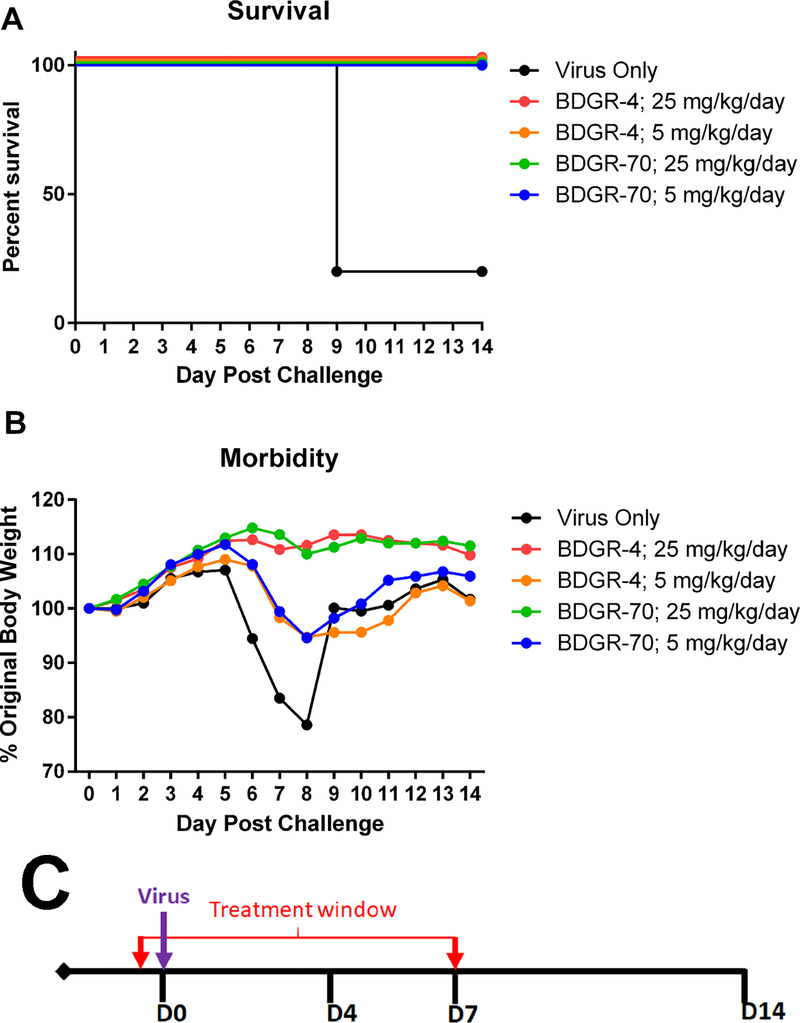
3.4. Prophylactic efficacy of BDGR-4 and BDGR-70 at 5 and 25 mg/kg/day BID for 8 days in C3H/HeN mice infected with VEEV TC-83
Based on the successful partial protection of mice from VEEV TC-83 with BDGR-4 and BDGR-70, we opted to extend dosing through the period when untreated mice begin to succumb to disease (approximately day 8); i.e. an additional 3 days. We posited that with an additional 3 days we could increase protection and reduce viral brain titers. Groups of C3H/HeN mice were treated with 5 or 25 mg/kg/day delivered BID with BDGR-4 or BDGR-70 or with placebo beginning 2 hours prior to VEEV TC-83 challenge and continuing for 8 days. Mice were monitored for survival and weight loss (Figure 4). BDGR-4 and -70 showed complete protection of mice at 25 mg/kg/day (Figure 4A). The recovery of mice that survived challenge was similar for both compounds as determined by body weight (Figure 4B). While both compounds showed similar levels of protection, BDGR-4 demonstrated greater solubility and microsomal stability, and hence this compound was chosen for subsequent evaluations.
Figure 4. Prophylactic efficacy of BDGR-4 and -70 tested in C3H/HeN mice and challenged intranasally with VEEV TC-83.
Groups of six C3H/HeN mice per treatment, vehicle, BDGR-4 or BDGR-70 at 25 mg/kg/day, were assessed for (A) survival and (B) morbidity over 14 days following a s.c. challenge with VEEV TC-83. (C) Treatments were administered i.p. in a volume of 0.1 ml in 25% PEG400/10% RH40/65% water starting at 2 hours prior to virus infection (D0) and were given BID through D7.
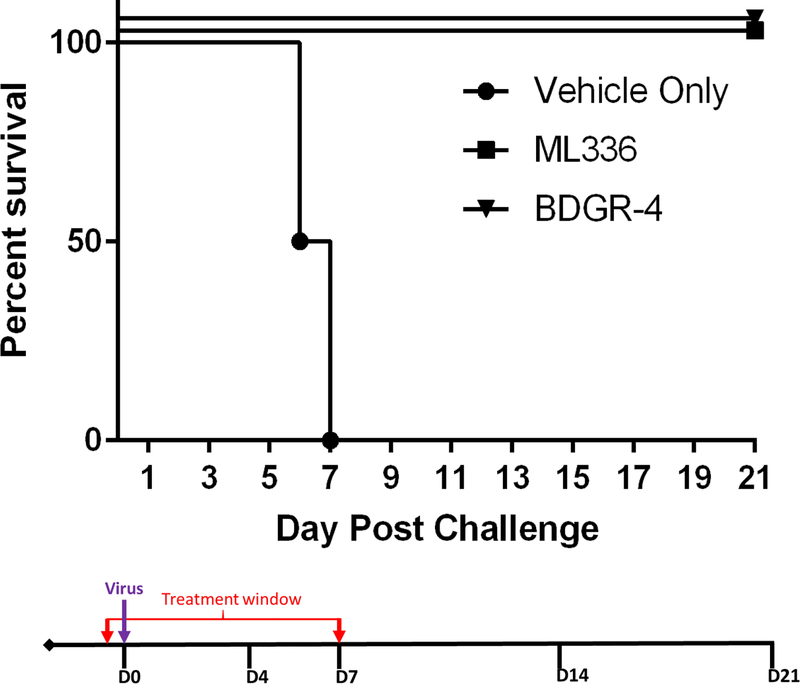
3.5. Prophylactic efficacy of ML336 and BDGR-4 at a single dose in BALB/c mice infected with 10LD50 of VEEV TrD
Based on the successful protection of mice from VEEV TC-83 with BDGR-4, we wanted to test its antiviral activity with the wildtype strain, VEEV TrD. Additionally, we wanted to know how ML336, our prototype compound, would compare. In our prior mouse efficacy study with ML336 (Schroeder et al., 2014), significant survival rates (71%) were observed with VEEV-TC-83 infected mice treated with 5 mg/kg/day of compound (administered twice a day at 2.5 mg/kg). Based on this prior study, we opted for a much higher concentration for this screen, 12.5 mg/kg, twice per day, and the longer 8 days of treatment, twice per day.
Groups of BALB/c mice were treated with 25 mg/kg/day with BDGR-4, ML336, or with placebo beginning 2 hours prior to 10LD50 VEEV TrD challenge by s.c.. Treatment was continued for 8 days, BID. Mice were monitored for survival (Figure 5) and weight loss (data not shown). BDGR-4 and ML336 showed 100% protection of the mice challenged with VEEV TrD (Figure 5). Based on the results of this study and in combination with the superior in vitro ADME profile compared to the analogs discussed herein, BDGR-4 was chosen to move forward for additional testing and evaluation.
Figure 5. Prophylactic efficacy of ML336 and BDGR-4 tested in BALB/c mice challenged with VEEV TrD.
Groups of four BALB/c mice per treatment, vehicle, ML336 or BDGR-4, were assessed for survival over 21 days following a s.c. challenge with 10LD50 VEEV TrD. ML336 or BDGR-4 were dosed at 12.5 mg/kg BID delivered by i.p.. Treatments were administered i.p. in a volume of 0.1 ml in 25% PEG400/10% RH40/65% water starting at 2 hours prior to virus infection (D0) and were given BID through D7.
3.6. Prophylactic and therapeutic efficacy of BDGR-4 at a single dose in BALB/c mice challenged with VEEV
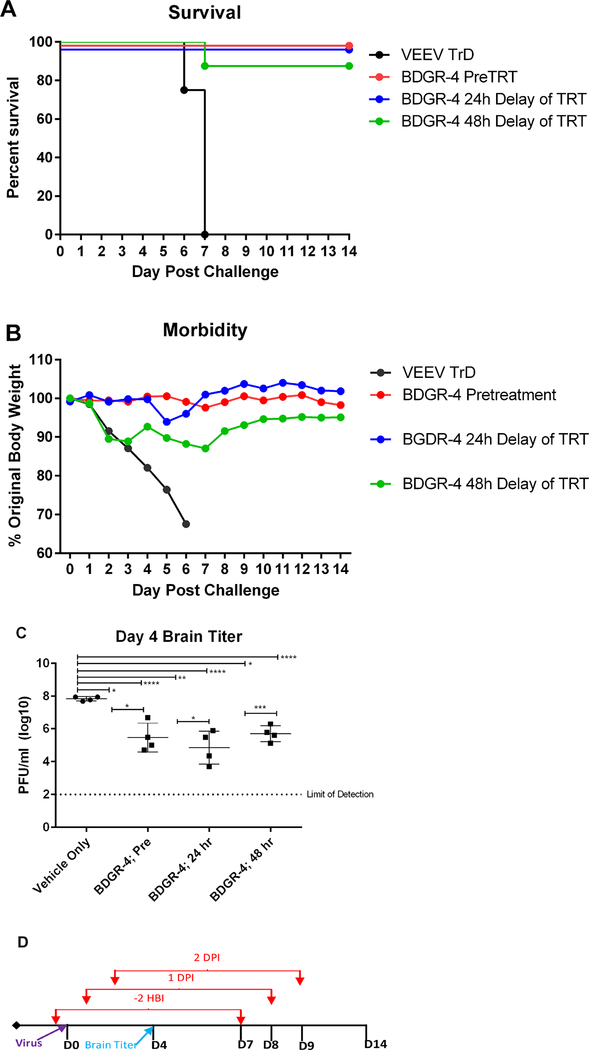
Based on the protection observed of mice at 25 mg/kg/day with BDGR-4 (Figure 5A) with 8 days of dosing, we tested the therapeutic potential of compound administration at 24 and 48 hpi. Groups of mice were treated with 25 mg/kg/d of BDGR-4 beginning 2 hours before, or 24 or 28 hours after virus challenge and continued for 8 days. Mice were monitored for survival (Figure 6A) and weight loss (Figure 6B). A cohort of mice was euthanized on 4 dpi from each group to collect brain tissue for virus titration (Figure 6C).
Figure 6. Prophylactic and therapeutic efficacy of BDGR-4 in BALB/c mice challenged with VEEV TrD.
Groups of eight BALB/c mice per treatment, vehicle, or BDGR-4 at 25 mg/kg/day, were assessed for (A) survival and (B) morbidity over 24 days following a s.c. challenge with 10LD50 VEEV TrD. (C) Four additional mice were used to determine the VEEV TrD titer by plaque assay on day 4. (D) All dosing was BID for a total of 8 days and delivered by i.p. with compound formulated in 25%PEG400/10%RH40/65%Water. Prophylactic doses started at 2 hours prior to virus challenge and therapeutic doses started at 24 or 48 hpi.
Treatment of mice with BDGR-4 at 25 mg/kg/day provided protection in all groups through day 21 with only a single animal succumbing to disease on 12 dpi in the 48-hour therapeutic group (Figure 6A). Weight change tended to increase slowly through 4 dpi. In placebo treated mice, a sharp decline in weights was observed beginning 1 dpi, which continued through 7 dpi. The groups of mice prophylactically-treated and treated 24 hpi with BDGR-4 continued to have similar levels of weight over time (Figure 6B). Virus titer in BDGR-4-treated animals (n=4 per group) ranged from 2–4 logs lower titers as compared to placebo-treated animals (Figure 6C).
From these studies we concluded that the level of the dose and the duration of dosing was optimal for therapeutic treatment when used at 24 hpi at 25 mg/kg/day. Future studies will examine additional dosing regimen approaches as this series of compounds is advanced for studies of alternate routes such as the oral and intravenous routes.
3.7. Assessment of Type I IFN induction and clinical chemistry following four days of BID dosing of BDGR-4.
Interferon is a potent inhibitor of New World alphaviruses in vivo and in vitro (Carpentier and Morrison, 2018; Frolov et al., 2012; Ryman and Klimstra, 2008), so it was essential that we determine the extent to which BDGR-4 induced Type-I IFNs. To assess the extent to which BDGR-4 induced a Type I IFN response, BABL/c mice were dosed with BDGR-4 or vehicle over the course of 4 days and IFNα and IFNβ levels were measured in the brain and serum (Table 2). The results demonstrate that BDGR-4 does not induce either of the Type I IFNs and therefore the effect of the drug on viral load is not due to the direct induction of type I IFNs by the drug. This is important since alphaviruses are highly sensitive to IFN Type 1. In addition, the results from clinical chemistry demonstrate that BDGR-4 dosing for 4 days does not affect the levels of liver or kidney enzymes demonstrating that it is not toxic for those organs (Table 2).
Table 2.
Assessment of Type I IFN and clinical chemistry following BDGR-4 dosing
| Analyte** | Vehicle* | BDGR-4* |
|---|---|---|
| IFNα serum (pg/ml) | 0 | 0 |
| IFNα brain (pg/ml) | 6 | 6 |
| IFNβ serum (pg/ml) | 0 | 0 |
| IFN β brain (pg/ml) | 0 | 0 |
| Alkaline Phosphatase (ALP:U/L) | 67.1±7.8 | 62.7±12.8 |
| Alanine Aminotransferase (ALT: U/L) | 28.3±8.4 | 23.0±4.9 |
| Aspartate Aminotransferase (AST:U/L) | 141.7±73.4 | 108±19.1 |
| Blood Urea Nitrogen (BUN:mg/dL) | 18.7±1.4 | 21.0±1.0 |
| Glucose (mg/dL) | 156.4±21.1 | 184.5±26.4 |
| Total Protein (g/dL) | 4.4±0.18 | 4.3±0.2 |
| Calcium (mg/dL) | 9.5±0.5 | 9.4±0.3 |
Mice were dosed with vehicle or BDGR4 (25mg/kg BID) by IP injections over 4 days.
Type I IFNs were measured in serum and brain homogenates using a multiplex Immunoassay with a lower limit of detection for IFNα of 2.81pg/ml and lower limit of detection for IFNβ of 3.39pg/ml). Results are represented as average ± S.D. n=4–10 samples.
3.8. In vitro and prophylactic efficacy of BDGR-4 at a single dose in C57BL/6 mice infected with EEEV
Our goal in development of a New World alphavirus therapeutic is to identify those small molecules that have the broadest spectrum of activity against V/E/WEEV. As such we tested BDGR-4 using the previously mentioned CPE and titer reduction assays against WEEV and EEEV. The EC50 values of BDGR-4 against WEEV and EEEV were 102 and 149 nM, respectively, with a 6-log titer reduction for WEEV (Table 3). Given the excellent in vitro antiviral activity, we moved BDGR-4 forward for testing in a lethal mouse model of EEEV. Because of the aggressive disease progression of EEEV in mice, we hypothesized that we may need a higher concentration of BDGR-4 to achieve significant protection. Hence, we doubled the amount administered to 25 mg/k, twice per day.
Table 3.
In vitro anti-WEEV (strain California) and anti-EEEV (strain FL93–939) activity of BDGR-4
| WEEV EC501 (nM) |
WEEV log titer reduction2 |
EEEV EC50 (nM) |
EEEV log titer reduction |
|
|---|---|---|---|---|
| BDGR-4 | 102.3 ± 37.8 | > 6.24* | 149.5 ± 24 | ND |
cytopathic effect (CPE) assay in VERO76 cells;
done at 18 h post infection using 5 μM compound concentration in VERO76 cells. ;
No progeny virus plaques were detected. ND = not determined.
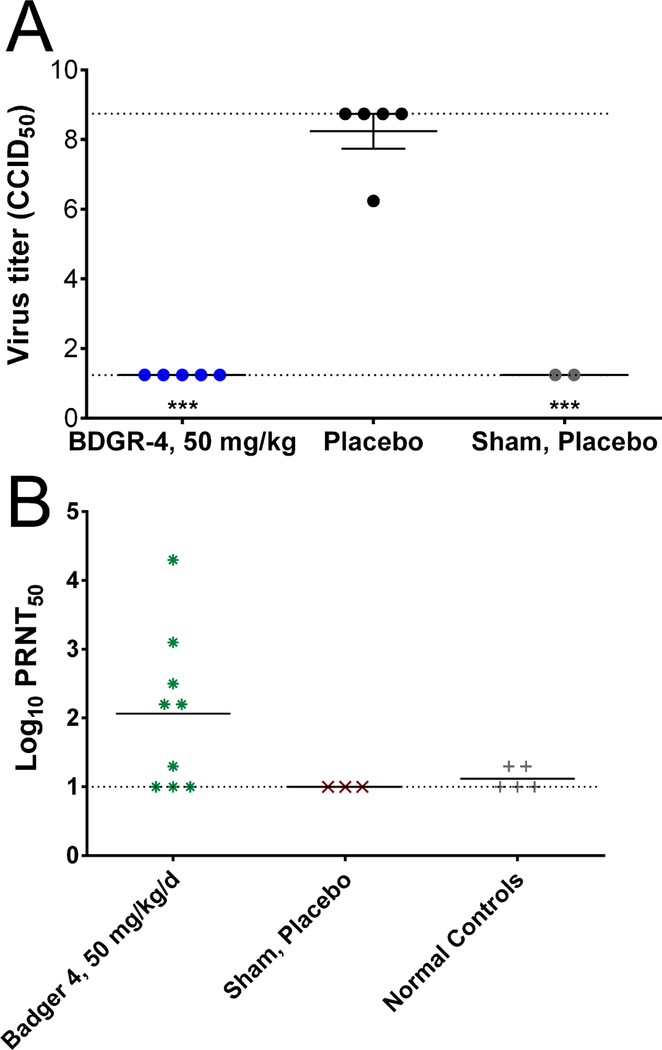
Groups of mice were treated with 50 mg/kg/day of BDGR-4 (n=10), or with placebo (n=15) beginning 2 hours prior to virus or sham-infected challenge and continuing for 8 days. Treatments were administered in a volume of 0.1 ml and were given twice a day at 25 mg/kg. Mice were monitored for survival and weight loss. A cohort of mice was euthanized on 6 dpi from the select groups to collect brain tissue for virus titration.
EEEV replicates faster in mice and hence, a higher dose of 50 mg/kg/day (25 mg/kg BID) was chosen for this study. Treatment of mice with BDGR-4 at 50 mg/kg/day provided protection through day 21 with only a single animal succumbing to disease on 12 dpi (Figure 7A). Weight change tended to increase slowly through 4 dpi. In placebo treated mice, a sharp decline in weights was observed beginning 5 dpi, which continued through 7 dpi. Mice treated with BDGR-4 continued to increase through to 7 dpi (Figure 7B). Normal controls and placebo-treated, sham-infected mice increased similarly to animals treated with compound (Figure 7B).
Figure 7. Prophylactic efficacy of BDGR-4 tested in C57BL/6 mice challenged with EEEV FL93–939.
(A) Survival of C57BL/6 mice infected by s.c. with EEEV and treated with various doses of BDGR-4. (B) Average percent weight change of animals treated with BDGR 4 and infected with EEEV. (C) Treatments were administered i.p. in a volume of 0.1 ml in 25% PEG400/10% RH40/65% water starting at 2 hours prior to virus infection (D0) and were given BID through D7 for a total of 8 days.
Virus titer was quantified in brain tissue obtained from a cohort of animals from select groups on 6 dpi. Virus titer in BDGR-4-treated animals was significantly reduced as compared with infection controls and was similar to titers in placebo-treated, sham-infected animals (Figure 8A). Lastly, we wanted to determine the immunological status of the animals to see if the animals mounted a neutralizing Ab response to virus infection.
Figure 8. Virus titers and neutralizing antibody levels from serum of surviving C57BL/6 mice after EEEV challenge of BDGR-4.
(A) Viral titers from brain tissue collected on 6 dpi from mice treated with BDGR-4. (B) Neutralizing antibody levels from mice sera collected 21 days following EEEV challenge of BDGR-4 treated mice. Upper and lower dashed lines represent upper and lower limits of assay detection, respectively. Dashed line represents limit of assay detection.
A measurable neutralizing antibody titer was observed in 6 of the 9 mice ranging from 1.5 to 4.5 log10 PRNT 50 (Figure 8B).
3.9. Concentration-based dynamics of emergence of drug resistance to BDGR-4
To determine the concentration of BDGR-4 that promotes resistance to TC-83, we passaged TC 83 in Vero76 cells in the presence of doubling concentrations of BDGR-4, starting at 50 nM and ending at 3200 nM, for a total of 7 concentrations. The approach is similar to that used by others to examine the emergence of mutations as they arise in vitro at various concentrations (Foll et al., 2014; Renzette et al., 2014). Three replicates for each concentration were sequenced using the Illumina NextSeq platform, and the sequences were aligned to the sequence of the viral stock before variant calling. As shown in Table 4, mutations did not emerge until 200 nM. Four mutations, G5414A, A5962T, A6331G, G6617A reached a majority in the population by 3200 nM; however, only the mutations at 5962 and 6331 resulted in amino acid changes, both in the nsP4 gene. Interestingly, multiple mutations resulting in the glutamine at position 210 in nsP4 that emerged though the three replicates were observed; notably, mutations at all positions at that codon were observed, all of which led to a mutation to a basic amino acid. Given a high enough concentration of the compound (32 to 64-fold greater than the EC50), TC-83 evolved resistance to BDGR-4. However, the penetrance of these variants never reached 100%. The greatest proportion of mutants at 3200 nM were at Q210R (61–79%).
Table 4.
Summary of point mutations and amino acid changes in VEEV TC83 that emerged at various concentrations of BDGR-4
| Nucleotide Change |
Amino Acid Change |
Gene | Replicate | 50 nM | 100 nM | 200 nM | 400 nM | 800 nM | 1600 nM | 3200 nM |
|---|---|---|---|---|---|---|---|---|---|---|
| G3033A | E452K | nsP2 | R1 | - | - | - | - | - | - | 21.4 |
| R1 | - | - | - | 30.51 | 31.43 | 64.53 | 73.86 | |||
| G5414A | A461A | nsP3 | R2 | - | - | 7.15 | 27.91 | 27.26 | 45.33 | 56.89 |
| R3 | - | - | 2.63 | 15.85 | 21.07 | 35.91 | 42.91 | |||
| R1 | - | - | 3.34 | 31.17 | 37.73 | 62.04 | 72.66 | |||
| A5962T | Y87F | nsP4 | R2 | - | - | 8.37 | 26.95 | - | 45.58 | 62.36 |
| R3 | - | - | 2.95 | 19.91 | 26.23 | 38.7 | 53.9 | |||
| C6330A | Q210K | nsP4 | R2 | - | - | - | - | - | - | 10.07 |
| R1 | - | - | - | - | - | 66.8 | 79.01 | |||
| A6331G | Q210R | nsP4 | R2 | - | - | - | - | - | - | 67.65 |
| R3 | - | - | - | - | - | - | 61.86 | |||
| R1 | - | - | 1.96 | 21.62 | 23.81 | 17.14 | 7.58 | |||
| A6332T | Q210H | nsP4 | R2 | - | - | - | 27.89 | - | - | - |
| R3 | - | - | 2.77 | 19.8 | 26.01 | - | - | |||
| A6341T | E213D | nsP4 | R2 | - | - | - | 10.74 | - | 4.21 | - |
| R3 | - | - | 0.65 | 4.34 | 4.06 | 1.67 | - | |||
| R1 | - | - | 2.52 | 28.32 | 37.32 | 57.49 | 66.97 | |||
| G6617A | R305R | nsP4 | R2 | - | - | 5.66 | 19.26 | 23.88 | 36.21 | 50.47 |
| R3 | - | - | 1.87 | 15.21 | 22.36 | 33.4 | 48.06 | |||
| G10639A | L213L | E1 | R2 | - | - | 1.53 | - | - | - | 12.43 |
| R3 | - | - | - | - | 5.97 | 10.71 | - |
Previously, we measured the resistance of VEEV TC-83 to the early hit compound, CID15997213 (Chung et al., 2014) and ML366 (Schroeder et al., 2014). In these studies, resistant viruses were selected by passaging VEEV TC-83 at a much higher concentration of compound and assessed only the passage 8. Specifically, CID15997213 and ML336 concentration was increased by 2.5 μM every other passage starting at 2.5 μM (Passage 0) and ending at 10 μM (Passage 8). Resistant viruses were plaque-purified and amplified in the presence of the compound at 5 μM, and these were sequenced by the Sanger method. Of the six plaques sequenced for the CID15997213-resistant VEEV-TC83, five carried a Y102C mutation and one had D116N within the amino-terminus of the nsP2 protein. In the study of ML336-resistant viruses, the Y102C mutation and D116N emerged, and a new mutation at Q210K in the nsP4. In our studies of BDGR-4, we observed a shift from the nsP2 resistance mutations (Y102C, D116N) to viruses with Q210 (K, R, H). previously, we did not assess the penetrance of the resistant virus phenotypes. As stated above, mutants did not reach greater than 61–79% of the population at 3200 nM. Unfortunately, the nsP4 resistant mutations fall under portions of their respective genes that do not a precise function or enzyme activity (Rubach et al., 2009). Confoundingly, the mutations at Y87 and Q210 in nsP4 are conserved residues throughout all alphaviruses, however, as yet we have not observed antiviral activity against Old World alphaviruses (data not shown).
4. Conclusions
Several benzamidine analogs of prototype ML336, a benzamidine anti-VEEV compound, were prepared and assessed for in vitro anti-VEEV activity, general cytotoxicity, solubility and microsomal stability. None of the compounds showed mammalian cytotoxicity liability (CC50 > 50 μM), and data obtained from CPE and titer reduction studies revealed nanomolar potency and robust attenuation of viral replication for the subset of compounds. Differences in microsomal stability and solubility differentiated the compounds, highlighting the best overall profile associated with BDGR-4.
Several of the analogs were tested initially in a lethal mouse model of VEEV TC-83. The best protection was noted by BDGR-4 and so this compound was advanced to evaluation in lethal mouse models of the wild type VEEV TrD and EEEV. In both models, mice survived challenge when treated with 25 mg/kg/day. Therapeutic treatment of mice with BDGR-4 beginning as late as 48 hours after VEEV infection provided 100% protection. The ability of BDGR-4 to protect mice from encephalitis is not due to the induction of IFNα or IFNβ as we did not detect either of these cytokines in the brain or sera of mice treated with the compound. In addition, there was no increase in liver or kidney enzymes in mice treated with BDGR-4 in comparison to vehicle treated mice suggesting that BDGR-4 did not have toxic side effects in mice.
A comprehensive compendium of antivirals against alphaviruses by Ching et al 2017 report several inhibitors acting at various stages of virus replication that are direct-acting or host-targeting (Ching et al., 2017). Of those reviewed, there were 14 publications of the New World alphaviruses, however, most of the publications were studies performed in vitro with most compounds showing micromolar activity (Ching et al., 2017; Markland et al., 2000) . Of these, only three studies to our knowledge have tested the efficacy of the compounds in mice, and none showed a substantial improvement in survival of the challenged mice. In conclusion, the BDGR-4 molecule may represent the first viable lead for therapeutic development for New World alphaviruses.
Lastly, our viral resistance studies presented herein and previously published ((Chung et al., 2016; Schroeder et al., 2014) suggest that this compound series directly targets polymerase function. It is known that nsP1–4 each contribute to viral replication. The first 97 amino acids of the alphaviruses nsP4 is predicted to have a disordered domain (Urakova et al., 2017), which may promote interactions with the other nonstructural proteins, nsP1–3, or host factors. Functional mapping of the nsP4 suggests residues within the terminal 500 amino acids of nsP4 that function as the finger domain (G159, Q191K, G324) and catalytic core (conserved GDD motif, 464–466) (Sawicki et al., 1990). The appearance of resistance mutations in the studies reported here and previously suggest the compound may lie in an area where nsP2 and nsP4 interact. For example, prior studies with Sindbis virus have reported interactions between the nsP1 and nsP4 in minus-strand synthesis (Fata et al., 2002) and nsP2 and nsP4 (Hahn et al., 1989). While we do not have evidence at this time, the binding of the compound into a structural pocket of nsP2–4 interactions, would potentially impact minus-strand synthesis. Current studies are in progress to map the interaction of ML336 with the nsp2 and 4.
Supplementary Material
Highlights.
Therapeutic treatment of mice with BDGR-4 beginning as late as 48 hours after VEEV infection provided 90% protection.
BDGR-4 improved survival, weight change and virus titer levels in the brain after prophylactic treatment.
Antiviral resistance of BDGR-4 mapped to amino acid changes in nsp2 and nsP4, and silent mutations in nsP3, nsP4 and E1.
5. Acknowledgements
We thank Joythi Parvathereddy for her technical support of the animal studies conducted at UTHSC Regional Biocontainment Laboratory. We thank CPM RBL staff at UofL and Kevin Bailey, Luci Wandersee and Eric Sefing for animal work at USU. This work was supported in part by NIH grant 5R01AI118814 to CBJ, DHC and JEG and contract HHSN272201000039I, Task order A11, from the Virology Branch, NIAID, NIH.
Glossary
- VEEV
Venezuelan equine encephalitis virus
- EEEV
Eastern equine encephalitis virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adcock RS, Chu YK, Golden JE, Chung DH, 2017. Evaluation of anti-Zika virus activities of broad-spectrum antivirals and NIH clinical collection compounds using a cell-based, high-throughput screen assay. Antiviral Res 138, 47–56. [DOI] [PubMed] [Google Scholar]
- Aguilar PV, Estrada-Franco JG, Navarro-Lopez R, Ferro C, Haddow AD, Weaver SC, 2011. Endemic Venezuelan equine encephalitis in the Americas: hidden under the dengue umbrella. Future Virol 6, 721–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher CH, 1994. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev 7, 89–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier KS, Morrison TE, 2018. Innate immune control of alphavirus infection. Current opinion in virology 28, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera JP, Forrester N, Wang E, Vittor AY, Haddow AD, Lopez-Verges S, Abadia I, Castano E, Sosa N, Baez C, Estripeaut D, Diaz Y, Beltran D, Cisneros J, Cedeno HG, Travassos da Rosa AP, Hernandez H, Martinez-Torres AO, Tesh RB, Weaver SC, 2013. Eastern equine encephalitis in Latin America. N Engl J Med 369, 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching KC, F.P.N. L, Chai CLL, 2017. A compendium of small molecule direct-acting and host-targeting inhibitors as therapies against alphaviruses. J Antimicrob Chemother 72, 2973–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D, Schroeder CE, Sotsky J, Yao T, Roy S, Smith RA, Tower NA, Noah JW, McKellip S, Sosa M, Rasmussen L, White EL, Aube J, Golden JE, 2010. ML336: Development of Quinazolinone-Based Inhibitors Against Venezuelan Equine Encephalitis Virus (VEEV), Probe Reports from the NIH Molecular Libraries Program, Bethesda; (MD). [PubMed] [Google Scholar]
- Chung DH, Golden JE, Adcock RS, Schroeder CE, Chu YK, Sotsky JB, Cramer DE, Chilton PM, Song C, Anantpadma M, Davey RA, Prodhan AI, Yin X, Zhang X, 2016. Discovery of a Broad-Spectrum Antiviral Compound That Inhibits Pyrimidine Biosynthesis and Establishes a Type 1 Interferon-Independent Antiviral State. Antimicrob Agents Chemother 60, 4552–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DH, Jonsson CB, Tower NA, Chu YK, Sahin E, Golden JE, Noah JW, Schroeder CE, Sotsky JB, Sosa MI, Cramer DE, McKellip SN, Rasmussen L, White EL, Schmaljohn CS, Julander JG, Smith JM, Filone CM, Connor JH, Sakurai Y, Davey RA, 2014. Discovery of a novel compound with anti-venezuelan equine encephalitis virus activity that targets the nonstructural protein 2. PLoS Pathog 10, e1004213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata CL, Sawicki SG, Sawicki DL, 2002. Modification of Asn374 of nsP1 suppresses a Sindbis virus nsP4 minus-strand polymerase mutant. J Virol 76, 8641–8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foll M, Poh YP, Renzette N, Ferrer-Admetlla A, Bank C, Shim H, Malaspinas AS, Ewing G, Liu P, Wegmann D, Caffrey DR, Zeldovich KB, Bolon DN, Wang JP, Kowalik TF, Schiffer CA, Finberg RW, Jensen JD, 2014. Influenza virus drug resistance: a time-sampled population genetics perspective. PLoS Genet 10, e1004185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov I, Akhrymuk M, Akhrymuk I, Atasheva S, Frolova EI, 2012. Early events in alphavirus replication determine the outcome of infection. J Virol 86, 5055–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn YS, Grakoui A, Rice CM, Strauss EG, Strauss JH, 1989. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: complementation group F mutants have lesions in nsP4. J Virol 63, 1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe DM, Howley PM, 2013. Fields virology, 6th ed. Wolters Kluwer/Lippincott Williams & Wilkins Health, Philadelphia, PA. [Google Scholar]
- Lindsey NP, Staples JE, Fischer M, 2018. Eastern Equine Encephalitis Virus in the United States, 2003–2016. Am J Trop Med Hyg 98, 1472–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markland W, McQuaid TJ, Jain J, Kwong AD, 2000. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob Agents Chemother 44, 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H, 1938. A simple method of estimating fifty per cent endpoints American J. Epidemiol. 27, 493–497. [Google Scholar]
- Renzette N, Caffrey DR, Zeldovich KB, Liu P, Gallagher GR, Aiello D, Porter AJ, Kurt-Jones EA, Bolon DN, Poh YP, Jensen JD, Schiffer CA, Kowalik TF, Finberg RW, Wang JP, 2014. Evolution of the influenza A virus genome during development of oseltamivir resistance in vitro. J Virol 88, 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas F, Diaz LA, Cardenas VM, Daza E, Bruzon L, Alcala A, De la Hoz O, Caceres FM, Aristizabal G, Martinez JW, Revelo D, De la Hoz F, Boshell J, Camacho T, Calderon L, Olano VA, Villarreal LI, Roselli D, Alvarez G, Ludwig G, Tsai T, 1997. Epidemic Venezuelan equine encephalitis in La Guajira, Colombia, 1995. J Infect Dis 175, 828–832. [DOI] [PubMed] [Google Scholar]
- Rubach JK, Wasik BR, Rupp JC, Kuhn RJ, Hardy RW, Smith JL, 2009. Characterization of purified Sindbis virus nsP4 RNA-dependent RNA polymerase activity in vitro. Virology 384, 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman KD, Klimstra WB, 2008. Host responses to alphavirus infection. Immunological reviews 225, 27–45. [DOI] [PubMed] [Google Scholar]
- Sabattini MS, Daffner JF, Monath TP, Bianchi TI, Cropp CB, Mitchell CJ, Aviles G, 1991. Localized eastern equine encephalitis in Santiago del Estero Province, Argentina, without human infection. Medicina (B Aires) 51, 3–8. [PubMed] [Google Scholar]
- Sawicki D, Barkhimer DB, Sawicki SG, Rice CM, Schlesinger S, 1990. Temperature sensitive shut-off of alphavirus minus strand RNA synthesis maps to a nonstructural protein, nsP4. Virology 174, 43–52. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Yao T, Sotsky J, Smith RA, Roy S, Chu YK, Guo H, Tower NA, Noah JW, McKellip S, Sosa M, Rasmussen L, Smith LH, White EL, Aube J, Jonsson CB, Chung D, Golden JE, 2014. Development of (E)-2-((1,4-dimethylpiperazin-2-ylidene)amino)-5-nitro-N-phenylbenzamide, ML336: Novel 2-amidinophenylbenzamides as potent inhibitors of venezuelan equine encephalitis virus. J Med Chem 57, 8608–8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakova N, Kuznetsova V, Crossman DK, Sokratian A, Guthrie DB, Kolykhalov AA, Lockwood MA, Natchus MG, Crowley MR, Painter GR, Frolova EI, Frolov I, 2017. beta-DN(4)-hydroxycytidine is a potent anti-alphavirus compound that induces high level of mutations in viral genome. J Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Winegar R, Manger ID, Forrester NL, 2012. Alphaviruses: population genetics and determinants of emergence. Antiviral Res 94, 242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.