Abstract
Fc γ receptor IIIa/CD16a is an activating cell surface receptor with a well-defined role in natural killer (NK) cell and monocyte effector function. The extracellular domain is decorated with five asparagine (N)-linked glycans; N-glycans at N162 and N45 directly contribute to high-affinity antibody binding and protein stability. N-glycan structures at N162 showed significant donor-dependent variation in a recent study of CD16a isolated from primary human NK cells, but structures at N45 were relatively homogeneous. In this study, we identified variations in N45 glycan structures associated with a polymorphism coding for histidine instead of leucine at position 48 of CD16a from two heterozygous donors. It is known that H48 homozygous individuals suffer from immunodeficiency and recurrent viral infections. A mass spectrometry analysis of protein isolated from the primary natural killer cells of individuals expressing both CD16a L48 and H48 variants demonstrated clear processing differences at N45. CD16a H48 displayed a greater proportion of complex-type N45 glycans compared to the more common L48 allotype with predominantly hybrid N45-glycoforms. Structures at the four other N-glycosylation sites showed minimal differences from data collected on donors expressing only the predominant L48 variant. CD16a H48 purified from a pool of monocytes similarly displayed increased processing at N45. Here, we provide evidence that CD16a processing is affected by the H48 residue in primary NK cells and monocytes from healthy human donors.
Keywords: Fc gamma receptor, glycoproteomics, immunodeficiency 20, N-glycan
Introduction
Natural killer (NK) cells are an important component of the innate immune system and perform two main effector functions: cytokine production and cell-mediated cytotoxicity (Lanier 2003). The immunomodulatory and cytotoxic capacities are stringently regulated by a cohort of activating and inhibitory receptors expressed on the NK cell surface (Vivier et al. 2008). The stimulatory, co-stimulatory or inhibitory signals originating from surface receptors upon interacting with the respective ligands reveals the pathological state of the interacting cell and directs the NK cell response. Fragment crystallizable (Fc)γRIIIa (CD16a) is one such activating receptor expressed by approximately 90% of circulating NK cells (Lanier et al. 1986). Binding of an antibody-opsonized target cell through the Fc region of Immunoglobulin G (IgG) is required to initiate antibody-dependent cell-mediated cytotoxicity (ADCC) (Perussia et al. 1984). Cell signaling by CD16a is also implicated in promoting NK cell proliferation and cytokine secretion (Fauriat et al. 2010; Pahl et al. 2018). Similar to NK cells, CD16a triggers ADCC, antibody-dependent cell-mediated phagocytosis and cytokine secretion on nonclassical monocytes (Yeap et al. 2016).
To date, only one functional NK cell immunodeficiency (#20) has been reported in humans that carry homozygous CD16a alleles encoding an L48H substitution (also referred to as L66H; Mace and Orange 2016). Three patients identified with immunodeficiency 20 suffer recurrent viral infections and exhibit normal NK cell counts, no defect in ADCC but reduced natural NK cell cytotoxicity (Jawahar et al. 1996; de Vries et al. 1996; Grier et al. 2012). A mechanistic study showed reduced cytotoxicity is potentially due to decreased surface expression of CD2 on NK cells isolated from two patients (Grier et al. 2012).
A considerable number of healthy individuals are expected to carry at least one copy of the autosomal recessive allele encoding CD16a H48 (8%) compared to 6% for R48 and 86% for L48 (de Haas et al. 1996), though a later study with more donors revealed lower frequencies of 2.7, 3.9 and 93.5%, respectively (Mahaweni et al. 2018). We focused on the CD16a H48 allotype because three individuals homozygous for the R48 allele were reportedly healthy (de Haas et al. 1996).
Our laboratory recently developed techniques to isolate CD16a from the primary NK cells or monocytes of a single healthy donor (Patel et al. 2018, 2019; Roberts et al. 2019). The site-specific N-glycosylation profile of NK cell CD16a revealed unique patterns at each of the five N-glycosylation sites. The distinct N-glycan structures at each site suggest potential site-specific functions. Considering the reports of CD16a H48 in immunodeficiency 20, we probed the N-glycan composition of the CD16a H48 allotype in healthy human donors to identify allotype-specific compositional differences. Such differences may provide mechanistic insight into critical interactions in the secretory pathway or at the cell surface that contribute to NK cell natural cytotoxicity but not ADCC.
The prevalence of the H48-encoding allele in the human population indicates that CD16a H48 protein should be accessible if the protein is expressed at the surface and a sufficient number of healthy donors are screened. Of the five CD16a N-glycans, only modifications at N45 and N162 contribute to antibody binding affinity (Shibata-Koyama et al. 2009; Patel et al. 2018). The N45-glycan stabilizes CD16a structure though composition at this site does not appear to impact receptor binding affinity unlike the N162-glycan (Subedi et al. 2017; Subedi and Barb 2018). Here, we used the recently developed method to characterize the processing of the naturally occurring CD16a H48 variant and identified differential N-glycan processing at N45 glycan in a heterozygous NK cell donor. These results proved comparable to the H48 allotype isolated from monocytes.
Materials and methods
NK cell and monocyte isolation
NK cells and monocytes were isolated using negative selection as described previously (Patel et al. 2018; Roberts et al. 2019). Briefly, contents of apheresis filters were diluted using phosphate buffered saline supplemented with 2% fetal bovine serum (FBS) and incubated with RosetteSep Enrichment Mixture (Stem Cell Tech) for 20 min. NK cells or monocytes were then separated from other tissues using Lymphoprep according to the manufacturer instructions (Stem Cell Tech). Cell yields and viability were determined with trypan blue staining; isolation quality was assessed with flow cytometry. Raw blood was collected from each sample for genotyping.
CD16a analysis
CD16a was isolated and digested as described by Patel et al. (2019). Briefly, cells were lysed with Tris-buffered saline containing dodecyl maltoside, AEBSF and oxidizing agents. The lysate was clarified by centrifugation and IgG removed with protein G Sepharose resin (GE Healthcare). CD16a was immunoprecipitated using 3G8-coupled agarose. The resin was washed of contaminating material and CD16a eluted with a mixture of acetonitrile, water and trifluoroacetic acid. CD16a was digested with chymotrypsin (Millipore Sigma) and GluC (Promega) in ammonium carbonate buffer. Glycopeptides were enriched using a glycopeptide enrichment kit (Millipore Sigma) and the peptide fraction was washed with water-saturated ethyl acetate to remove residual detergent. Glycopeptide and peptide fractions were analyzed over a C18-nLC system coupled to a Q-Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer (Thermo) as described in the supplement. Data were analyzed using Byonic (Protein Metrics) and custom R scripts.
Results
CD16a isolated from CD16a L/H48 donors
An apheresis filter collected from a healthy 23-year-old male produced approximately 85 × 106 NK cells (hereafter referred to as “Donor 6”). These NK cells appeared phenotypically normal with respect to CD16 and CD56 staining and isolated CD16a migrated in an SDS-PAGE gel at rate comparable to other healthy donors previously described (data not shown). Similarly, about 139 × 106 monocytes were isolated from a 35-year-old healthy female donor and the rate of monocyte CD16a migration in an SDS-PAGE gel was comparable to previously reported rate (Roberts et al. 2019). Sequencing CD16a cDNA confirmed both donors had at least one H48-CD16a coding allele (Figure 1). Additionally, the NK cell donor expressed only the V158-coding allele though the monocyte donor expressed multiple CD16a alleles encoding both V158 and F158. Mass spectrometry analysis of CD16a peptide fraction from NK cell donor also confirmed the presence of peptides from both CD16a L48 and H48 (Figure 1B and C).
Fig. 1.
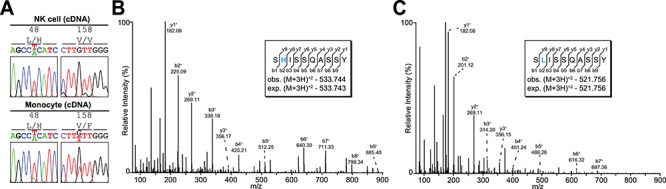
Donors with the L48H coding polymorphism and L/H48-CD16a. (A) CD16a cDNA sequencing shows NK cell and monocyte donor genotypes. The parent ion mass and b/y-ion series in MS2 spectra confirm the presence of L48 (B) and H48 (C) in NK cell CD16a.
Differential N45-glycan processing in NK cells
The CD16a L/H48 polymorphism is located three residues from an N-glycosylation site (N45) and, thus, may impact the local environment that contributes to N-glycan processing. Chymotrypsin and GluC digestion of full-length CD16a in the presence of detergent produced three N45-containing peptides (Figure 2). The smallest, peptide 1 does not distinguish between L or H at 48 position; however, the larger peptides with L at position 48 (peptide 2) or H at position 48 (peptide 3) are distinguishable based on the mass of intact glycopeptide and fragment ions (Figure 2B and C). Thus, N-glycan species present on peptide 3 represent the glycosylation profile at N45 of the CD16a H48 allotype while peptide 2 represents species present on CD16a L48. Glycopeptide analysis of NK cell CD16a from the H48-expressing Donor 6 revealed 32 and 15 unique N45 glycoforms on peptides 2 and 3, respectively (Supplementary MSExcel Worksheet). Though these peptides likely ionize with different efficiencies, the relative abundance of each glycoform among all identified glycoforms of the same peptide (peptides 2 or 3) served as the basis for comparison. A larger percentage of the H48 peptides (53% vs. 21%) include the addition of 79.9 Da that is likely a phosphate moiety associated with the N-glycan as we previously described though it is unclear if this addition represents an adduct or a covalent modification (Patel et al. 2019).
Fig. 2.
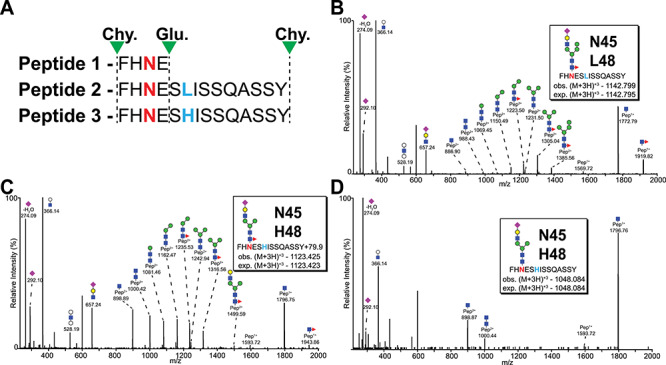
MS2 spectra of two distinct peptides carrying N45 glycosylation site along with L/H at position 48. (A) Three peptides are generated as a result of Chymotrypsin (Chy.) and GluC (Glu.) digestion of CD16a. Peptides 2 and 3 carry both the N45 glycosylation site (red N) and L/H at position 48 (blue L and H) and are produced by the missed GluC cleavage at E46. (B) MS2 spectra for peptide 2 carrying a hybrid type N-glycan at N45 from the NK cell donor. Hybrid type N-glycan on peptide 3 from NK cell (C) and monocyte (D).
A hybrid N-glycan structure provided the greatest ion intensities for both peptides 2 and 3; however, there were several differences between the L48 and H48 glycopeptides. The N45-L48 glycan from the heterozygous NK cell Donor 6 showed signs of restricted glycan processing (64% hybrid and oligomannose type) that proved comparable to that observed with peptide 2 in a previous report of five CD16a L48 homozygous NK cell donors (77 ± 9% hybrid and oligomannose type) (Figure 3; Patel et al., 2019). On the contrary, hybrid forms were less prevalent on the N45-H48 glycopeptide (47% of total intensity) while complex types proved more abundant (53%) with no detected oligomannose-type N-glycans. A comparison of the ten most abundant glycoforms on peptide 2 with peptide 3 from Donor 6 highlighted the extent of greater N45 glycan processing on peptide 3; the second most abundant form on peptide 3 was a tetraantennary di-sialylated complex type N-glycan (Figure 3A). Moreover, other glycoforms on peptide 3 include tetraantennary structures with poly-LacNAc repeats (2 of 10 most abundant glycoforms) which are not observed in the 10 most abundant forms on peptide 2 of this donor as well as on peptide 2 from other donors (Figure 3; Patel et al., 2019). These observations indicate the processing of the CD16a H48 allotype is distinct from CD16a L48 in the NK cells of the same heterozygous donor and NK cells isolated from donors homozygous for the CD16a L48 allele.
Fig. 3.
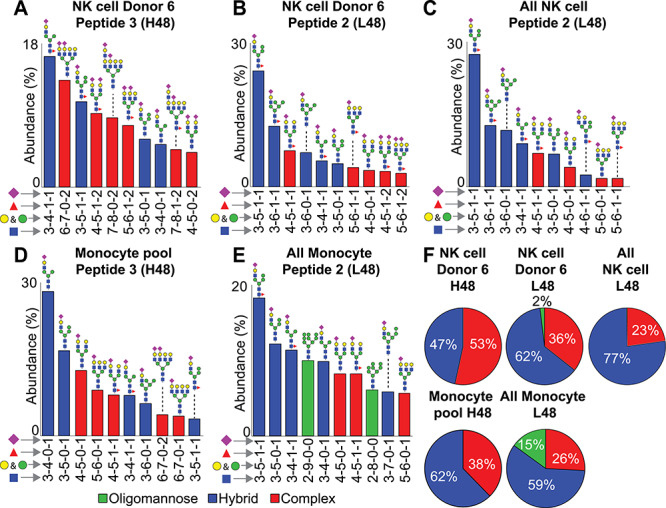
Differential N45 glycan processing due to L48H substitution. Relative abundance of the ten most common glycans identified at N45 in peptide 3 (A) peptide 2 (B) from a single NK cell donor. Pie charts represent the distribution of all N-glycan types identified. (C) and (D) represent a similar comparison between peptide 3 from pooled monocyte samples and peptide 2 from all monocyte samples, respectively. “All NK cell” and “All monocyte” peptide 2 data from (Patel et al. 2019; Roberts et al. 2019). Isobaric species were not distinguished.
The L/H48 polymorphism minimally affected NK cell processing at the four remaining CD16a N-glycosylation sites
The differential processing of the N45-glycan could be a result of factors intrinsic to Donor 6 and not specifically limited to this site. If this is true then a considerable fraction of N-glycans identified at the other four CD16a N-glycosylation sites from Donor 6 would also show distinct N-glycan processing in comparison to other NK cell donors. We compared the three most abundant N-glycan species at each site (representing over 59% of the relative intensity of all identified N-glycans) between Donor 6 and the five homozygous CD16a L48 donors (Patel et al. 2019). Negligible differences appeared in the relative abundance of identified N-glycan species in a comparison of the five previously analyzed CD16a L48 donors with the abundance from Donor 6 (Figure 4). Thus, the differential N-glycan processing observed at N45 for the L/H48 NK cell donor was not shared at the other four CD16a N-glycosylation sites.
Fig. 4.
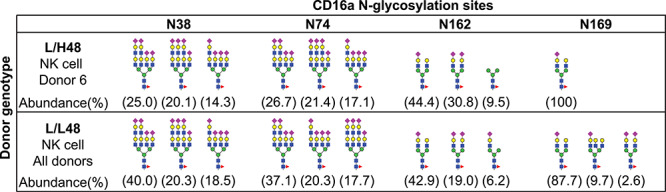
N-glycan processing at four other CD16a sites is not affected by H48. Three most abundant N-glycan species and their relative abundance identified in donor with L/H48 and L/L48 genotype. Intensities of the following glycopeptides were used to calculate the relative abundance: KCQGAYSPEDNSTQW (N38), RCQTNLSTLSDPVQLE (N74), VGSKNVSSE/CRGLVGSKNVSSE (N162) and TVNITITQGL (N169). Isobaric species were not distinguished.
Differential N45-glycan processing in monocytes
Due to the reduced abundance of CD16a on monocytes relative to NK cells, we pooled samples from three donors in our previous analysis of monocyte CD16a processing without prior knowledge of CD16a allotypes (Roberts et al. 2019). In one pool, PCR analysis of individually prepared cDNA from all three donors confirmed the identity of one donor with L/H48-CD16a. The generation of this pool introduces the problem that the L48 peptides from the one donor expressing the CD16a H48 allotype cannot be isolated from the other two donors, so comparisons were made to all monocyte CD16a L48 peptides we recently identified using ten independent donors (Supplementary MSExcel Worksheet). Even though the intensity of hybrid type N45-H48 glycopeptides (peptide 3; 62%) proved comparable to N45-L48 glycopeptides (peptide 2; 59%), we observed a striking loss of oligomannose type N-glycans on the N45-H48 glycopeptides (Figure 3C and D). Moreover, we also identified a higher abundance of complex type N45 glycans on the N45-H48 glycopeptides (38% compared to 26% for L48) along with the presence of tetraentennary complex-type N-glycans among the ten most abundant species that were not observed with the N45-L48 glyopeptides (Figure 3C and D). We likewise found comparable processing of the N38, N74, N162 and N169-glycans. Thus, the processing of CD16a on monocytes, including the increased processing at N45 for the H48 donors, proved analogous to processing observed from the NK cell Donor 6.
Discussion
There are several examples of N-glycosylation site gain or loss due to DNA polymorphisms; however, there are relatively few examples of changes in glycan structures on endogenous human protein due to DNA polymorphisms (Mazumder et al. 2012; Ding et al. 2015; Fan et al. 2018). One recent example was described in a study of fetuin (Lin et al. 2019). Here, we describe variation in N-glycosylation processing that is linked to an amino acid difference.
NK cells from Donor 6 expressed both L48 and H48 variants and displayed normal cell surface CD16 and CD56 staining. However, we observed considerable differences in N45-glycan processing from CD16a H48 that included the higher abundance of complex type N-glycans, specifically tetraantennary complex type glycoforms. Additionally, there was a noticeable absence of oligomannose type N-glycans at this site. We observed differential processing of the N45 glycan but not at any of the other four sites, thus these differences could be attributed to L48H substitution and not the donor. CD16a H48 expressed by monocytes likewise revealed comparable features. These observations suggest H48 removes a factor that specifically restricts N45 glycan processing in CD16a L48.
Disruption of intramolecular interactions between the N45 glycan and CD16a polypeptide due to a histidine at 48 position may explain differences in processing at N45. Molecular dynamics simulations revealed intramolecular contacts formed between the N-linked GlcNAc on the N45 glycan and E68 and D64 that stabilize the secondary structure of residues between 40 to 55 and 60 to 70 (Subedi et al. 2017). The location of the N45 glycan on the apex of a loop formed by residues 42 to 50 positions the N-glycan to make these interactions and it can be speculated that any disruptions in the loop structure (such as L48H substitution) can disrupt glycan-polypeptide contacts, exposing the glycan to glycan remodeling enzymes in the protein secretory pathway.
Another possible explanation for the observed differences could be disruption of an intermolecular interaction between CD16a and CD2. According to one estimate, around 70% of CD56dim/CD16+ NK cells express surface CD2, a co-activating receptor commonly associated with priming NK cells (Angelo et al. 2015). A recent study proposed that the H48 substitution decreased the CD2-CD16a interaction, reducing CD2 export and spontaneous cytotoxicity in immunodeficiency 20 patients (Grier et al. 2012). Additionally, a synergistic relationship between CD2 and CD16a signaling was recognized in NK cells suggesting both receptors might interact directly or are part of a multiprotein signaling complex (Liu et al. 2016). It is tempting to speculate that L48 CD16a is required for cis-interactions between CD16a and CD2 molecules and H48 disrupts the interaction resulting in increased N45 processing, however, the CD2-CD16a binding interface is not known. Furthermore, CD2 is expressed by a limited number of CD14bright monocytes, which do not express CD16 (Crawford et al. 1999).
Changes in N-glycosylation can impact function (Varki 2017). Individual’s age, gender and disease state are known to associate with protein N-glycan composition (reviewed in Gudelj et al. 2018) but here we demonstrate that CD16a polymorphisms represent another source of variation which affects N-glycosylation. It is our belief that characterization of protein glycosylation will be required to characterize the functionally diverse responses attributable to donor-specific variability. CD16a variability is already known to impact therapeutic efficacy (Koene et al. 1997; Wu et al. 1997; Cartron et al. 2002). Associating CD16a variability to mechanistic and clinically relevant outcomes represents a significant challenge. Donor- and site-specific N-glycan analysis of endogenous proteins can provide valuable initial insights into naturally occurring variation within human population and can be applied to design targeted intervention strategies.
Grant Support
National Institutes of Health under Awards No. R01 GM115489 (NIGMS) and R21 AI142122 (NIAID).
Supplementary Material
Acknowledgment
We thank donors and staff at DeGowin Blood Center (Iowa City, IA) and LifeServe Blood Center (Ames and Des Moines, IA) for providing materials, and also to Joel Nott for the MS analysis (ISU Protein Facility) and Dr. Sean Rigby (ISU Flow Cytometry Facility) for performing the flow cytometry measurements.
References
- Angelo LS, Banerjee PP, Monaco-Shawver L, Rosen JB, Makedonas G, Forbes LR, Mace EM, Orange JS. 2015. Practical NK cell phenotyping and variability in healthy adults. Immunol. Res. 62:341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. 2002. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 99:754–758. [DOI] [PubMed] [Google Scholar]
- Crawford K, Gabuzda D, Pantazopoulos V, Xu J, Clement C, Reinherz E, Alper CA. 1999. Circulating CD2+ monocytes are dendritic cells. J. Immunol. 163:5920–5928. [PubMed] [Google Scholar]
- Ding Q, Yang L, Dinarvand P, Wang X, Rezaie AR. 2015. Protein C Thr315Ala variant results in gain of function but manifests as type II deficiency in diagnostic assays. Blood. 125:2428–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Hu Y, Yan C, Goldman R, Pan Y, Mazumder R, Dingerdissen HM. 2018. Loss and gain of N-linked glycosylation sequons due to single-nucleotide variation in cancer. Sci. Rep. 8:4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauriat C, Long EO, Ljunggren H-G, Bryceson YT. 2010. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 115:2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier JT, Forbes LR, Monaco-Shawver L, Oshinsky J, Atkinson TP, Moody C, Pandey R, Campbell KS, Orange JS. 2012. Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity. J. Clin. Invest. 122:3769–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudelj I, Lauc G, Pezer M. 2018. Immunoglobulin G glycosylation in aging and diseases. Cell. Immunol. 333:65–79. [DOI] [PubMed] [Google Scholar]
- de Haas M, Koene HR, Kleijer M, de Vries E, Simsek S, van Tol MJ, Roos D, von dem Borne AE. 1996. A triallelic Fc gamma receptor type IIIA polymorphism influences the binding of human IgG by NK cell Fc gamma RIIIa. J. Immunol. 156:2948–2955. [PubMed] [Google Scholar]
- Jawahar S, Moody C, Chan M, Finberg R, Geha R, Chatila T. 1996. Natural killer (NK) cell deficiency associated with an epitope-deficient Fc receptor type IIIA (CD16-II). Clin. Exp. Immunol. 103:408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. 1997. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 90:1109–1114. [PubMed] [Google Scholar]
- Lanier LL. 2003. Natural killer cell receptor signaling. Curr. Opin. Immunol. 15:308–314. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. 1986. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J. Immunol. 1950:4480–4486. [PubMed] [Google Scholar]
- Lin Y-H, Zhu J, Meijer S, Franc V, Heck AJR. 2019. Glycoproteogenomics: a frequent gene polymorphism affects the glycosylation pattern of the human serum Fetuin/α-2-HS-glycoprotein. Mol. Cell. Proteomics. 18:1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LL, Landskron J, Ask EH, Enqvist M, Sohlberg E, Traherne JA, Hammer Q, Goodridge JP, Larsson S, Jayaraman J, et al. 2016. Critical role of CD2 co-stimulation in adaptive natural killer cell responses revealed in NKG2C-deficient humans. Cell Rep. 15:1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace EM, Orange JS. 2016. Genetic causes of human NK cell deficiency and their effect on NK cell subsets. Front. Immunol. 7:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaweni NM, Olieslagers TI, Rivas IO, Molenbroeck SJJ, Groeneweg M, Bos GMJ, Tilanus MGJ, Voorter CEM, Wieten L. 2018. A comprehensive overview of FCGR3A gene variability by full-length gene sequencing including the identification of V158F polymorphism. Sci. Rep. 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder R, Morampudi KS, Motwani M, Vasudevan S, Goldman R. 2012. Proteome-wide analysis of single-nucleotide variations in the N-glycosylation Sequon of human genes. PLoS One. 7:e36212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl JHW, Koch J, Götz J-J, Arnold A, Reusch U, Gantke T, Rajkovic E, Treder M, Cerwenka A. 2018. CD16A activation of NK cells promotes NK cell proliferation and memory-like cytotoxicity against cancer cells. Cancer Immunol. Res. 6:517–527. [DOI] [PubMed] [Google Scholar]
- Patel KR, Nott JD, Barb AW. 2019. Primary human natural killer cells retain proinflammatory IgG1 at the cell surface and express CD16a Glycoforms with donor-dependent variability. Mol. Cell. Proteomics. 18:2178–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KR, Roberts JT, Subedi GP, Barb AW. 2018. Restricted processing of CD16a/Fc γ receptor IIIa N-glycans from primary human NK cells impacts structure and function. J. Biol. Chem. 293:3477–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B, Trinchieri G, Jackson A, Warner NL, Faust J, Rumpold H, Kraft D, Lanier LL. 1984. The Fc receptor for IgG on human natural killer cells: Phenotypic, functional, and comparative studies with monoclonal antibodies. J. Immunol. 133:180–189. [PubMed] [Google Scholar]
- Roberts JT, Patel KR, Barb AW. 2019. Site-specific N-glycan analysis of antibody-binding Fc γ receptors from primary human monocytes. Mol. Cell. Proteomics. 19:362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata-Koyama M, Iida S, Okazaki A, Mori K, Kitajima-Miyama K, Saitou S, Kakita S, Kanda Y, Shitara K, Kato K, et al. 2009. The N-linked oligosaccharide at Fc gamma RIIIa Asn-45: An inhibitory element for high Fc gamma RIIIa binding affinity to IgG glycoforms lacking core fucosylation. Glycobiology. 19:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi GP, Barb AW. 2018. CD16a with oligomannose-type N-glycans is the only “low-affinity” Fc γ receptor that binds the IgG crystallizable fragment with high affinity in vitro. J. Biol. Chem. 293:16842–16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi GP, Falconer DJ, Barb AW. 2017. Carbohydrate-polypeptide contacts in the antibody receptor CD16A identified through solution NMR spectroscopy. Biochemistry. 56:3174–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. 2017. Biological roles of glycans. Glycobiology. 27:3–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. 2008. Functions of natural killer cells. Nat. Immunol. 9:503–510. [DOI] [PubMed] [Google Scholar]
- de Vries E, Koene HR, Vossen JM, Gratama JW, von dem Borne AE, Waaijer JL, Haraldsson A, de Haas M, van Tol MJ. 1996. Identification of an unusual Fc gamma receptor IIIa (CD16) on natural killer cells in a patient with recurrent infections. Blood. 88:3022–3027. [PubMed] [Google Scholar]
- Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, Salmon JE, Kimberly RP. 1997. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J. Clin. Invest. 100:1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap WH, Wong KL, Shimasaki N, Teo ECY, Quek JKS, Yong HX, Diong CP, Bertoletti A, Linn YC, Wong SC. 2016. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 6:srep34310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


