Abstract
Two maintenance mechanisms with separate neural systems have been suggested for verbal working memory: articulatory-rehearsal and non-articulatory maintenance. Although lesion data would be key to understanding the essential neural substrates of these systems, there is little evidence from lesion studies that the two proposed mechanisms crucially rely on different neuroanatomical substrates. We examined 39 healthy adults and 71 individuals with chronic left-hemisphere stroke to determine if verbal working memory tasks with varying demands would rely on dissociable brain structures. Multivariate lesion–symptom mapping was used to identify the brain regions involved in each task, controlling for spatial working memory scores. Maintenance of verbal information relied on distinct brain regions depending on task demands: sensorimotor cortex under higher demands and superior temporal gyrus (STG) under lower demands. Inferior parietal cortex and posterior STG were involved under both low and high demands. These results suggest that maintenance of auditory information preferentially relies on auditory-phonological storage in the STG via a nonarticulatory maintenance when demands are low. Under higher demands, sensorimotor regions are crucial for the articulatory rehearsal process, which reduces the reliance on STG for maintenance. Lesions to either of these regions impair maintenance of verbal information preferentially under the appropriate task conditions.
Keywords: articulatory rehearsal, lesion–symptom mapping, nonarticulatory maintenance, verbal working memory, working memory demands
Introduction
Working memory plays an important role in language processing by temporarily storing and keeping information active for further processing (Daneman and Carpenter 1980; Baddeley 2003b; Gernsbacher and Kaschak 2006). How information is maintained in working memory is still a matter of debate. Verbal working memory in Baddeley’s seminal model (Baddeley and Hitch 1974; Baddeley 2003b) consists of a phonological store and an articulatory rehearsal process that keeps the information active through subvocal speech. Prior lesion and imaging studies have suggested that simple maintenance of verbal information relies on a nonarticulatory mechanism that refreshes the information in the short-term store, instantiated in auditory/phonological regions of the temporal lobe (Buchsbaum et al. 2005; Leff et al. 2009). It remains unclear if subvocal rehearsal is only engaged for more demanding tasks and if the reliance on auditory-phonological maintenance regions would be reduced under those circumstances. A better understanding of the maintenance mechanisms in verbal working memory and their neural correlates is crucial in the development of habilitation and rehabilitation techniques for impairments of language and other cognitive abilities, which rely on verbal working memory.
Cognitive Models of Working Memory
Several models of working memory have been proposed in the literature (see the reviews by Baddeley and Hitch 2019; Chai et al. 2018). A main controversy among different theories is whether they define working memory as a system separate from long-term memory (LTM) (Waugh and Norman 1965; Atkinson and Shiffrin 1968; Baddeley 2012; Barrouillet and Camos, 2014, 2015) or as a subset of LTM (Cowan 1999). One of the most salient models that considers working memory as a separate system is the multicomponent model introduced by Baddeley and Hitch (1974). According to Baddeley and Hitch (1974), working memory consists of two specialized storage components and one processing component. Based on this model, the “phonological loop” and “visuo-spatial sketchpad” are two storage systems responsible for storing and maintaining verbal-acoustic and visual information, respectively. Maintenance of information is suggested to be achieved through rehearsal within the storage components as well as attentional and inhibitory processes performed by a central executive (Baddeley 2003a, 2012). Central executive functions in this model focus attention, divide attention between multiple tasks, and switch between tasks (Baddeley 2012). A fourth component, the “episodic buffer,” was also later added by Baddeley to the multicomponent model of working memory. The episodic buffer is proposed to be capable of holding different types of information; it serves to link the different components of working memory to each other and also to link working memory to LTM and perception (Baddeley 2012).
In contrast to the multicomponent model, state-based models do not consider working memory as a storage system separate from LTM (Cowan 1999; Engle et al. 1999; Li et al. 2014; D'Esposito and Postle 2015). Working memory in these models is an activated state of LTM (in the same long-term store). They mainly define working memory as the processes important in keeping information active for further processing by allocating attention to internal representations in LTM or inhibiting unrelated information. Based on Cowan’s embedded-processes model, working memory refers to a subset of the stored information in LTM that is activated and is in the scope of attention (Cowan 1999). Despite differences in how state-based and multicomponent theories define relationships between working memory and LTM, they mostly agree that working memory has a limited capacity and that information can decay with time and interference (Camos et al. 2009). Therefore, maintenance mechanisms are required to keep the information active for further processing.
One proposed maintenance mechanism is a domain-general attention-based rehearsal process (i.e., attentional refreshing) that keeps different types of information in an active state through shifting the focus to target information (Postle et al. 2004) or thinking briefly of already activated information (Johnson 1992; Johnson et al. 2002; Raye et al. 2007). This process involves increasing the activation of information, which has recently been presented or retrieved, to keep it accessible for further processing. This process can be applied to information from different modalities such as verbal and visual information in short-term store (Camos et al. 2018). Another proposed maintenance mechanism is an articulatory rehearsal process specific to maintenance of verbal information in working memory (Baddeley and Hitch 1974). Baddeley (2012) suggested that attentional refreshing maintains information in the episodic buffer while maintenance in the phonological loop occurs through articulatory rehearsal. Similarly, some recent theories (e.g., Time-Based Resource-Sharing model; Barrouillet and Camos 2007) have suggested that the two mechanisms of refreshing and rehearsal work separately from each other. Studies have shown that under different task demands, adults can favor one or the other method (Johnson et al. 2002; Hudjetz and Oberauer 2007; Camos et al. 2009, 2011, 2018; Mora and Camos, 2013, 2015; Camos 2015; Lucidi et al. 2016). Camos et al. (2011) suggested that attentional demands of the task as well as phonological characteristics of the items to be remembered determine the usage of articulatory rehearsal versus refreshing. In less attentionally demanding tasks, or when the items are phonologically confusable (phonologically similar items), refreshing would be the more effective maintenance mechanism. Refreshing is believed to be highly demanding, as attention must be focused on the target information during the maintenance stage. Therefore, under higher attentional demands, rehearsal would be preferred, as it requires minimal attention. However, if the items are phonologically similar and confusable, rehearsal would not be an effective strategy anymore as it relies on phonological features of the items. This hypothesis suggests that different brain regions, serving either refreshing or rehearsal mechanisms, may be crucial for maintenance of information in working memory under different task demands.
Neural Basis of Verbal Working Memory
Neuroimaging studies have provided evidence for different theories of working memory through examining brain regions involved in different working memory tasks. Some studies have suggested that a single domain-general neural network is responsible for maintenance of all types of information (e.g., verbal, visual), while domain-specific stores might be involved in encoding of information in working memory (Li et al. 2014). However, results from several neuroimaging studies suggest a multicomponent neuroanatomical model of working memory in which visual and verbal working memory are associated with distinct brain areas (Jonides et al. 2006; Smith and Jonides 1997), and two different but interacting neural networks are involved in nonarticulatory maintenance and the articulatory rehearsal process of verbal working memory (Paulesu et al. 1993; Gruber 2001; Gruber and von Cramon 2003; Gruber and Goschke 2004; Trost and Gruber 2012).
Many studies have suggested a role for parietal cortex in verbal working memory (Paulesu et al. 1993; Gruber 2001; Gruber and von Cramon 2003; Chai et al. 2018). Findings from a series of PET experiments (Awh et al. 1996; Smith and Jonides 1997) suggest that areas in the left parietal cortex are involved in storage or retrieval of verbal information in working memory. Areas in the frontal lobe, including premotor and supplementary motor cortex, Broca’s area, and cerebellum, are suggested to be involved in an articulatory rehearsal mechanism (Awh et al. 1996; Fiez et al. 1996; Smith and Jonides 1997). It has been proposed that when more demanding computation is performed on information in working memory (e.g., n-back test), dorsolateral prefrontal cortex (PFC) is recruited, reflecting the involvement of the central executive (Awh et al. 1996; Smith and Jonides 1997; Tanji and Hoshi 2008; D'Esposito and Postle 2015).
Similarly, functional magnetic resonance imaging (fMRI) studies of normal adults have shown that left-hemisphere articulatory motor areas, including Broca’s area and premotor cortex as well as the cortex along the left intraparietal sulcus, are involved in the rehearsal process of verbal working memory (Chein and Fiez 2001; Gruber 2001; Trapp et al. 2014). Gruber (2001) showed that if articulatory rehearsal is suppressed through a concurrent verbal task, a different prefronto-parietal network is engaged. Under rehearsal suppression, activations were found in bilateral anterior prefrontal and inferior parietal areas. It was suggested that these activations could be associated with executive processing used to resolve domain-specific interference in verbal working memory. Left inferior parietal lobule (particularly supramarginal gyrus) has been considered to play an essential role in storage of phonological information (Paulesu et al. 1993; Gruber 2001; Gruber and von Cramon 2001). Since this area was not active during the rehearsal condition in Gruber’s (2001) study, it was suggested that phonological storage is not continuously refreshed by a rehearsal process. Rather, it gets activated when rehearsal is no longer possible. In such a case, an alternative nonarticulatory maintenance mechanism would replace the rehearsal process. This was later supported by results from a functional connectivity study showing a negative interaction between rehearsal-related premotor areas and prefrontal and parietal areas associated with nonarticulatory maintenance (Gruber et al. 2007). Similarly, Raye et al. (2002) and Johnson et al. (2005) associated activations of the dorsolateral PFC to the nonarticulatory domain-general maintenance process of refreshing a just activated representation in working memory. They suggested that the refreshing process is performed through an interaction of dorsolateral PFC with other areas that temporarily store representations (short-term stores; e.g., parietal cortex for visually presented words and auditory areas for auditory information) (Raye et al. 2002; Johnson et al. 2005).
Consistent with fMRI studies, lesion studies have provided some evidence for the involvement of two different neural systems in maintenance and rehearsal of information in verbal working memory (Vallar et al. 1997; Leff et al. 2009; Trost and Gruber 2012; Ivanova et al. 2018). A case study (Trost and Gruber 2012) reported impairment of articulatory rehearsal in a patient with a lesion to Broca’s area and a nonarticulatory maintenance deficit in a patient with bifrontopolar lesion. Other studies, however, have found lesions in different brain regions to be associated with deficits of nonrehearsal maintenance. In a large lesion mapping study, Leff et al. (2009) showed that left posterior superior temporal gyrus (STG) plays a crucial role in simple recall of auditory information (forward digit span task). In their voxel-based morphometry analyses, they controlled for different language abilities (i.e., speech perception, repetition, production, comprehension) with the purpose of isolating working memory storage from executive functions and language-related processing. Similarly, using auditory working memory tasks, Vallar et al. (1997) reported a phonological storage deficit in a patient with lesions to the inferior parietal and middle and superior temporal gyri and a rehearsal deficit in a patient with lesions to subcortical premotor and Rolandic regions. A recent lesion–symptom mapping (LSM) study found distinct brain regions to be involved in performing working memory tasks with different task demands (Ivanova et al. 2018). Using two different working memory tasks (i.e., complex span and word N-back tasks), Ivanova et al. (2018) found left IFG, insula, and subcortical white matter to be involved in the complex span task and superior and middle temporal gyri to be involved in the N-back task. The authors interpreted these findings as reflecting the reliance on rehearsal and cue-dependent selection in the complex span task and updating/auditory recognition in the N-back task. A limitation of this study was that the two tasks differed substantially in the language and cognitive processes required for performance, making the interpretation of the observed lesion dissociations somewhat uncertain.
In summary, functional neuroimaging in healthy participants supports the theory that there are two mechanisms for maintenance of verbal information in working memory, which can be used depending on task demands: (1) a rehearsal mechanism that relies on motor and premotor cortices and their interactions with prefrontal and parietal regions and (2) a nonarticulatory auditory-phonological mechanism that relies on representations stored in posterior temporal or inferior parietal cortex, which are activated by attention and control regions in bilateral frontal lobes (Gruber 2001; Raye et al. 2002; Johnson et al. 2005; Trapp et al. 2014). Two lesion mapping studies (Leff et al. 2009; Ivanova et al. 2018) support the importance of posterior STG for nonarticulatory maintenance in forward digit span and N-back tasks, and a few lesion case studies suggest that this storage mechanism is dissociable from the rehearsal mechanism, which relies on the motor system (Vallar et al. 1997; Trost and Gruber 2012). There are no large-scale lesion mapping studies using carefully matched tasks to demonstrate that the two proposed mechanisms of maintaining verbal information in working memory are dissociable and are engaged differentially depending on task demands. To address these issues, we used multivariate LSM of left-hemisphere stroke survivors to identify brain regions in which lesions impair maintenance of verbal information in working memory under two different task conditions: simple recall of auditory information (forward digit span) and recall in a backward order (backward digit span), which requires manipulation of information and thus has higher working memory demands. To isolate domain-specific mechanisms of verbal working memory, we controlled for patients’ spatial working memory abilities using scores from forward and backward spatial span tasks, which share the same general cognitive demands as digit span tasks but differ in domain-specific processing. We hypothesized that simple immediate recall of auditory information relies on the auditory-phonological areas of the brain, while extra processing and manipulation of information in backward recall require articulatory rehearsal and thus rely on the articulatory motor areas of the brain in addition to sensory motor interface and auditory-phonological regions.
Methods
Participants
Participants included 71 native English speakers with chronic left-hemisphere stroke (>6 months) and 39 age- and education-matched healthy controls without any history of significant neurological or psychiatric disorders. Stroke patients had no history of other significant neurological or psychiatric disorders or speech and language impairments prior to the stroke. The study was approved by the Georgetown University IRB, and all participants provided informed consent. Patients’ demographic information and group characteristics are provided in Table 1.
Table 1.
Patients’ demographic information and group characteristics
| Mean age (SD) | 59.46 (9.88) |
| Handedness | 4 Left, 3 ambidextrous, 64 right |
| Gender | 46 M/25 F |
| Mean education in years (SD) | 16.21 (2.99) |
| Mean months since stroke (SD) | 47.54 (36.10) |
| Mean lesion volume (SD) | 101.28 (80.20) cm3 |
| Mean The Philadelphia Naming Test (PNT) (SD) | 35.42 (20.34) |
| Mean the Western Aphasia Battery (WAB) auditory-verbal comprehension—yes/no questions (SD) | 55.69 (4.51) |
| Mean WAB auditory word recognition (SD) | 43.72 (6.88) |
Behavioral Testing
All participants (stroke survivors and controls) completed four working memory tasks: forward digit span, backward digit span, forward spatial span, and backward spatial span. In forward and backward digit span tasks, sequences varying in length from 2 to 9 digits were read by the examiner, and the participant was instructed to recite the digits immediately after each sequence was read, either in the same order as presented (forward digit span) or in reverse order (backward digit span). Digits were presented at a rate of 1 per second, and for each task, two sequences per length (16 sequences in total) were tested in increasing order of length. Testing stopped when participants provided incorrect responses on both sequences of a given length. The score for each task was the total number of sequences recited correctly aloud (maximum score = 16 for 16 correctly recited sequences).
For spatial span, the Corsi block-tapping task (Corsi 1972) was used. The examiner touched sequences of 2–9 cubes on a board of 10 cubes at a rate of 1 cube per second. Participants were asked to touch the cubes in the same order as the examiner in the forward spatial span task and in the reverse order in the backward spatial span task. Two sequences were presented at each length (16 sequences in total) in increasing order of length. Testing was discontinued when the participant provided incorrect responses for both sequences at a given length, and the score was the total number of sequences performed correctly (maximum score = 16 for 16 correctly performed sequences). Behavioral data from the healthy adults were used as a measure of normal scores to determine the degree of impairment on these tasks in the stroke survivors.
Linear mixed-effects modeling, implemented in R, was used to analyze working memory scores to test if 1) patients performed worse than controls, 2) patients’ performance was differentially affected in digit span versus spatial span tasks, and 3) patients’ performance was differentially affected in forward versus backward versions of the tasks. The analysis examined the fixed effects of group (patient or control), task (digit span or spatial span), and task version (forward or backward). All relevant interactions between fixed effects were tested. Random effects for participant were also included in the analysis. Model fitting was performed in a backward-stepwise iterative fashion, followed by forward fitting of maximal random effects structure. Model fitting was independently supported by model fitness comparisons using Akaike information criterion and Bayesian information criterion.
Image Acquisition
Scanning was performed on the stroke survivors at the Center for Functional and Molecular Imaging, Georgetown University Medical Center. MRI data were acquired using a 3.0T Siemens TIM Trio scanner. Images were acquired using a high-resolution 3D T1-weighted sequence (repetition time [TR] = 1900 ms, echo time [TE] = 2.56 ms, flip angle = 9°, 160 contiguous 1 mm sagittal slices, field of view = 250 × 250 mm, matrix size = 246 × 256, voxel size = 1 mm3) and a 3D T2-weighted sequence (TR = 3200 ms, TE = 450 ms, number of excitations (NEX) = 2; matrix 192 × 192; 144 slices, 1.25 mm cubic voxels).
Lesion Tracing and Spatial Normalization
Lesions were manually traced on the T1-weighted images in native space, using T2-weighted images for guidance on white matter damage, using ITK-SNAP 3.6 (http://www.itksnap.org) by a board-certified neurologist (P.T.). Native space MPRAGEs and lesion tracings were warped to Montreal Neurological Institute (MNI) space using the Clinical Toolbox Older Adult Template as the target template (Rorden et al. 2012) via a custom pipeline. First, brain parenchyma was extracted from each native space image by applying a mask intended to minimize the clipping of gray matter edges. The initial mask was generated by combining the lesion tracing image (binarized) with white and gray matter tissue probability maps generated by the unified segmentation procedure in SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) applied to the original native space image, cost-function masked with the lesion tracing. The resulting mask was blurred and inverted to remove nonbrain tissue from the image. The resulting brain-extracted image was then normalized using Advanced Normalization Tools software (ANTs, http://picsl.upenn.edu/software/ants/; Avants et al. 2009). Lesion masking was used at each step of the ANTs process. After bias field correction was applied, normalization proceeded using a typical ANTs procedure, including a rigid transform step, an affine transform step, and a nonlinear SyN step. Next, the output of this initial ANTs warp was recursively submitted to three additional applications of the SyN step. Finally, the resulting linear (rigid and affine) and 4 nonlinear warp fields were concatenated, and the original native space MPRAGE and lesion tracings were transformed to the template space using BSpline interpolation. This iterative application of nonlinear warping was intended to improve normalization of expanded ventricles and displaced deep structures in individuals with large lesions. The normalized lesion tracings were finally downsampled to 2.5 mm3. Brain areas that were lesioned in the 71 participants are demonstrated in the lesion overlap map (Fig. 1).
Figure 1.
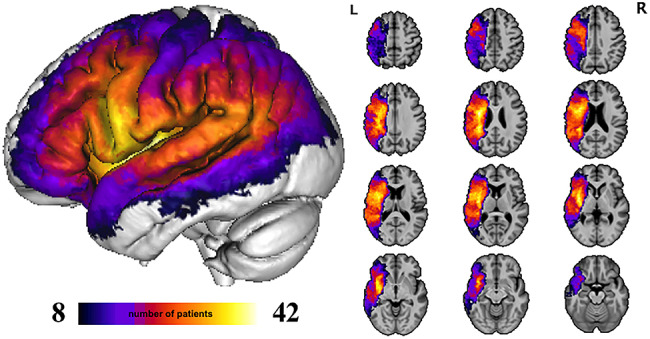
Lesion overlap map, N = 71.
Lesion–Symptom Mapping
We implemented support vector regression-LSM (SVR-LSM) (Zhang et al. 2014) using a MATLAB-based toolbox (DeMarco and Turkeltaub 2018) running under MATLAB R2017a (The MathWorks, Inc.). SVR-LSM applies a machine learning based algorithm to find lesion-symptom relationships more sensitively and specifically than traditional mass-univariate LSM approaches (Mah et al. 2014). In traditional mass-univariate LSM, an independent lesion–symptom relationship model is estimated at each voxel. The “univariate” part of the name refers to the fact that one predictor variable (voxel location) is considered at a time, and the “mass” part of the name refers to the fact that many models are constructed, one for each voxel in the analysis. Such an approach cannot consider lesion–symptom relationships that involve patterns that extend beyond a single voxel. The primary advantage of using a multivariate approach such as SVR over a mass-univariate approach for LSM is that it estimates the lesion–symptom map at all voxels simultaneously in a single model. Thus, patterns of lesion–symptom relationship that exist across multiple voxels are available to the analysis.
In many ways, the analysis is similar to a typical mass-univariate approach. The analysis estimates a model relating one or more predictors measured from a sample dataset to an outcome also measured from that sample, with the goal of understanding which predictor or predictors explain that outcome. The sample consists of a group of patients with brain lesions, and the sample size corresponds to the number of subjects. The outcome to be predicted corresponds to the behavior measured from each subject. Each predictor consists of a list of the voxel values at a single voxel’s spatial coordinate in the traced lesions for the group. Whereas the mass-univariate approach includes as the predictor the voxel value at a single spatial coordinate across the patient group, the multivariate approach includes all predictors (i.e., voxels) at once.
The traditional support vector machine (SVM) classifier is similar to logistic regression in that it classifies training data. But unlike traditional regression in which all points influence the solution, an SVM model retains only the vital subset of training samples (i.e., lesion datasets), referred to as support vectors, that lie near an optimal separating hyperplane. SVR adapts the traditional SVM to a regression framework, which estimates a continuously valued (rather than categorical) multivariate function. Here, the SVR approach is augmented with a kernel function that allows a model to be estimated for data that is not linearly separable. The kernel function is used to transform the feature data (voxels) into a high-dimensional space (where number of dimensions equals the number of voxel features) in which an optimal linear separating hyperplane is estimated. Whereas SVM/SVR is typically used to construct a model for predicting properties of novel, unobserved data, SVR-LSM takes advantage of a property of the SVM that allows the hyperplane solution to be backprojected into a pseudodataset in the original feature space that can be interrogated for patterns of interest and tested for statistical reliability as one would the results of a traditional mass-univariate analysis.
Like any auditory task that involves speech processing, performing an auditory digit span task requires a number of linguistic subprocesses at sublexical (e.g., acoustic, phonetic, phonological) and lexical-semantic levels (Hickok and Poeppel 2007) as well as nonlinguistic cognitive sub-processes (e.g., inhibition, attention). Therefore, performing a digit span task would involve a network of brain regions, damage to any of which could cause deficits in subprocesses leading to impaired performance in digit span tasks. To isolate maintenance of verbal information and remove the effects of domain-general working memory functions common to both spatial and verbal tasks, spatial span scores were included as a covariate in all of the analyses. This was accomplished prior to estimating each SVR model by covarying the spatial span scores out of both the behavioral data and voxelwise lesion tracing data using a nuisance model (DeMarco and Turkeltaub 2018). Voxels that were lesioned in fewer than 10% of participants were excluded from the analyses.
SVR-LSM was first used to identify lesions associated with lower scores in 1) forward digit span controlling for forward spatial span and 2) backward digit span controlling for backward spatial span. Two additional analyses examined lesions associated with lower scores in 1) forward digit span controlling for both forward spatial span and backward digit span and 2) backward digit span controlling for both backward spatial span and forward digit span. These contrasts remove the effects of sensory, motor, linguistic, and working memory functions common and equally important to both forward and backward digit span tasks (e.g., audition, phonological access, speech production) and separate the areas more important for maintenance of verbal information in each digit span task compared to the other. For many behaviors, lesion size confounds the analysis because larger lesions generally result in more severe behavioral impairments, regardless of location. Thus, the confound of lesion volume was controlled in all analyses by regressing the lesion volume out of both behavioral scores and lesion mask data on a voxelwise basis, a method that provides rigorous control of lesion volume and is more specific than alternative approaches (DeMarco and Turkeltaub 2018). Voxelwise β-values were thresholded at P < 0.005 based on a voxelwise null distribution generated from 10 000 permutations of the behavioral scores. To correct for multiple comparisons, family wise error rate was controlled at 0.05 using a cluster-extent threshold determined from the same 10 000 permutations. Permutation-based multiple comparisons correction methods are considered the gold standard for LSM because they account for the lesion autocorrelation structure inherent to the datasets (Nichols and Holmes 2002; Kimberg et al. 2007; Mirman et al. 2016; Wilson 2017).
Results
Behavioral Results
Controls scored higher on all tasks compared to patients (Table 2), demonstrating that patients as a group were impaired on all tasks. Patients’ performance was significantly better on forward digit span compared to backward digit span (t(70) = 10.532, P < 0.001), with every patient having a forward digit span score equal or greater than their backward digit span score. Relationships among the scores are shown in Table 3 and Figure 2.
Table 2.
The range and mean of the scores in each working memory task
| Forward digit span score | Backward digit span score | Forward spatial span score | Backward spatial span score | |
|---|---|---|---|---|
| Controls | ||||
| Range | 7–16 | 4–12 | 4–12 | 4–11 |
| Mean (SD) | 10.92 (2.50) | 7.92 (2.19) | 8.13 (1.95) | 7.69 (1.72) |
| Patients | ||||
| Range | 0–12 | 0–8 | 1–11 | 0–11 |
| Mean (SD) | 5.13 (3.39) | 2.48 (2.18) | 6.49 (2.22) | 5.38 (2.32) |
| Comparison of controls vs. patients | ||||
| t value | 10.218 | 12.502 | 4.001 | 5.913 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 |
| df | 98.93 | 71.81 | 87.50 | 98.67 |
Span scores are the total number of sequences recited/performed correctly during each task.
Table 3.
Correlation coefficients and significance levels
| Forward digit span | Backward digit span | Forward spatial span | |
|---|---|---|---|
| Controls | |||
| Forward digit span | |||
| Backward digit span | 0.537*** | ||
| Forward spatial span | 0.105 | 0.212 | |
| Backward spatial span | 0.154 | 0.133 | 0.617*** |
| Patients | |||
| Forward digit span | |||
| Backward digit span | 0.794*** | ||
| Forward spatial span | 0.451*** | 0.466*** | |
| Backward spatial span | 0.409*** | 0.537*** | 0.730*** |
* * * P < 0.001.
Figure 3.
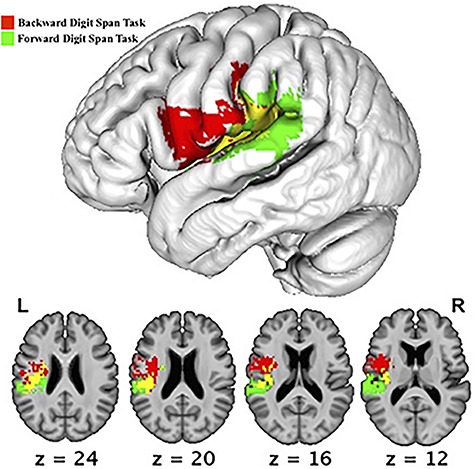
SVR-LSM results showing lesions associated with reduced forward digit span performance controlling for forward spatial span performance (green) and reduced backward digit span performance controlling for backward spatial span performance (red). Overlapping areas are demonstrated in yellow. All voxelwise P < 0.005, cluster corrected family-wise error <0.05, 10 000 permutations. Z is the MNI coordinate of the axial slice being displayed.
Figure 2.
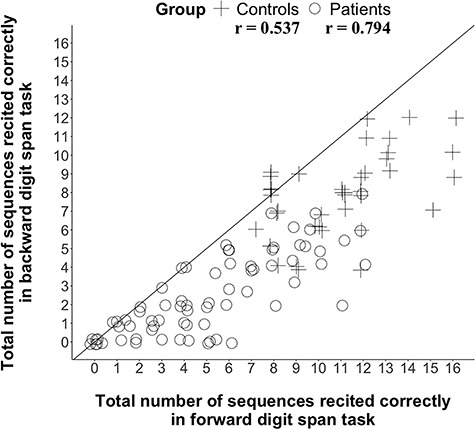
Participants’ performance in forward and backward digit span tasks. Span scores are the total number of sequences recited correctly during each span task. These values provide a more precise measure of working memory than the span (the longest sequence at which an individual is successful at least once).
A series of linear mixed-effects models were used to analyze participants’ scores on the four working memory tasks. The final linear mixed model for working memory scores is presented in Table 4. Participant was included as a random effect (SD = 1.71) in the final model. There was a significant interaction between task and task version (t = −5.515, P < 0.001) showing that across both patients and controls, participants’ performance was less affected by the version of the task in spatial span tasks compared to the digit span tasks. There was also a significant two-way interaction between task and group. Although patients scored lower in all tasks compared to the control group (t = −13.716, P < 0.001), their performance was less affected in the spatial span tasks compared to the digit span tasks (t = 10.45, P < 0.001). This is consistent with the reliance of all tasks on a shared common process, likely executive control, with additional processes that are sensitive to left-hemisphere lesions required for digit span tasks, likely the maintenance of verbal information. The three-way interaction of group, task, and version was not significant and was thus removed from the final model.
Table 4.
Coefficients and P values of the final linear mixed effects model for working memory scores
| Effects | Coefficient | Standard error | t value | P value |
|---|---|---|---|---|
| (Intercept) | 8.2008 | 0.3569 | 22.978 | <0.001 |
| Task | −0.6287 | 0.3312 | −1.898 | 0.06 |
| Version | 2.7404 | 0.2399 | 11.424 | <0.001 |
| Group | −5.7717 | 0.4208 | −13.716 | <0.001 |
| Task × version | −1.8722 | 0.3394 | −5.515 | <0.001 |
| Task × group | 3.7057 | 0.3546 | 10.45 | <0.001 |
LSM Results
In our first set of multivariate lesion mapping analyses, we examined areas of the brain that were crucially involved in maintenance of verbal information in working memory in tasks with either low processing demands (i.e., forward digit span) or high demands (i.e., backward digit span). We controlled for spatial span scores to isolate domain-specific processing involved in maintenance of verbal information from domain-general processing that is shared between verbal and spatial working memory in each task condition (low and high demands). These analyses identified distinct areas involved in maintenance of verbal information for each digit span task, with pre- and primary motor cortex and somatosensory cortex and underlying white matter only for backward digit span (corrected P < 0.05, shown in red in Fig. 3) and posterior STG and underlying white matter only for forward digit span (corrected P < 0.05, shown in green in Fig. 3). Both tasks relied on regions in the supramarginal gyrus, parietal operculum, and posterior insula along with the white matter underlying these regions (shown in yellow in Fig. 3).
Figure 4.
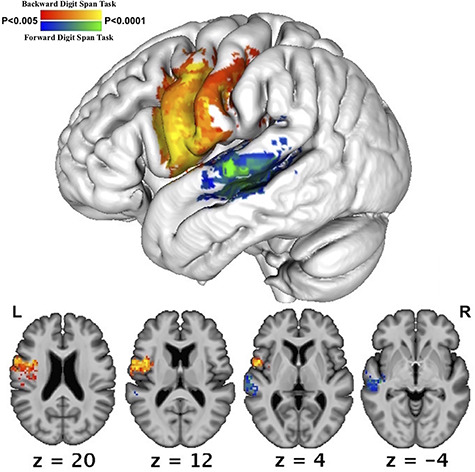
SVR-LSM results showing lesions associated with reduced forward digit span performance controlling for forward spatial span and backward digit span performance (blue-green) and reduced backward digit span performance controlling for backward spatial span and forward digit span performance (red-yellow). All voxelwise P < 0.005, cluster corrected family-wise error <0.05, 10 000 permutations. Z is the MNI coordinate of the axial slice being displayed.
Next, we confirmed the apparent dissociation in localization of forward and backward digit span by repeating the above analyses and adding each digit span task as a control variable in the SVR-LSM analysis of the other. Doing so ensures that findings cannot relate to deficits in auditory comprehension or speech production, as these abilities are required for both digit span tasks. These analyses confirmed the difference observed in the previous analyses; maintenance of verbal information during backward digit span relied more heavily on pre-motor, motor, and somatosensory cortex (shown in red-yellow in Fig. 4) and maintenance of information during forward digit span preferentially relied on STG (shown in blue-green in Fig. 4).
Discussion
Our findings demonstrate that lesions in auditory areas of STG affect maintenance of verbal information more strongly for forward compared to backward digit span, whereas lesions in motor and somatosensory cortex impair maintenance of verbal information more severely for backward compared to forward digit span. This neuroanatomical double dissociation demonstrates that verbal information is maintained in working memory using different mechanisms depending on the task demands. The cortex at the temporoparietal junction might be a shared substrate for both tasks, likely serving as an interface between auditory and motor maintenance systems depending on the task demands.
Short-Term Maintenance of Verbal Information in Left STG
LSM results showed that immediate recall of verbal information without any manipulation (i.e., forward digit span task) preferentially relied on STG. This finding is consistent with results from previous fMRI and lesion studies that used auditorily presented stimuli (Buchsbaum et al. 2005; Ivanova et al. 2018; Leff et al. 2009; Vallar et al. 1997). In contrast, STG has not been associated with verbal working memory in studies using visually presented stimuli (i.e., presenting digits visually in digit span tasks), suggesting that different stimulus characteristics may lead to different use of the maintenance mechanisms. Auditory-verbal information is suggested to have direct access to phonological storage while visual information may enter phonological storage after rehearsal (Vallar et al. 1997). This dissociation has been supported by fMRI findings (Buchsbaum et al. 2005), demonstrating that retrieval and short-term maintenance of auditory information relies on auditory-phonological memory in STG and superior temporal sulcus while maintenance of visually presented verbal information as well as longer maintenance of auditory information (e.g., delay recall) relies on articulatory processes in premotor, motor, and sensorimotor areas.
Using forward digit span as a task with lower demands and backward digit span as a task with higher demands, and controlling for processing that is equally important for the two tasks, we examined how reliance on STG is affected by task demands. We expected STG and articulatory motor areas to have equally important roles in verbal working memory under higher working memory demands. However, our results showed that under the higher working memory demands of the backward digit span task, maintenance of information relies more heavily on motor areas. This was further supported by the direct contrast of backward and forward digit spans showing the essential role of STG in forward digit span even after controlling for backward digit span. The absence of STG from the results of backward digit span analysis implies that verbal working memory tasks with higher demands rely more heavily on a separate mechanism that reduces the reliance on short-term storage in STG. Therefore, although STG might play a role in both tasks, the use of the other maintenance mechanism (i.e., rehearsal) in backward digit span task reduces the reliance on this area. While a lesion in STG could severely impair performance in forward digit span task, it would cause less severe impairment in performing backward digit span task, as a separate process would be the main maintenance mechanism in performing this task. This result is consistent with the findings from a recent LSM study (Ivanova et al. 2018) showing the crucial role of STG only in a less demanding N-back task and not in a more demanding complex span task.
Since STG has been reported to be active during passive auditory tasks (Binder et al. 2000) and has been suggested to be involved in lexical phonological access (Graves et al. 2008), it could be argued that this area plays a role in encoding of verbal information or that it is the auditory lexicon. However, by controlling for backward digit span in our second set of analyses, we removed the effects that could be caused by any deficit in processing of auditory information and lexical access shared between the two tasks. If the effect of STG lesions were simply to damage the auditory lexicon, any lesion in this area would be expected to equally impair performance in both forward and backward digit span tasks as the access to the auditory representation of the digits would be disrupted. This is consistent with Leff et al.’s (2009) lesion study showing lack of correlation between the structural integrity of the left STG and single-word comprehension. This does not preclude a role for the STG in storage of auditory lexical forms, but perhaps the right STG can support this ability when the left side is damaged. This bilateral capacity for auditory lexical access is supported by findings that single word auditory comprehension is often relatively preserved after left-hemisphere anesthesia or in chronic lesions (Hickok and Poeppel, 2000, 2004, 2007; Hickok et al. 2008; Rogalsky et al. 2008, 2011).
Our results do suggest, however, that left STG plays a crucial role in short-term storage and maintenance of auditory information in verbal working memory. Working memory impairment on forward digit span was associated with left STG lesions, even after controlling for backward digit span performance, which has identical auditory comprehension demands. This demonstrates that the STG region associated with forward digit span performance here cannot be responsible for access to or storage of auditory lexical representations, since lesions to this region preferentially impair forward digit span and not backward span. This might imply that the nonarticulatory maintenance (attentional refreshing) does not occur merely through directing attention toward the same auditory representations that are the basis of lexical access, in which case no dissociation between these abilities could be observed due to lesions. In other words, verbal working memory might rely on a short-term store in left STG separate from the long-term lexicon, perhaps storing either copies of auditory representations activated in LTM or pointers to representations in LTM (Norris 2017). The representations in this short-term store can be refreshed through a domain-general attentional refreshing process (Camos et al. 2018). An alternative explanation is that both the right and left STG are capable of supporting lexical access, but the representations in right STG decay too quickly to be used for working memory, whereas the left STG representations decay more slowly, making them capable of supporting short-term maintenance under low-demand conditions. In this case, a unilateral left STG lesion would not severely impact auditory comprehension but would impact maintenance of information in verbal working memory, consistent with our findings.
A Rehearsal Mechanism Reliant on Left Sensorimotor Regions
As we expected based on the previous functional imaging literature and the few prior lesion cases, we found that pre- and primary motor cortex is crucial for maintenance of verbal information in working memory under higher demands, but not low demands. This finding provides strong lesion evidence to support the role of these areas in the articulatory rehearsal process in verbal working memory (Vallar et al. 1997; Gruber 2001; Gruber and von Cramon 2003; Gruber and Goschke 2004; Trost and Gruber 2012). Therefore, these motor areas might be involved in subvocal rehearsal through covert inner speech, which refreshes the information in verbal working memory.
Both overt and some forms of inner speech rely on motor areas (Paulesu et al. 1993; Huang et al. 2002; Indefrey and Levelt 2004; Wilson et al. 2004; Price 2012). Since our digit span tasks required spoken responses, lesions to motor areas might be expected to impair performance in both tasks. However, motor areas were absent from our results for the forward digit span task. Moreover, controlling for forward digit span performance in the analysis of backward digit span should have removed any lesion effects related to impairment in overt speech production. Rather, the finding that lesions in motor cortex specifically impair backward digit span suggests a difference between the mechanisms involved in the production of overt speech and inner speech used for rehearsal. Previous behavioral and lesion studies have provided evidence for a dissociation of inner and overt speech (Geva et al. 2011a, 2011b; Hayward et al. 2016; Fama et al. 2017, 2019). It is possible that different error monitoring mechanisms used in overt versus inner speech underlie this difference. While overt speech can benefit from auditory feedback for error monitoring, inner speech can only benefit from forward models of representations (Tourville and Guenther 2011) or other internal signals (Nozari et al. 2011). Therefore, if motor cortex plays a role in error monitoring or repair via internal models, the absence of auditory feedback in inner speech might result in poorer error detection/repair during the rehearsal process. Internal errors during subvocal rehearsal might accumulate, causing loss of information in working memory maintenance even when overt speech, although impaired, is adequate for comprehensible production. It is also possible that the right motor cortex is able to execute motor actions with enough fidelity to produce comprehensible speech but not enough for the more demanding task of rehearsing speech internally.
Alternatively, “rehearsal” could occur through activation and refreshing of articulatory representations, without explicit subvocal articulation. Pre- and primary motor areas have been suggested to play a role in storage of articulatory motor representations of speech. Based on this view, articulatory maintenance in verbal working memory requires auditory phonological representations in posterior STG to be mapped onto articulatory motor representations stored in motor areas (Hickok and Poeppel 2000, 2004). It is possible that the right hemisphere could compensate for the left hemisphere during overt speech. However, analogous to the suggestion above regarding auditory/phonological representations in STG, the motor representations in the right hemisphere might have shorter decay rates such that they are adequate for comprehensible overt speech execution, but decay too quickly for the refreshing process to occur for working memory maintenance.
Regardless of whether the rehearsal process requires explicit subvocal articulation or implicit refreshing of articulatory representations, the relative lack of reliance of backward digit span on the STG suggests that the rehearsal process might not critically rely on reactivation of auditory forms in a motor-auditory feedback cycle, but rather on motor and perhaps kinesthetic representations. This interpretation relies on a negative result, the lack of reliance of backward digit span on the STG, so should be considered speculative until further research is conducted. However, this conclusion is consistent with a functional connectivity study showing that during a task requiring rehearsal, increased activation of areas involved in the rehearsal process (e.g., left ventral premotor area) was coupled with decreased activation in prefrontal and parietal regions, which have been suggested to play roles in nonarticulatory maintenance of visual-phonological information (Gruber et al. 2007).
Conditions Determining the Use of the Two Maintenance Systems
Our results demonstrate that the degree of reliance on the rehearsal mechanism during verbal working memory tasks depends on task demands. Under lower task demands (e.g., immediate recall), nonarticulatory maintenance, which relies heavily on short-term stores might be sufficient, while under higher working memory demands (e.g., higher attentional demands, manipulation of information), articulatory rehearsal is required to prevent information decay. The use of the rehearsal mechanism reduces the reliance on short-term stores and the nonarticulatory maintenance mechanism. It is logical that the cognitive demands of backward digit span may be too great to be served by short-term maintenance only and must engage a separate rehearsal mechanism. However, it is curious that individuals with STG lesions apparently could not fully compensate for damage in the auditory maintenance system by relying instead on rehearsal for the forward digit span task. That we identified a neuroanatomical substrate specific to simple maintenance at all, when controlling for the more demanding working memory task, is surprising in this context and may suggest that rehearsal is relatively inefficient compared to STG-based auditory maintenance when task demands are low. This notion is supported by the absolute scores on the two digit span tasks. Across all patients, the score on forward digit span was equal to or greater than the backward span score. Left STG lesions thus do not result in worse performance on forward digit span than backward digit span, but rather a loss of the typical advantage for forward compared to backward span. This pattern is consistent with the auditory maintenance mechanism being most efficient for low-demand working memory tasks, but rehearsal being available for less efficient maintenance when the more efficient auditory system is damaged. Alternatively, patients with left STG lesions might also have damage to other areas that are involved in articulatory rehearsal, which does not enable them to rely on articulatory rehearsal mechanism.
It also remains unclear what aspect of the backward digit span task necessitates engagement of the rehearsal mechanism. One possibility is that the additional executive control demand of reversing the order of digits drives the reliance on rehearsal. This is consistent with the findings of Camos et al. (2011), suggesting that rehearsal is preferred under higher attentional demands, as it requires minimal attention. Another possibility is that the longer period of time required to perform this manipulation drives the reliance on rehearsal. If so, then similar results would be observed simply by enforcing a delay in the response or increasing inter-stimulus intervals, without requiring any manipulation of the information in working memory. Previous studies have provided evidence for the use of rehearsal in span tasks with delayed recall/longer inter-stimulus intervals (Vallar et al. 1997; Mora and Camos 2013). More research will be needed to fully understand the conditions under which the two mechanisms of maintenance are engaged.
Role of Temporoparietal Junction in Working Memory
Our SVR-LSM results revealed significant lesion–behavior associations at the junction of the temporal and parietal lobes, including supramarginal gyrus, in both forward and backward digit span tasks. Several studies on verbal working memory have also found involvement of inferior parietal regions (Vallar et al. 1997; Gruber 2001; Gruber and von Cramon 2003; Ravizza et al. 2004; D'Esposito et al. 2006; Muller and Knight 2006; Leff et al. 2009; Allen et al. 2011; Trost and Gruber 2012; Lacey et al. 2017). It has been suggested that this area plays a role as a sensory–motor interface (Hickok and Poeppel 2004; Buchsbaum et al. 2005; Binder et al. 2009; Hickok et al. 2009; Hickok 2012; Rogalsky et al. 2015). Auditory information must be mapped onto articulatory representations for the articulatory rehearsal process in backward digit span and in the last stage prior to speech production for the recall of the digits in the forward digit span, potentially explaining the involvement of this region in both tasks.
Alternatively, this area might act as a temporary store for the articulatory phonological representations (Gow 2012), which are used in articulatory rehearsal in backward digit span and in generating spoken responses in both forward and backward digit span tasks. Another possible role of the temporoparietal junction, which could explain its involvement in both tasks, is that it is this region (rather than the STG) that serves as the auditory phonological lexicon. Access to the auditory phonological lexicon is required for lexical retrieval in both forward and backward digit span tasks. Previous studies have provided evidence for the involvement of this area in long-term storage of new vocabulary and have considered it as the phonological lexicon (Breitenstein et al. 2005). If auditory-lexical representations are retrieved from the lexicon in the regions of the temporoparietal junction, then depending on task demands, they may be either temporarily stored in STG or sent to the rehearsal loop where they are transformed into articulatory representations.
Limitations
Although several studies have suggested a role for prefrontal regions in verbal working memory (Gruber 2001; Gruber and von Cramon 2003; Gruber and Goschke 2004; Trost and Gruber 2012; Ivanova et al. 2018), we did not find evidence for the involvement of prefrontal regions in maintenance of verbal information in either low or high working memory demand conditions. Negative results should always be interpreted cautiously, but our lack of findings in PFC is consistent with prior evidence that bilateral prefrontal areas are involved in verbal working memory such that unilateral prefrontal lesions do not cause working memory deficits (D'Esposito et al. 2006; see the review by D'Esposito and Postle 1999). This study did not include participants with lesions outside the left hemisphere, and further studies including right-hemisphere and cerebellar stroke survivors and individuals with bilateral lesions would be useful, particularly to address the role of PFC in working memory. Our results also implicated some deep white matter regions in the digit span tasks when they were not directly contrasted with each other. This may simply reflect the sensory input and motor output requirements of the tasks, but further investigations using connectome-based LSM methods would help to clarify the contributions of white matter tracts to verbal working memory maintenance.
Conclusions
The findings from this study demonstrate that two separate mechanisms are involved in maintenance of auditory information in verbal working memory: a nonarticulatory maintenance mechanism that critically relies on left STG and an articulatory rehearsal mechanism that relies more heavily on left sensorimotor areas. Unilateral left-hemisphere lesions are sufficient to disrupt either of these processes. We suggest that left STG is the short-term store for auditory-phonological information. Under lower working memory demands, refreshing information in STG is sufficient for maintenance, while under higher working memory demands, articulatory rehearsal in sensorimotor areas is required to keep the information activated for further processing.
Funding
National Institute of Health/The National Institute on Deafness and Other Communication Disorders (R01DC014960 and R03DC014310 to P.T., F31DC014875 to M.F.); National Institute of Health/National Center for Advancing Translational Sciences via the Georgetown-Howard Universities Center for Clinical and Translational Science (KL2TR000102 to P.T., TL1TR001431 to A.D.); the Doris Duke Charitable Foundation (2012062 to P.T.).
Notes
We thank Katherine Spiegel, Laura Hussey, Lauren Taylor, Jessica Friedman, and Molly Stamp for contributing to data collection and organization.
Conflict of Interest: The authors report no competing interests.
References
- Allen H, Ledgeway T, Kelly N, Hutchinson C, Blundell J. 2011. Divided attention impairs motion perception in older adults. J Vis. 11(11):97. [Google Scholar]
- Atkinson RC, Shiffrin RM. 1968. Human memory: a proposed system and its control processes In: Kenneth WS, Janet Taylor S, editors. Psychology of learning and motivation. New York: Academic Press, pp. 89–195. [Google Scholar]
- Avants B, Tustison N, Song G. 2009. Advanced normalization tools (ANTS). Insight J. V1: 1–35. [Google Scholar]
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. 1996. Dissociation of storage and rehearsal in verbal working memory: evidence from positron emission tomography. Psychol Sci. 7(1):25–31. [Google Scholar]
- Baddeley AD. 2003a. Working memory and language: an overview. J Commun Disord. 36(3):189–208. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. 2003b. Working memory: looking back and looking forward. Nat Rev Neurosci. 4(10):829–839. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. 2012. Working memory: theories, models, and controversies. Annu Rev Psychol. 63(1):1–29. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. 1974. Working memory In: Bower GH, editor. Psychology of learning and motivation. New York: Academic Press, pp. 47–89. [Google Scholar]
- Baddeley AD, Hitch GJ. 2019. The phonological loop as a buffer store: an update. Cortex. 112:91–106. [DOI] [PubMed] [Google Scholar]
- Barrouillet P, Camos V. 2007. The time-based resource-sharing model of working memory In: Osaka N, Logie RH, D'Esposito M, editors. The cognitive neuroscience of working memory. Hove:UK: Oxford University Press, pp. 59–80. [Google Scholar]
- Barrouillet P, Camos V. 2014. On the proper reading of the TBRS model: reply to Oberauer and Lewandowsky (2014). Front Psychol. 5:1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrouillet P, Camos V. 2015. Working memory: loss and reconstruction. Hove: Psychology Press. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. 2009. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 19(12):2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, Possing ET. 2000. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 10(5):512–528. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Jansen A, Deppe M, Foerster A-F, Sommer J, Wolbers T, Knecht S. 2005. Hippocampus activity differentiates good from poor learners of a novel lexicon. NeuroImage. 25(3):958–968. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Olsen RK, Koch P, Berman KF. 2005. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron. 48(4):687–697. [DOI] [PubMed] [Google Scholar]
- Camos V. 2015. Storing verbal information in working memory. Curr Dir Psychol Sci. 24(6):440–445. [Google Scholar]
- Camos V, Johnson M, Loaiza V, Portrat S, Souza A, Vergauwe E. 2018. What is attentional refreshing in working memory. Ann N Y Acad Sci. 1424(1):19–32. [DOI] [PubMed] [Google Scholar]
- Camos V, Lagner P, Barrouillet P. 2009. Two maintenance mechanisms of verbal information in working memory. J Mem Lang. 61(3):457–469. [Google Scholar]
- Camos V, Mora G, Oberauer K. 2011. Adaptive choice between articulatory rehearsal and attentional refreshing in verbal working memory. Mem Cogn. 39(2):231–244. [DOI] [PubMed] [Google Scholar]
- Chai WJ, Abd Hamid AI, Abdullah JM. 2018. Working memory from the psychological and neurosciences perspectives: a review. Front Psychol. 9(401), 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein JM, Fiez JA. 2001. Dissociation of verbal working memory system components using a delayed serial recall task. Cereb Cortex. 11(11):1003–1014. [DOI] [PubMed] [Google Scholar]
- Corsi PM. 1972. Human memory and the medial temporal regions of the brain. Diss Abstr Int. 34: 819–B. [Google Scholar]
- Cowan N. 1999. An embedded-processes model of working memory In: Miyake A, Shah P, editors. Models of working memory: mechanisms of active maintenance and executive control. New York, NY, US: Cambridge University Press, pp. 62–101. [Google Scholar]
- D'Esposito M, Cooney JW, Gazzaley A, Gibbs SE, Postle BR. 2006. Is the prefrontal cortex necessary for delay task performance? Evidence from lesion and FMRI data. J Int Neuropsychol Soc. 12(2):248–260. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR. 1999. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 37:1303–1315. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR. 2015. The cognitive neuroscience of working memory. Annu Rev Psychol. 66(1):115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. 1980. Individual differences in working memory and reading. J Verbal Learn Verbal Behav. 19(4):450–466. [Google Scholar]
- DeMarco AT, Turkeltaub PE. 2018. A multivariate lesion symptom mapping toolbox and examination of lesion-volume biases and correction methods in lesion-symptom mapping. Hum Brain Mapp. 39(11):4169–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. 1999. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. J Exp Psychol Gen. 128(3):309–331. [DOI] [PubMed] [Google Scholar]
- Fama ME, Hayward W, Snider SF, Friedman RB, Turkeltaub PE. 2017. Subjective experience of inner speech in aphasia: preliminary behavioral relationships and neural correlates. Brain Lang. 164:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama ME, Snider SF, Henderson MP, Hayward W, Friedman RB, Turkeltaub PE. 2019. The subjective experience of inner speech in aphasia is a meaningful reflection of lexical retrieval. J Speech Lang Hear Res. 62(1):106–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE. 1996. A positron emission tomography study of the short-term maintenance of verbal information. J Neurosci. 16(2):808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernsbacher MA, Kaschak M. 2006. Discourse processing In: Keith B, editor. Encyclopedia of language & linguistics. Oxford: Elsevier, pp. 654–660. [Google Scholar]
- Geva S, Bennett S, Warburton EA, Patterson K. 2011a. Discrepancy between inner and overt speech: implications for post-stroke aphasia and normal language processing. Aphasiology. 25(3):323–343. [Google Scholar]
- Geva S, Jones PS, Crinion JT, Price CJ, Baron JC, Warburton EA. 2011b. The neural correlates of inner speech defined by voxel-based lesion-symptom mapping. Brain 134(Pt 10):3071–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow DW., Jr 2012. The cortical organization of lexical knowledge: a dual lexicon model of spoken language processing. Brain Lang. 121(3):273–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gupta P. 2008. The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. J Cogn Neurosci. 20(9):1698–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber O. 2001. Effects of domain-specific interference on brain activation associated with verbal working memory task performance. Cereb Cortex. 11(11):1047–1055. [DOI] [PubMed] [Google Scholar]
- Gruber O, Goschke T. 2004. Executive control emerging from dynamic interactions between brain systems mediating language, working memory and attentional processes. Acta Psychol. 115(2):105–121. [DOI] [PubMed] [Google Scholar]
- Gruber O, Müller T, Falkai P. 2007. Dynamic interactions between neural systems underlying different components of verbal working memory. J Neural Transm. 114(8):1047. [DOI] [PubMed] [Google Scholar]
- Gruber O, Cramon DY. 2001. Domain-specific distribution of working memory processes along human prefrontal and parietal cortices: a functional magnetic resonance imaging study. Neurosci Lett. 297(1):29–32. [DOI] [PubMed] [Google Scholar]
- Gruber O, Cramon DY. 2003. The functional neuroanatomy of human working memory revisited. Evidence from 3-T fMRI studies using classical domain-specific interference tasks. NeuroImage. 19(3):797–809. [DOI] [PubMed] [Google Scholar]
- Hayward W, Snider SF, Luta G, Friedman RB, Turkeltaub PE. 2016. Objective support for subjective reports of successful inner speech in two people with aphasia. Cogn Neuropsychol. 33(5–6):299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. 2012. The cortical organization of speech processing: feedback control and predictive coding the context of a dual-stream model. J Commun Disord. 45(6):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Okada K, Barr W, Pa J, Rogalsky C, Donnelly K, Barde L, Grant A. 2008. Bilateral capacity for speech sound processing in auditory comprehension: evidence from Wada procedures. Brain Lang. 107(3):179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Okada K, Serences JT. 2009. Area Spt in the human planum temporale supports sensory-motor integration for speech processing. J Neurophysiol. 101(5):2725–2732. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. 2000. Towards a functional neuroanatomy of speech perception. Trends Cogn Sci. 4(4):131–138. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. 2004. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 92(1–2):67–99. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. 2007. The cortical organization of speech processing. Nat Rev Neurosci. 8(5):393–402. [DOI] [PubMed] [Google Scholar]
- Huang J, Carr TH, Cao Y. 2002. Comparing cortical activations for silent and overt speech using event-related fMRI. Hum Brain Mapp. 15(1):39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudjetz A, Oberauer K. 2007. The effects of processing time and processing rate on forgetting in working memory: testing four models of the complex span paradigm. Mem Cogn. 35(7):1675–1684. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. 2004. The spatial and temporal signatures of word production components. Cognition. 92(1–2):101–144. [DOI] [PubMed] [Google Scholar]
- Ivanova MV, Dragoy O, Kuptsova SV, Yu Akinina S, Petrushevskii AG, Fedina ON, Turken A, Shklovsky VM, Dronkers NF. 2018. Neural mechanisms of two different verbal working memory tasks: a VLSM study. Neuropsychologia. 115:25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK. 1992. MEM: mechanisms of recollection. J Cogn Neurosci. 4(3):268–280. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, Sanislow CA. 2005. Using fMRI to investigate a component process of reflection: prefrontal correlates of refreshing a just-activated representation. Cogn Affect Behav Neurosci. 5(3):339–361. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Reeder JA, Raye CL, Mitchell KJ. 2002. Second thoughts versus second looks: an age-related deficit in reflectively refreshing just-activated information. Psychol Sci. 13(1):64–67. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE, Berman MG. 2006. What has functional neuroimaging told us about the mind? So many examples, so little space. Cortex. 42(3):414–417. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Coslett BH, Schwartz MF. 2007. Power in voxel-based lesion-symptom mapping. J Cogn Neurosci. 19(7):1067–1080. [DOI] [PubMed] [Google Scholar]
- Lacey EH, Skipper-Kallal LM, Xing S, Fama ME, Turkeltaub PE. 2017. Mapping common aphasia assessments to underlying cognitive processes and their neural substrates. Neurorehabil Neural Repair. 31(5):442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Crinion JT, Seghier ML, Grogan A, Green DW, Price CJ. 2009. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain 132(Pt 12):3401–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Christ SE, Cowan N. 2014. Domain-general and domain-specific functional networks in working memory. NeuroImage. 102:646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucidi A, Langerock N, Hoareau V, Lemaire B, Camos V, Barrouillet P. 2016. Working memory still needs verbal rehearsal. Mem Cogn. 44(2):197–206. [DOI] [PubMed] [Google Scholar]
- Mah YH, Husain M, Rees G, Nachev P. 2014. Human brain lesion-deficit inference remapped. Brain 137(Pt 9):2522–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Landrigan J-F, Kokolis S, Verillo S, Ferrara C. 2016. Permutation-Based Cluster Size Correction for Voxel-Based Lesion-Symptom Mapping. arXiv:1606.00475 [Q-Bio, Stat]. http://arxiv.org/abs/1606.00475.
- Mora G, Camos V. 2013. Two systems of maintenance in verbal working memory: evidence from the word length effect. PLoS One. 8(7):e70026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora G, Camos V. 2015. Dissociating rehearsal and refreshing in the maintenance of verbal information in 8-year-old children. Front Psychol. 6(11), 11. doi: 10.3389/fpsyg.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller NG, Knight RT. 2006. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 139(1):51–58. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. 2002. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 15(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris D. 2017. Short-term memory and long-term memory are still different. Psychol Bull. 143(9):992–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozari N, Dell GS, Schwartz MF. 2011. Is comprehension necessary for error detection? A conflict-based account of monitoring in speech production. Cogn Psychol. 63(1):1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. 1993. The neural correlates of the verbal component of working memory. Nature. 362(6418):342–345. [DOI] [PubMed] [Google Scholar]
- Postle BR, Awh E, Jonides J, Smith EE, D'Esposito M. 2004. The where and how of attention-based rehearsal in spatial working memory. Cogn Brain Res. 20(2):194–205. [DOI] [PubMed] [Google Scholar]
- Price CJ. 2012. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 62(2):816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. 2004. Functional dissociations within the inferior parietal cortex in verbal working memory. NeuroImage. 22(2):562–573. [DOI] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Greene EJ, Johnson MR. 2007. Refreshing: a minimal executive function. Cortex. 43(1):135–145. [DOI] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Reeder JA, Greene EJ. 2002. Neuroimaging a single thought: dorsolateral PFC activity associated with refreshing just-activated information. NeuroImage. 15(2):447–453. [DOI] [PubMed] [Google Scholar]
- Rogalsky C, Love T, Driscoll D, Anderson SW, Hickok G. 2011. Are mirror neurons the basis of speech perception? Evidence from five cases with damage to the purported human mirror system. Neurocase. 17(2):178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Pitz E, Hillis AE, Hickok G. 2008. Auditory word comprehension impairment in acute stroke: relative contribution of phonemic versus semantic factors. Brain Lang. 107(2):167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Poppa T, Chen K-H, Anderson SW, Damasio H, Love T, Hickok G. 2015. Speech repetition as a window on the neurobiology of auditory-motor integration for speech: a voxel-based lesion symptom mapping study. Neuropsychologia. 71:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. 2012. Age-specific CT and MRI templates for spatial normalization. NeuroImage. 61(4):957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. 1997. Working memory: a view from neuroimaging. Cogn Psychol. 33(1):5–42. [DOI] [PubMed] [Google Scholar]
- Tanji J, Hoshi E. 2008. Role of the lateral prefrontal cortex in executive Behavioral control. Physiol Rev. 88(1):37–57. [DOI] [PubMed] [Google Scholar]
- Tourville JA, Guenther FH. 2011. The DIVA model: a neural theory of speech acquisition and production. Lang Cogn Process. 26(7):952–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Mueller K, Lepsien J, Krämer B, Gruber O. 2014. Different neural capacity limitations for articulatory and non-articulatory maintenance of verbal information. Exp Brain Res. 232(2):619–628. [DOI] [PubMed] [Google Scholar]
- Trost S, Gruber O. 2012. Evidence for a double dissociation of articulatory rehearsal and non-articulatory maintenance of phonological information in human verbal working memory. Neuropsychobiology. 65(3):133–140. [DOI] [PubMed] [Google Scholar]
- Vallar G, Di Betta AM, Silveri MC. 1997. The phonological short-term store-rehearsal system: patterns of impairment and neural correlates. Neuropsychologia. 35(6):795–812. [DOI] [PubMed] [Google Scholar]
- Waugh NC, Norman DA. 1965. Primary memory. Psychol Rev. 72(2):89–104. [DOI] [PubMed] [Google Scholar]
- Wilson SM. 2017. Lesion-symptom mapping in the study of spoken language understanding. Lang Cogn Neurosci. 32(7):891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M. 2004. Listening to speech activates motor areas involved in speech production. Nat Neurosci. 7:701. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kimberg DY, Coslett HB, Schwartz MF, Wang Z. 2014. Multivariate lesion-symptom mapping using support vector regression. Hum Brain Mapp. 35(12):5861–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]


