Abstract
Background.
Von Hippel-Lindau disease (VHL) is a rare autosomal dominant syndrome diagnosed for 1 out of 36000–45000 newborns and 90% of the patients have a clinical manifestation before 65 years of age. Affected individuals have an increased risk of developing tumours in several organs or their systems. The most common tumours are retinal or central nervous system hemangioblastomas (60–80%) and VHL-associated renal lesions. Contrast-enhanced computer tomography (CECT) is the gold standard for the diagnosis and characterization of renal tumours. The best treatment option for VHL syndrome-caused renal tumours are nephron-sparing treatment techniques (cryotherapy, radiofrequency, or microwave ablation), which require imaging control. All these innovative treatment techniques are extremely important for VHL patients, because they increase the quality of life by staving off renal dialysis and preventing distant metastases.
Case report.
Our case report presents a 16-year-old female with multiple renal cysts observed on ultrasound examination and clinically and molecularly diagnosed with Von Hippel-Lindau syndrome (deletion of the entire VHL gene). After that, for past 11 years multiple renal tumours were removed by cryoablation and patient monitoring on contrast-enhanced magnetic resonance (MRI) and CECT control scans was conducted.
Conclusions.
Active multidisciplinary patient follow-up, routine radiological examinations, and correct treatment tactics allow controlling the progression of renal cell carcinoma and other tumours associated with VHL syndrome, maintaining a normal organ function for a long time, and preventing distant metastases and fatal disease outcomes.
Keywords: VHL syndrome, clear cell RCC, cryoablation
Abstract
VON HIPPEL-LINDAU SINDROMAS IR INKSTŲ NAVIKAI: RADIOLOGINĖ DIAGNOSTIKA IR GYDYMO GALIMYBĖS. KLINIKINIO ATVEJO ANALIZĖ IR MOKSLINĖS LITERATŪROS APŽVALGA
Santrauka
Apžvalga. Von Hippel-Lindau (VHL) yra retas dominantinis autosominis sindromas, diagnozuojamas 1 iš 36 000–45 000 naujagimių, kliniškai jis pasireiškia 90 % sergančiųjų sulaukus 65 metų. Šiuo sindromu sergantys asmenys turi didesnę navikų išsivystymo riziką keliuose organuose ar jų sistemose. Dažniausiai pasitaikantys navikai yra tinklainės ar centrinės nervų sistemos hemangioblastomos (60–80 %) ir su VHL susiję inkstų pažeidimai. Kompiuterinė tomografija (KT) yra auksinis diagnostikos standartas inkstų navikų atveju. Geriausias VHL sindromu sergančių pacientų inkstų navikų gydymo būdas yra nefronus tausojantys gydymo metodai (krioterapija, radiodažnuminė terapija arba mikrobangų abliacija), kuriems reikalinga radiologinių tyrimų kontrolė. Visi šie gydymo metodai yra labai svarbūs VHL sindromu sergantiems pacientams, nes pagerina jų gyvenimo kokybę, atitolindami inkstų dializę ir užkirsdami kelią tolimų metastazių atsiradimui.
Atvejo aprašymas. Aprašoma 16-metų pacientė, kuriai ultragarsinio tyrimo metu buvo pastebėtos kelios inkstų cistos, o genetiniais ir molekuliniais tyrimais buvo diagnozuotas Von Hippel-Lindau sindromas. Vėliau, per 11 metų, pacientei buvo diagnozuoti daugybiniai inkstų navikai, kurie buvo pašalinti taikant krioabliaciją, ligos kontrolei buvo atliekami kompiuterinės tomografijos (KT) ir magnetinio rezonanso (MRT) tyrimai.
Išvados. Multidisciplininis aktyvus paciento stebėjimas, radiologiniai tyrimai ir teisinga gydymo taktika leidžia kontroliuoti inkstų karcinomų ir kitų navikų, susijusių su VHL sindromu, progresavimą, ilgą laiką palaikyti normalią organų funkciją, užkirsti kelią tolimoms metastazėms ir mirtinų ligų, kurias gali sukelti VHL sindromas, baigtims.
Raktažodžiai: VHL sindromas, renoceliulinė karcinoma, krioabliacija
BACKGROUND
Von Hippel-Lindau disease (VHL) is a rare autosomal dominant syndrome, which typically appears due to various mutations in the VHL gene located on the short arm of chromosome 3. VHL syndrome is diagnosed for 1 out of 36000–45000 newborns and 90% of the patients have a clinical manifestation until 65 years of age (1, 2). Affected individuals have an increased risk of developing tumours in several organs or their systems, including the central nervous system, kidneys, adrenals, the pancreas or the reproductive system. Clinical manifestation and the type of the disease depend on the type of germline mutation (3). The most common tumours are retinal or central nervous system hemangioblastomas (60–80%) and VHL-associated renal lesions that may vary from simple cysts to multiple and bilateral renal cell carcinomas (RCCs) (24–45%). In affected individuals, the most common renal RCC is clear cell renal cell carcinoma (ccRCC), which is usually diagnosed at 25–40 years of age and is one of the most common causes of death among VHL patients (3). CcRCCs under 3 cm are usually asymptomatic for a long period of time and at a low risk of metastasizing in VHL patients; however, in most cases they are reciprocal, multiple, and prone to relapse (3, 4). It is important to recognize patients with VHL syndrome because they have to be observed for the whole life to prolong their lifetime and improve the quality of their life. Contrast-enhanced computer tomography (CECT) is the gold standard for the characterization of renal tumours; likewise, other radiological imaging techniques such as ultrasound (US), magnetic resonance imaging (MRI), or positron emission tomography (PET) are also used in malignant renal tumours detection (5–7). CECT, US, or MRI are important tools not only for RCCs diagnostics but also for the treatment, as radical nephrectomy is not recommended for patients with VHL. Nowadays other innovative nephron-sparing treatment techniques (cryotherapy, radiof-requency or microwave ablation), which require imaging control, are used (8). All these innovative treatment techniques are extremely important for VHL patients, because they increase the quality of life by staving off renal dialysis and preventing distant metastases (3).
CASE REPORT
In December 2008, a 16-year-old female patient came for the first genetic consultation because of the predisposition to VHL genetic mutation. Patient genetic testing was indicated when multiple renal cysts were observed on ultrasound examination, and due to a possible inheritance of the genetic syndrome. The patient was clinically and molecularly diagnosed with von Hippel-Lindau syndrome (deletion of the entire VHL gene). In the course of the following five years, renal cysts were followed up by abdominal contrast-enhanced MRI once a year, according to international recommendations (36). After five years, intra-parenchymal hyper vascular tumour of 14 mm was observed in the middle third of the left kidney (Fig. A).The patient was sent to the National Cancer Institute (NCI), where cryoablation was suggested as the least traumatic minimal invasive procedure. CECT detected six tumours in the kidneys – three in the left kidney, three in the right kidney, and multiple renal cysts on both sides. At this stage, it was decided to perform a biopsy and cryoablation of the largest tumour in the middle third of the left kidney. Postoperative CECT showed the ablation margin to be sufficient. The histological report showed moderate grade G2 by WHO II0 by Fuhrman ccRCC. Few months later, cryoablation of the largest tumours in the lower poles of the left and right kidneys was done. Left nephrostomy was established for ureteric protection by warm solution infusion during freezing cycles. After ablation, a long-term ureteric stent was placed in the left ureter to prevent stenosis. It was removed due to fever two months later. After one-year, CECT showed a decrease in ablation zones and no signs of local recurrence were found, although a new tumour in the upper part of the left kidney was found (9 mm in size) and it was decided to have a follow-up. After two years, a control contrast-enhanced abdominal MRI was performed that showed four tumours – one in the left kidney and three in the right one. It was decided to perform cryoablation of two largest tumours in the right kidney and, few months later, of single tumour in the left kidney. The small tumour in the middle third of the right kidney was left for the follow-up. The patient underwent cryoablation of right kidney tumours and the left kidney tumour one month later. On control CECT, no new tumours were found; there was only one 10 mm tumour in middle part of the right kidney that was left for the follow-up. Few years later, control CECT (Fig. C, D) showed an enlarging 18 mm tumour in the middle third of the right kidney. In addition, several new tumourous structures of up to 15 mm in size were observed in the kidneys. The follow-up strategy for all tumours was approved. After four years, four enlarging tumours in the kidneys were found on CECT (Fig. E) – one in the left kidney and three in the right one. It was decided to perform cryoablation of three tumours of the right kidney (Fig. F, G).
Fig. 1.
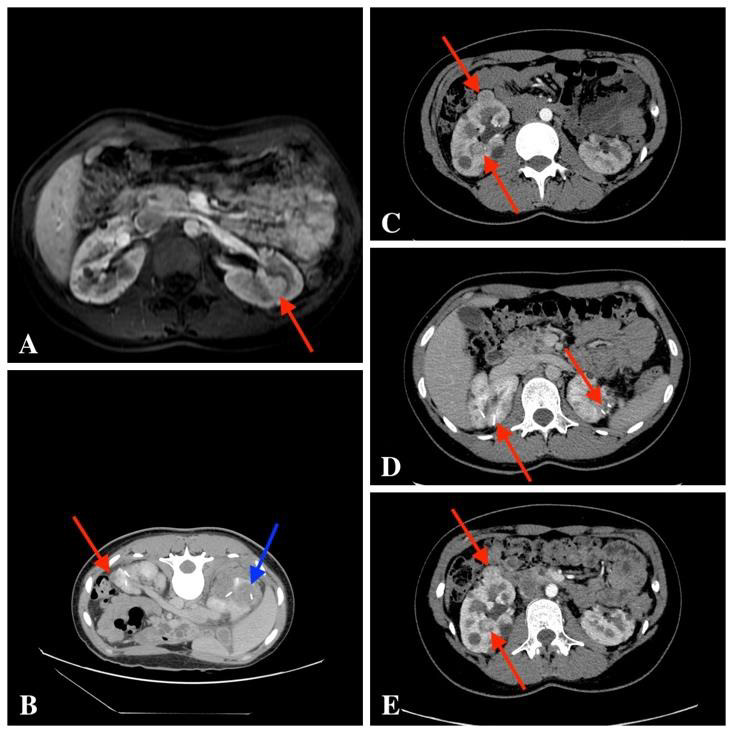
Fig. A. Contrast-enhanced MRI scan T1 weighted image, intraparenchymal 14 mm hypervascular tumour was observed in the middle third of the left kidney
Fig. B. Post-cryo-CECT, fresh ablation zone (blue arrow) and the resorbed ablation zone (red arrow)
Fig. C. CECT scan: multiple tumours in the kidneys. The enlarging 18 mm tumour in the middle third of the right kidney; in addition, several new tumourous structures of up to 15 mm in both kidneys
Fig. D. CECT scan shows previous ablation areas
Fig. E. CECT scan shows growing kidney tumours – four enlarging tumours in the kidneys were found: one in the left kidney and three in the right one
Fig. 2.
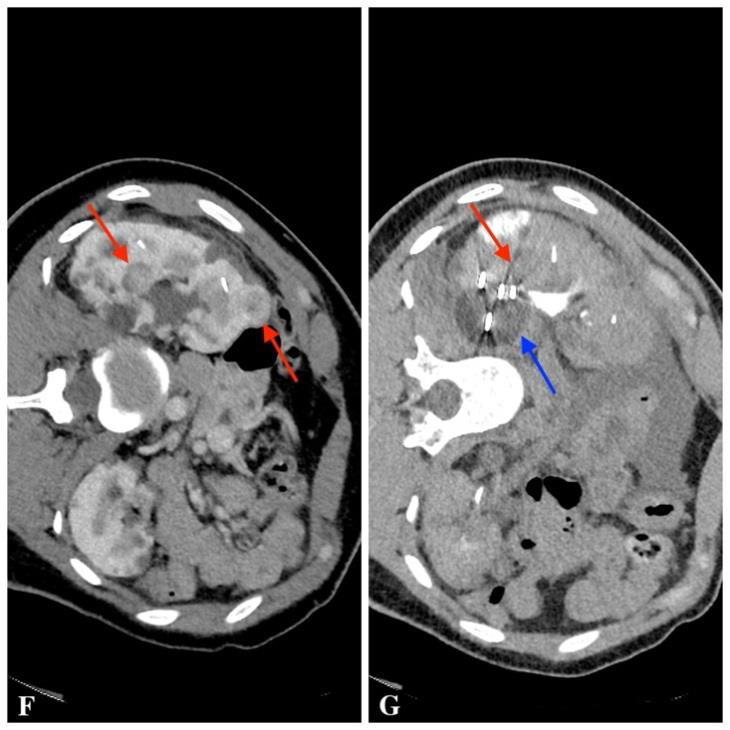
Fig. F. CECT scan before cryoablation of three tumours of the right kidney
Fig. G. CECT scan during cryoablation: the monitored ablation probe (red arrow) and a tumour-containing “ice bubble” (blue arrow)
The patient was also actively monitored by neurologists, neurosurgeons, and other professionals in accordance with international VHL syndrome surveillance guidelines (36). During this monitoring, hemangioblastoma of the right cerebellar hemisphere was detected, which was removed by posterior trepanation. Later, after control MRI examination, CNS tumours were detected in the upper and left lateral part of the cerebellum, a mass at the C7 vertebra level was also observed. Surgical treatment was contraindicated due to the high risk of neurological complications, so the patient was treated with radiotherapy. During a subsequent control scan, an ovarian cyst was found and right ovarian endometrioid cyst was confirmed after laparoscopic cystectomy. No other malignancies were found.
It is recommended to continue active monitoring of existing and/or newborn kidney tumours by conducting an annual abdominal contrast-enhanced MRI and cryoablation if needed to prevent distant metastases and fatal disease. An annual neurologist consultation, a complete neurological examination, and monitoring of CNS dynamics of the existing and/or newly emerging tumours should be indicated, with CNS MRI performed every two years. It is also recommended to perform an ophthalmoscopy and an audiogram every year. Plasma hormone testing (methanephrine, normetanephrine, chromogranin A) and blood pressure monitoring is needed (the patient had a low risk of developing pheochromocytoma) (36).
DISCUSSION
Von Hippel-Lindau disease is a rare autosomal dominant syndrome. Most patients with confirmed VHL syndrome have a positive family history, but the literature indicates that up to 20% of newly diagnosed cases may occur de novo (without a positive family history). That is why it is important to know how to identify this syndrome so that reasonable tumour detection and treatment could be start (9).
Among patients with VHL syndrome, pathological kidney masses can range from simple cysts to solid malignant tumours. Each kidney can have as many as 1100 cysts and 600 malignancies of renal cell carcinomas (10). According to different references, RCCs account for 3% of all malignant tumours in adults and 85% of all primary renal tumours (11). Also, in more than 75% of patients kidney carcinomas and cysts are multiple and reciprocal localization (12). As many as 5% of all RCCs are hereditary, with VHL syndrome being the most common cause; in most cases, renal cell carcinomas and multiple renal cysts start to develop between the ages of 25 and 40, but are rarely the first manifestations of VHL syndrome (13).
Differentiation of ccRCCs (75% of all RCCs) from other non-ccRCCs plays an important role in clinical practice due to differences in the genetic expression, variety of symptoms, the treatment choice, and prognosis of survival (14, 15). A detailed analysis of 328 patients with pathological renal disease indicates that in 23 subjects with VHL, 58% of tumours had carcinomas, all of which (100%) were ccRCCs. The remaining pathological findings in the kidney were either simple (31%) or atypical (7%) cysts (16). VHL syndrome-associated ccRCCs usually have a low degree of differentiation and slow growth of 0.2 cm to 2.2 cm annually, averaging 1.6 cm per year and very rarely metastatic to 3 cm in size (17). As the tumours are becoming bigger, the risk of metastasis to the lymph nodes, bones, lungs, the liver, and the CNS increases. VHL syndrome-associated RCCs and cysts can remain asymptomatic for a long time, as can common kidney cysts. As the disease progresses, major renal carcinomas may present with lateral pain, haematuria and/or a palpable tumour mass in the lateral area (18).
The primary goals of the diagnosis of VHL are active monitoring of the manifestation of the disease from childhood, early diagnosis and treatment of the syndrome-related tumours. Continuous monitoring of the patient plays an important role not only in the identification of new tumours but also requires the constant monitoring of small, asymptomatic tumours. CECT scans are the gold standard test method for diagnosis of kidney pathology and the clinical stage of tumour. CT scan sensitivity reaches more than 90% in the diagnosis of renal tumours smaller than 2 cm and almost 99.9% when the tumour is larger than 2 cm, but diagnosis of RCCs sensitivity ranges from 60% to 79% and specificity ranges from 44% to 99.9%. That is why radiological diagnosis of renal tumours must be confirmed by a histological examination (19, 20). The main advantage of CT examination over MRI or UG is the absorption of X-rays, which are expressed in Hounsfield units (different types of kidney cancer – light cells, papillary cells, and chromophobe cells – have different densities) (21). Usually, ccRCCs exhibit exophytic growth and are seen in CT images as a heterogeneous derivative due to the characteristic necrotic, cystic, or haemorrhagic intranodal areas (22, 23). It is reported in the literature that as many as 15% of all RCCs may have a cystic component (24).
Although CT is considered to be the most sensitive method for the diagnosis of kidney tumours, regular monitoring of patients with VHL syndrome by MRI and UG is preferred because of the reduced radiation exposure. MRI is based on the interaction of a strong magnetic field with the body’s use of hydrogen ions and therefore has no toxic radiation effects. Contrast-enhanced MRI is also indicated in patients who cannot undergo CECT due to iodine allergy. MRI is considered the optimal method for differentiation of complicated cystic masses and solid tumours in the kidneys (25). Therefore, it is recommended that abdominal MRI be performed annually to monitor the progression of renal tumours (5–7).
In today’s medical practice, kidney-sparing minimally invasive surgery is the gold standard in the treatment of RCCs with the primary goal of preventing distant metastases and maintaining a normal renal function.
Kidney-sparing surgery should replace traditional full nephrectomy when the size of the kidney tumour does not exceed 3–4 cm. However, very common partial nephrectomies have an adverse effect on the renal function, may lead to renal failure and require early dialysis; therefore, optimal intervals between surgical interventions and targeted patient selection should be maintained. For patients with VHL syndrome, due to multiple and persistent RCCs, the most optimal treatment for RCCs – radical surgery – is not recommended. In current practice, surgical resection of RCCs associated with VHL syndrome has been replaced by modern kidney-sparing treatments for small, multiple, and asymptomatic RCCs. These minimally invasive procedures include: microwave ablation, radio frequency ablation (RFA), cryoablation, and stereotaxic radiotherapy (a non-invasive method of treatment of external radiation therapy with low toxicity, in which the dose of ionizing radiation irradiates the pathological kidney foci). Among patients with VHL, the long-term efficacy of stereotactic radiotherapy is not precisely known due to the lack of research and irreversible electroporation (an alternative and promising approach for the treatment of renal tumours at the clinical trial stage) (26–30).
Microwave ablation is a minimally invasive treatment of renal tumours, which creates a thermal field leading to pathological tissue necrosis by means of special antennas and electromagnetic microwaves (930–2450 MHz). Microwave ablation is considered to have an advantage over RFA treatment because of the use of more powerful energy waves that produce a greater thermal effect. It is also stated that microwave ablation can be used when the kidney tumour is localized in the renal collecting system and other ablation techniques make the procedures more complicated (28, 31).
RFA is a minimally invasive method of treating renal tumours. It uses high frequency (460–55 kHz) alternating current transmitted to the tumour via a thin needle electrode. During the procedure, the electric current turns into radio frequency waves and the pathological tissue is damaged due to the high temperature released (32).
Cryoablation is a minimally invasive method of treating renal tumours, in which the catheter with a refrigerant is delivered and the pathological focus is frozen. The resulting intracellular crystals damage the integrity of the cell wall and other physiological processes and cause the cell to die. The location and the size of the kidney tumour have the greatest influence on the success of the procedure. Cryoablation treatment of a tumour located in the central part of the kidney that extends more than 4 cm is most often unsuccessful (27). Procedures are performed under CT, MRI, or UG control percutaneously or laparoscopically. Both RFID and cryoablation show high treatment efficacy and low complication rates during interventions. Most kidney tumours of up to 3 cm in size are effectively destroyed, but tumours larger than 4 cm can be treated by several iterations of the ablation procedure. It is recommended that the efficacy of the treatment be evaluated daily by CECT or MRI. The effectiveness of treatment is indicated by the formation of a 0.5–1 cm ablation zone around the tumour (33).
Renal transplantation is successfully performed in patients with VHL syndrome who require reciprocal nephrectomy for multiple, late-stage RCCs and renal failure. Kidney transplantation is not yet widespread as a treatment alternative for VHL syndrome due to a lack of research and the widespread belief that post-transplant immunosuppression may increase the likelihood of returning new tumours. However, in one study of 32 patients with VHL syndrome and 32 patients without renal transplant syndrome, no significant differences were observed between the two groups when comparing the survival prognosis or the changes in the renal function over the 4-year active follow-up period (34).
Systemic drug therapy is also applied today in medical practice for VHL syndrome-associated RRCs. Suppression of the expression of the endothelial growth factor (VEGF) by tyrosine kinase inhibitors (sunitinib, sorafenib, pazopanib and axitinib, etc.) is thought to help reduce the size of renal cellular carcinomas and the frequency of surgical interventions. In a clinical study of 15 patients with VHL syndrome, Jonasch et al. observed a partial response rate of 33% with sunitinib (35).
CONCLUSIONS
VHL syndrome is a rare genetic disorder characterized by the uncontrolled manifestation of tumours in various organs or systems, requiring close monitoring of patients. Early diagnosis of the disease by molecular tests for at-risk groups and monitoring of disease progression through international programmes offer the potential to reduce early VHL-related mortality, which currently averages 49 years. The most common tumours leading to disability and premature mortality are hemangioblastoma, RCC, and pheochromocytoma. Computed tomography (CT) imaging is considered to be the most sensitive test method for recognizing asymptomatic RCCs, but routine MRI or ultrasound to reduce the dose of radiation received is more common. Currently, the most popular treatment methods include kidney-sparing treatments: RFID, microwave or cryoablation, and other emerging modern methods or kidney transplantation. Medication is also available to prevent the progression of the disease and distant metastasis.
We can conclude that active multidisciplinary patient follow-up, routine radiological examinations, and correct treatment tactics enable control of the progression of renal cell carcinoma and other VHL syndrome-associated tumours, to maintain normal organ function for a long time, and to prevent distant metastases and fatal disease outcomes.
Audrius Untanas, Mantas Trakymas, Indrė Lekienė, Rūta Briedienė
References
- Evans DG Howard E Giblin C Clancy T Spencer H Huson SM et al.. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010. February; 152A (2): 327–32. [DOI] [PubMed] [Google Scholar]
- Burhan H Burhan* H Ansari AB Shah M Lateef N Kazmi M et al.. The genetic and clinical aspects of Von Hippel-Lindau syndrome. Int J Hematol Ther. 2018. January 31; 4(1): 1–7. [Google Scholar]
- Hes FJ, Höppener JW, Luijt RB van der, Lips CJ. Von Hippel-Lindau disease. Hered Cancer Clin Pract. 2005. November 15; 3(4): 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashouri K, Mohseni S, Tourtelot J, Sharma P, Spiess PE. Implications of Von Hippel-Lindau syndrome and renal cell carcinoma. J Kidney Cancer VHL. 2015. September 25; 2(4): 163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbrun ME Remer EM Casalino DD Beland MD Bishoff JT Blaufox MD et al.. ACR Appropriateness Criteria indeterminate renal mass. J Am Coll Radiol JACR. 2015. April; 12(4): 333–41. [DOI] [PubMed] [Google Scholar]
- Vikram R Beland MD Blaufox MD Moreno CC Gore JL Harvin HJ et al.. ACR appropriateness criteria renal cell carcinoma staging. J Am Coll Radiol JACR. 2016. May; 13(5): 518–25. [DOI] [PubMed] [Google Scholar]
- Kay FU, Pedrosa I. Imaging of solid renal masses. Radiol Clin North Am. 2017. March; 55(2): 243–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BK, Kim CK, Park SY, Shen S-H. Percutaneous radiofrequency ablation of renal cell carcinomas in patients with von Hippel Lindau disease: indications, techniques, complications, and outcomes. Acta Radiol Stockh Swed 1987. 2013. May; 54(4): 418–27. [DOI] [PubMed] [Google Scholar]
- Aufforth RD Ramakant P Sadowski SM Mehta A Trebska-McGowan K Nilubol N et al.. Pheochromocytoma screening initiation and frequency in von Hippel-Lindau syndrome. J Clin Endocrinol Metab. 2015. Dec; 100(12): 4498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittiboina P, Lonser RR. Von Hippel–Lindau disease. Handb Clin Neurol. 2015; 132: 139–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L Xu B Wang Y Liu C Lu K Huang Y et al.. Advanced renal cell carcinoma associated with von Hippel-Lindau disease: a case report and review of the literature. Oncol Lett. 2015. August 1; 10(2): 1087–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilg CA Neumann HP Gläsker S Schäfer O Ardelt PU Schwardt M et al.. Growth kinetics in von Hippel-Lindau-associated renal cell carcinoma. Urol Int. 2012; 88(1): 71–8. [DOI] [PubMed] [Google Scholar]
- Varshney N, Kebede AA, Owusu-Dapaah H, Lather J, Kaushik M, Bhullar JS. A review of von Hippel-Lindau syndrome. J Kidney Cancer VHL. 2017. August 2; 4(3): 20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JJ Purdue MP Signoretti S Swanton C Albiges L Schmidinger M et al.. Renal cell carcinoma. Nat Rev Dis Primer. 2017. Mar 9; 3: 17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S Stawiski EW Pavía-Jiménez A Modrusan Z Kapur P Jaiswal BS et al.. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat Genet. 2015. January; 47(1): 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraf F, Chauveau D, Chrétien Y, Richard S, Grünfeld JP, Droz D. Renal lesions in von Hippel-Lindau disease: immunohistochemical expression of nephron differentiation molecules, adhesion molecules and apoptosis proteins. Histopathology. 2000. May; 36(5): 457–65. [DOI] [PubMed] [Google Scholar]
- Diagnostic approach, differential diagnosis, and management of a small renal mass – UpToDate [Internet]. [cited 2019. May 4]. Available from: https://www.uptodate.com/contents/diagnostic-approach-differential-diagnosis-and-management-of-a-small-renal-mass [Google Scholar]
- Crespigio J Berbel LCL Dias MA Berbel RF Pereira SS Pignatelli D et al.. Von Hippel–Lindau disease: a single gene, several hereditary tumors. J Endocrinol Invest. 2018. January 1; 41(1): 21–31. [DOI] [PubMed] [Google Scholar]
- Kay FU, Pedrosa I. Imaging of solid renal masses. Radiol Clin North Am. 2017. March; 55(2): 243–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH Sun HY Hwang J Hong SS Cho YJ Doo SW et al.. Diagnostic accuracy of contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging of small renal masses in real practice: sensitivity and specificity according to subjective radiologic interpretation. World J Surg Oncol. 2016. October 12; 14(1): 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JR, Margolis D, Sauk S, Pantuck AJ, Sayre J, Raman SS. Clear cell renal cell carcinoma: discrimination from other renal cell carcinoma subtypes and oncocytoma at multiphasic multidetector CT. Radiology. 2013. May; 267(2): 444–53. [DOI] [PubMed] [Google Scholar]
- Gurel S, Narra V, Elsayes KM, Siegel CL, Chen ZE, Brown JJ. Subtypes of renal cell carcinoma: MRI and pathological features. Diagn Interv Radiol Ank Turk. 2013. August; 19(4): 304–11. [DOI] [PubMed] [Google Scholar]
- Low G, Huang G, Fu W, Moloo Z, Girgis S. Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol. 2016. May 28; 8(5): 484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon AD, Pedrosa I. Imaging and screening of kidney cancer. Radiol Clin North Am. 2017. November; 55(6): 1235–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel GM, Hindman N, Bosniak MA. Evaluation of cystic renal masses: comparison of CT and MR imaging by using the Bosniak classification system. Radiology. 2004. May; 231(2): 365–71. [DOI] [PubMed] [Google Scholar]
- Allasia M Soria F Battaglia A Gazzera C Calandri M Caprino MP et al.. Radiofrequency ablation for renal cancer in von Hippel-Lindau syndrome patients: a prospective cohort analysis. Clin Genitourin Cancer. 2017. August 10. [DOI] [PubMed] [Google Scholar]
- Yamanaka T Yamakado K Yamada T Fujimori M Takaki H Nakatsuka A et al.. CT-guided percutaneous cryoablation in renal cell carcinoma: factors affecting local tumor control. J Vasc Interv Radiol JVIR. 2015. August; 26(8): 1147–53. [DOI] [PubMed] [Google Scholar]
- Yu J Zhang G Liang P Yu X Cheng Z Han Z et al.. Midterm results of percutaneous microwave ablation under ultrasound guidance versus retroperitoneal laparoscopic radial nephrectomy for small renal cell carcinoma. Abdom Imaging. 2015. October; 40(8): 3248–56. [DOI] [PubMed] [Google Scholar]
- Wersäll PJ Blomgren H Lax I Kälkner K-M Linder C Lundell G et al.. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2005. October; 77(1): 88–95. [DOI] [PubMed] [Google Scholar]
- Olweny EO, Kapur P, Tan YK, Park SK, Adibi M, Cadeddu JA. Irreversible electroporation: evaluation of nonthermal and thermal ablative capabilities in the porcine kidney. Urology. 2013. March. [DOI] [PubMed] [Google Scholar]
- Chan P Vélasco S Vesselle G Boucebci S Herpe G Debaene B et al.. Percutaneous microwave ablation of renal cancers under CT guidance: safety and efficacy with a 2-year follow-up. Clin Radiol. 2017. September; 72(9): 786–92; 81(3): 679–84. [DOI] [PubMed] [Google Scholar]
- Iannuccilli JD, Dupuy DE, Beland MD, Machan JT, Golijanin DJ, Mayo-Smith WW. Effectiveness and safety of computed tomography-guided radiofrequency ablation of renal cancer: a 14-year single institution experience in 203 patients. Eur Radiol. 2016. June; 26(6): 1656–64. [DOI] [PubMed] [Google Scholar]
- Park BK, Kim CK, Park SY, Shen S-H. Percutaneous radiofrequency ablation of renal cell carcinomas in patients with von Hippel Lindau disease: indications, techniques, complications, and outcomes. Acta Radiol Stockh Swed 1987. 2013. May; 54(4): 418–27. [DOI] [PubMed] [Google Scholar]
- Clinical features, diagnosis, and management of von Hippel-Lindau disease – UpToDate [Internet]. [cited 2019. May 4]. Available from: https://www.uptodate.com/contents/clinical-features-diagnosis-and-management-of-von-hippel-lindau-disease [Google Scholar]
- Jonasch E McCutcheon IE Waguespack SG Wen S Davis DW Smith LA et al.. Pilot trial of sunitinib therapy in patients with von Hippel-Lindau disease. Ann Oncol Off J Eur Soc Med Oncol. 2011. December; 22(12): 2661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.vhl.org/wp-content/uploads/2017/07/Active-Surveillance-Guidelines.pdf. [Google Scholar]


