Abstract
Background.
Population-based EUROCARE-5 studies demonstrated that childhood cancer survival rates in Lithuania were 10–20% lower than the European mean. We aimed to analyse the change in the outcome of treatment of paediatric malignancies in Lithuania over 30 years.
Methods.
A single-centre retrospective analysis of children below 18 years of age treated for cancer at Vilnius University Hospital Santaros Klinikos between 1982 and 2011 was carried out. The minimal requirement of 5-year follow-up after diagnosis was specified for survival estimation. The vital status was assessed using data from the population-based Lithuanian Cancer Registry. To evaluate changes over time, the entire cohort was split into three groups according to the time of diagnosis: 1982–1991, 1992–2001, and 2002–2011.
Results.
A total of 1268 children met the inclusion criteria. The shortest median follow-up was 8.9 (IQR 6.4–11.5) years for patients treated in the third decade. The 5-year overall survival of the entire cohort increased from 37.3% (95% CI 30.2–44.3) in 1982–1991 to 70.7% (95% CI 66.4–74.1) in 2002–2011 (p < 0.0001). The same trend was evident when calculated separately for leukaemia (p < 0.0001), lymphoma (p < 0.0005), and solid tumours (p < 0.004). The percentage of cure rose from zero in the early years of the period analysed to 80% in 2010 and 2011. The improvement in the treatment outcome was attributable to the reduction of treatment-related mortality from 45.8% in 1982–1991 to 12.4% in 2002–2011 and disease recurrence from 30.4% to 19.6% for the same periods, respectively.
Conclusions.
Significant progress in the cure rate of children treated for cancer at our institution was observed over 30 years. Collaborative national and international clinical and research efforts are crucial to ensure further advances in care and cure.
Keywords: childhood cancer, survival, Lithuania
Abstract
VAIKŲ VĖŽIO IŠEIČIŲ PAGERĖJIMAS LIETUVOJE PER 30 METŲ
Santrauka
Įvadas. Remiantis populiacinių EUROCARE-5 analizių duomenimis, nuo vėžio gydytų Lietuvos vaikų išgyvenamumas 10–20 % mažesnis nei Europos vidurkis. Mes siekėme išanalizuoti vaikų vėžio gydymo išeičių pokyčius per 30 metų.
Metodai. Atlikta retrospektyvinė vieno centro analizė, į kurią įtraukti vaikai iki 18 metų, gydyti nuo vėžio 1982–2011 m. Vilnius universiteto ligoninės Santaros klinikose (VULSK). Pacientų išgyvenamumas vertintas praėjus mažiausiai penkeriems metams nuo diagnozės nustatymo. Gyvybės statusas vertintas remiantis Lietuvos vėžio registro duomenimis. Pokyčių per 30 metų analizei pacientai buvo suskirstyti į tris grupes priklausomai nuo diagnozės nustatymo metų: 1982–1991, 1992–2001 ir 2002–2011.
Rezultatai. 1268 vaikai atitiko įtraukimo kriterijus. Trumpiausias vidutinis pacientų, gydytų paskutiniuoju dešimtmečiu, stebėjimo laikas siekė 8,9 (IQR 6,4–11,5) metų. Visos tyrimo kohortos bendras penkerių metų išgyvenamumas padidėjo nuo 37,3 % (95 % PI 30,2–44,3) 1982–1991 m. iki 70,7 % (95 % PI 66,4–74,1) 2002–2011 m. (p < 0,0001). Analogiška tendencija išliko skaičiuojant išgyvenamumą atskirai leukemijų (p < 0,0001), limfomų (p < 0,0005) ir solidinių navikų (p < 0,004) grupėse. Pasveikusiųjų pacientų procentas padidėjo nuo nulio ankstyvais gydymo metais iki 80 % gydytų 2010 ir 2011 metais. Gydymo išeičių pagerėjimas sietinas su mirtingumo dėl toksinių komplikacijų sumažėjimu nuo 45,8 % 1982–1991 m. iki 12,4 % 2002–2011 m. ir sumažėjusiu ligos recidyvo dažniu nuo 30,4 % iki 19,6 % atitinkamais laikotarpiais.
Išvados. Vaikų, gydytų nuo vėžio VULSK, išgyvenamumas per 30 metų reikšmingai pagerėjo. Norint užtikrinti tolesnę pažangą gydant vaikų vėžį, būtinas nacionalinis ir tarptautinis bendradarbiavimas tiek teikiant gydymo paslaugas, tiek ir mokslo srityje.
Raktažodžiai: vaikų vėžys, išgyvenamumas, Lietuva
INTRODUCTION
Childhood cancer is a rare disease. Cancer does not fall into the top 100 of the most-reported paediatric diseases encountered across outpatient visits in a children’s hospital (1). Despite being rare, childhood cancer remains a major public health issue in Europe (2). Whilst overall survival rates have reached 80% in the highest-performing European countries, more than 6,000 young people die each year of cancer, disproportionately from Eastern European countries. Cancer continues to be the leading cause of death from disease of children beyond one year of age in Europe and North America, surpassed only by accidents (3).
Series of population-based EUROCARE-5 studies demonstrate that survival rates of childhood cancer in Lithuania are 10–20% lower than the European mean (4–6). Poorer outcome indicators were related to the inferior health expenditure in the country (below 2,000 USD per capita1) and restricted access to international funding aimed at supporting research activities and collaboration (7). Incomplete cancer registration could partially contribute to inferior survival estimates (8). Considerable advances in the outcome of treatment in Lithuanian were reported in population-based studies on childhood leukaemia (9, 10) and single-centre analyses on solid tumours (11–13). However, the survival rate in paediatric malignancies taken as a whole was not addressed.
Within the current study, we aimed to analyse the change in the treatment outcome over 30-year observation period. Long-term survival beyond five years was targeted in all cancer types diagnosed and treated at the major paediatric oncology centre in Lithuania.
METHODS
Study population
A single-centre retrospective analysis was carried out from November 2014 to December 2019. Children below 18 years of age diagnosed with cancer (ICD-10-AM codes C00-C96) between 1982 and 2011 were included. The study time frame was set up with the aim to evaluate long-term survival defined as at least 5-year follow-up after diagnosis.
The study population was identified through a review of the institutional database of the Centre for Paediatric Oncology and Haematology at Vilnius University Hospital Santaros Klinikos (VUHSK), the major paediatric oncology centre in Lithuania. The patients’ baseline characteristics were extracted from the institutional database. Electronic medical records were consulted to retrieve missing information. The retrieved cases were cross-checked with the Lithuanian Cancer Registry (LCR) using personal identification code unique for each individual.
Follow-up
The vital status of the study group was assessed as of 31 December 2016 by passive follow-up, using data from the LCR, which is a population-based cancer registry. The LCR collects personal and demographic information (place of residence, sex, date of birth, vital status), as well as information on diagnosis (cancer site, date of diagnosis, method of cancer verification) and death (date of death, cause of death) of all cancer patients (including children) in Lithuania.
Statistical analysis
The probability of the overall survival was chosen as a primary endpoint to assess the treatment outcome. The overall survival was defined as the time from diagnosis to death from any cause or to the end of follow-up.
In order to evaluate the changes in the survival rates over the 30-year time period, the included cases were split into three groups according to the time of diagnosis: 1982 to 1991, 1992 to 2001, and 2002 to 2011. We calculated 5-, 10-, 15-, and 20-year survival for all malignant neoplasms, and separately for leukaemias, lymphomas, and solid tumours.
The overall survival was estimated by the Kaplan-Meier method. The statistical difference between the survival curves was determined using the log-rank test. P < 0.05 indicated a statistically significant difference. All statistical analyses were performed using Stata Statistical Software v.11.0. (StataCorp. 2009. Stata Statistical Software: Release 11.0. College Station, TX, USA).
Ethics
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and national research committee, with the 1964 Helsinki Declaration and its later amendments, or comparable ethical standards. The study was approved by the Vilnius Regional Committee of Biomedical Research (Approval No.2019/10-1154-645) and granted a waiver of informed consent.
RESULTS
Baseline characteristics
A total of 1293 children below the age of 18 years were diagnosed and treated for cancer at Vilnius University Hospital Santaros Klinikos between 1982 and 2011. Since 25 patients without follow-up information were ineligible for assessment, 1268 subjects were included in the final analysis. The number of patients who met the inclusion criteria differed across the three periods: it increased from 227 in 1982–1991 to 550 in 2002–2011, thus 43.3% of cases were treated during the last period (for details on baseline characteristic, refer to Table 1).
Table 1.
Distribution of cancer cases considered in the analysis (N = 1268) by period of diagnosis
| Diagnosis | Period of diagnosis | ||
|---|---|---|---|
| 1982–1991 | 1992–2001 | 2002–2011 | |
| Leukaemia | |||
| ALL | 181 | 253 | 190 |
| AML | 32 | 41 | 43 |
| CML | 5 | 5 | 7 |
| JMML | 2 | 3 | |
| MDS* | 1 | 2 | |
| Lymphoma | |||
| Hodgkin lymphoma | 2 | 62 | 52 |
| Non-Hodgkin lymphoma | 6 | 49 | 51 |
| Solid tumours | |||
| Neuroblastoma | 1 | 20 | 37 |
| Wilms tumours | 25 | 34 | |
| Soft tissue sarcomas | 7 | 35 | |
| Ewing sarcoma/PNET | 7 | 28 | |
| Osteosarcoma | 6 | 24 | |
| Germ cell tumours | 1 | 15 | |
| Retinoblastoma | 2 | 8 | |
| Liver tumours | 5 | 4 | |
| Malignant vascular tumours | 2 | 3 | |
| Adrenocortical carcinoma | 1 | 3 | |
| Other very rare entities | 2 | 9 | |
| CNS tumours | 2 | ||
| Total | 227 | 491 | 550 |
| Adverse event (n, % within analysed time period) | |||
| No event | 53 (23.3) | 261 (53.2) | 370 (67.3) |
| Treatment-related mortality | 104 (45.8) | 109 (22.1) | 68 (12.4) |
| Disease relapse/progression | 69 (30.4) | 112 (22.8) | 108 (19.6) |
| Secondary cancers | 1 (0.4) | 7 (1.4) | 3 (0.5) |
| Others** | 0 (0) | 2 (0.4) | 1 (0.2) |
| (25–75% IQR) | (25.0–26.3) | (15.5–18.9) | (6.4–11.5) |
| [min-max] | [24.1–29.6] | [13.2–19.8] | [5.0–15.8] |
| Alive at the time of evaluation, (n, % within the analysed time period) | 66 (29.1) | 282 (57.4) | 404 (73.4) |
Abbreviations: ALL – acute lymphoblastic leukaemia, AML – acute myeloblastic leukaemia, CML – chronic myeloid leukaemia, CNS – central nervous system, IQR – interquartile range, JMML – juvenile myelomonocytic leukaemia, MDS – myelodysplastic syndrome (* refractory anaemia with excess of blasts in transformation), PNET – peripheral neuroectodermal tumours.
** “Others” refers to parental refusal to treat a child (n = 2) and suicide (n = 1).
Despite increasing absolute patient number over the analysed period, the treatment groups did not differ with regard to the distribution by sex: a slight male predominance was observed in the whole cohort (55.3%) and was consistent throughout the three decades. However, the mean age (± SD) at diagnosis increased gradually from 5.8 ± 4.1 to 6.7 ± 4.7 and to 7.9 ± 5.7 years in the first, second, and third decade, respectively.
Leukaemia (all leukaemic phenotypes were included) was the predominant type of cancer (765 (60.3%) out of 1268 patients) across the entire cohort. The 1982–1992 patient group consisted almost exclusively of leukaemia (218 children, 96.0%) with a negligible proportion of lymphoma cases (8 children, 3.5%) and only one patient with a solid tumour (0.4%). The number of solid tumours increased from 78 (15.9%) cases in 1992 to 2001 to 221 (40.2%) cases in 2002 to 2011.
The median follow-up time of all included subjects was 13.2 (25–75% interquartile range (IQR) 8.5–18.4) years. The shortest median follow-up of patients treated in the third decade exceeded five years and accounted for 8.9 (IQR 6.4–11.5) years (Table 1).
Treatment outcome
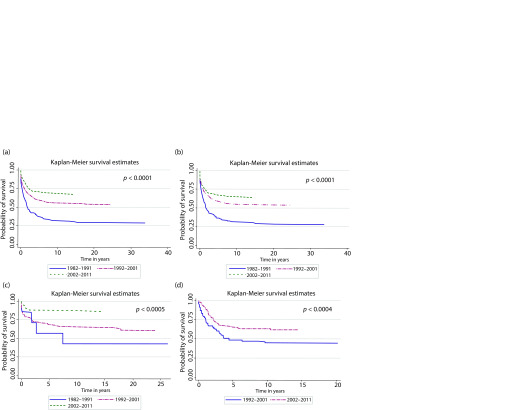
Analysis of the treatment outcome demonstrated a significant improvement in overall survival over the 30-year period. The cure rates increased significantly when calculated for the entire cohort (the overall 5-year survival rate increased from 37.3% in 1982–1991 to 70.7% in 2002–2011, p < 0.0001) (Fig. 1a) as well as calculated separately for leukaemia (p < 0.0001), lymphoma (p < 0.0005), and solid tumour (p < 0.004) groups (Fig. 1b-d). The 5-year and 10-year survival estimates for all aforementioned groups are out-lined in Table 2. The positive trend remained traceable at five and ten years across treatment periods. In the solid tumour group, survival estimates were compared only between the second and third treatment periods as there was only one patient diagnosed with a solid tumour in 1982 to 1911.
Table 2.
Five- and 10-year survival rates and 95% confidence interval by period of diagnosis and diagnosis group
| Survival rates | All malignant tumours | Leukaemia | Lymphoma | Solid tumours |
|---|---|---|---|---|
| 5-year | ||||
| 1982–1991 | 37.3 (30.2–44.3) | 36.1 (29.0–43.3) | 57.1 (17.2–83.7) | – |
| 1992–2001 | 59.5 (55.0–63.7) | 58.6 (52.8–64.0) | 69.4 (59.9–77.0) | 48.7 (37.3–59.2) |
| 2002–2011 | 70.7 (66.4–74.1) | 66.9 (60.6–72.4) | 88.2 (80.2–93.1) | 65.7 (58.7–71.9) |
| 10-year | ||||
| 1982–1991 | 32.0 (25.3–39.0) | 31.2 (24.4–38.3) | 42.9 (9.8–73.4) | – |
| 1992–2001 | 55.8 (51.2–60.1) | 54.9 (49.0–60.4) | 65.8 (56.1–73.8) | 44.9 (33.7–55.5) |
| 2002–2011 | 68.5 (64.3–72.2) | 65.2 (58.8–70.9) | 86.0 (76.5–91.9) | 63.5 (56.4–69.8) |
The improvement in cure rates was attributable to the reduction in adverse events (Table 1): treatment-related mortality due to infections and drug-induced toxicity dropped down from 45.8% in 1982–1991 to 22.1% in 1992–2001 and, ultimately, to 12.4% in 2002–2011. Similarly, the number of patients who succumbed to cancer relapse or progression decreased from 30.4% to 22.8% and, finally, to 19.6% in 1982 to 1991, 1992 to 2001, and 2002 to 2011, respectively. Consequently, the number and the percentage of survivors alive beyond five years from diagnosis increased from 66 (29.1%) in 1982 to 1992 to 404 (73.4%) in 2002 to 2011. Ten survivors (seven of them treated in the second decade) were diagnosed with a second cancer. The evolution of survival estimated over the 30-year study period is summarized in Table 3: a slight decrease in the cure rate estimated at 5, 10, 15, and 20 years was detected across all evaluated patient groups.
Table 3.
Survival rates and 95% confidence interval by diagnosis group
| Survival rates | All malignant tumours | Leukaemia | Lymphoma | Solid tumours | |
|---|---|---|---|---|---|
| 5-year | 61.1 (58.3–63.8) | 56.0 (52.3–59.6) | 77.7 (71.6–82.7) | 61.1 (55.2–66.6) | |
| 10-year | 56.8 (54.9–60.5) | 52.5 (48.7–56.1) | 74.5 (68.2–79.9) | 58.0 (51.8–63.6) | |
| 15-year | 56.6 (53.7–59.4) | 51.2 (47.3–54.9) | 73.8 (67.3–79.2) | 57.3 (51.1–63.0) | |
| 20-year | 55.7 (52.7–58.7) | 50.9 (47.0–54.6) | 69.7 (60.9–76.9) |
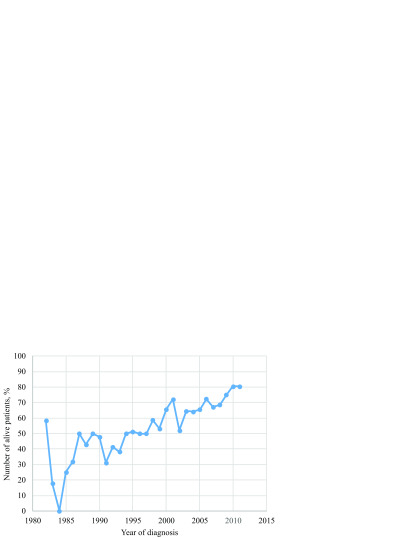
The aforementioned improvement translated in the growing percentage of survivors over the analysed three decades (Fig. 2). The percentage of patients alive at least five years after diagnosis – a cut-off definition for a long-term remission – increased from zero in the early years of treatment and reached 80% in 2010 and 2011.
Fig. 1.
Overall survival of children treated for cancer between 1982 and 2011 by period of diagnosis.
(a) All cancer types (N = 1268), (b) All types of leukaemia (n = 768), (c) All types of lymphoma (n = 222), (d) Solid tumours (n = 299)
Fig. 2.
Percentage of patients treated from 1982 to 2011 and alive at the end of follow-up (N = 1268)
DISCUSSION
The current study is the first attempt to analyse the outcome of treatment of all childhood cancers treated at Vilnius University Hospital Santaros Klinikos irrespective of the particular type of malignancy. The cancer-specific research focused on leukaemia (9, 10) and solid tumours (11–13) were reported previously. In line with our previous publications, a significant improvement in treatment outcome over decades was demonstrated: the cure rates rose dramatically from 37.3% in 1982 to 1991 to 59.5% in 1092 to 2001 and to 70.5% in 2002 to 2011. These findings replicate data published by the population-based EUROCARE-5 study that showed improvement of 5-year relative survival in Eastern Europe from 65% in 1999–2001 to 70% in 2005–2007 (4). The same study depicted graphically the 5-year survival for Lithuanian children diagnosed with cancer at the age of 0–14 years and treated from 2000 to 2007 approximately as 65% (exact survival rates per country were not provided). The rate was almost 15% lower than the European average survival of 79.1% (4). Despite minor differences in inclusion criteria as compared to the EUROCARE-5 study, we were able to demonstrate that the cure rates of children treated in 2010 and 2011 reached 80%.
The main reason of significant advances was the decrease in treatment-related mortality (from 45.8% to 12.4%) and the relapse rate (from 30.4% to 19.6%). Several factors contributed to the reduction of adverse events and a gradual increase in cure as depicted in Fig. 2. Political and economic transformation of Lithuania over the last three decades ensured a transition from an upper middle- to a high-income country, which translated into better national funding for the health system. Consequently, modern diagnostic, treatment, and supportive care facilities became available on a routine basis enabling a tremendous survival breakthrough in such a toxicity-sensitive disease as paediatric acute myeloblastic leukaemia (9). An additional fundamental issue was continuous staff education, gaining knowledge and experience, the possibility to learn, and become closer to the expert centres. Positive socioeconomic development has had a beneficial impact on the treatment outcome as observed by other research initiatives that reported substantially lower survival rates seen in most low- and middle-income countries compared to high-income countries (14, 15).
The major advantage of our study is its big sample size (1268 subjects were included) that enabled us to show reliable results. Our previous cancer-specific clinical research was limited to small numbers ranging from 40 to 60 of enrolled patients despite extensive study periods between 2000 and 2018 (9, 11–13). Treating childhood cancer in a small country with a limited number of patients that paediatric oncologists encounter in their daily practice is especially challenging. Therefore, collaborative initiatives are of a paramount importance to ensure the best care and survival as well as to obtain solid research output. An example of such a benefit was reported by Vaitkevičienė et al.: since 2008, when VUHSK joined an academic international clinical trial conducted by the Nordic Society of Paediatric Haematology and Oncology, the event-free survival of children with acute lymphoblastic leukaemia has improved from 71% in 2003 to 2008 to 82% in 2009 to 2012 (10). Thus, the enrolment of patients in cancer-specific academic clinical trials should become a strategic priority to ensure the best care and cure as endorsed by the European Standards of Care for Children with Cancer (www.siope.eu).
The major shortcoming of the current study is its limitation to a single-centre review. In Lithuania, two tertiary-care hospitals (VULHSK and the Hospital of the Lithuanian University of Health Sciences Kauno Klinikos (LUHSKK)) provide health care services to children affected by cancer. Of note, childhood leukaemias (all types) are centralized at VUHSK, therefore current treatment outcomes of haematological malignancies represent population-based data. However, solid tumours are split between the two centres. Thus, an improvement in solid tumour cancer cannot be considered nation-wide and should be interpreted with caution.
The increasing number of enrolled children across treatment periods reflected changes in the organization of paediatric oncology care in Lithuania. In the 1980s, the childhood malignancies treated at VUHSK were confined exclusively to leukaemia; therefore solid tumours were underrepresented in the first decade. Children with solid cancers were treated in the adult oncology centre. However, paediatric solid tumours differ substantially from adult malignancies and require a dedicated paediatric oncology team and facilities. From the early 1990s, paediatric tumours, with the exception of CNS tumours, became centralized in the paediatric oncology centre of VUHSK. CNS tumours were traditionally concentrated at LUHSKK (16); currently, however, all solid tumours, including CNS tumours, are treated in both centres.
The primary aim of our study was assessment of long-term survival beyond five years. The extensive follow-up period allowed us to estimate even longer survival rates at 10, 15, and 20 years. An important consequence of this effort was an evidence of a slight decrease in the survival rates beyond the traditional 5-year evaluation time-point. Among the survivors, we found ten patients who developed a second cancer; most of them were treated from 1992 to 2011. Radio- and chemotherapy-induced secondary malignant neoplasms have a cumulative incidence rate of 19% after 30 years of diagnosis, with a median latency period of ten years (17, 18). There are emerging data to the effect that disease recurrence can compromise the cure beyond five years. A recent review of the Surveillance, Epidemiology, and End Results (SEER) database reported a substantial number of the disease-related adverse events occurring between five and ten years in patients diagnosed with cancers at the age of 15–39 years from 1975 to 2011 (19). Thus, a longer follow-up for potential disease recurrence beyond five years after diagnosis is warranted (assuming potential limitations such as emigration, change of health care provider, reluctance of being contacted by medical stuff etc.). Another important issue to be considered is the quality of life after cancer treatment. Among long-term survivors, 62% have at least one treatment-induced chronic health condition, whilst 28% have a severe or life-threatening problem (20). This could explain increasing late mortality found in our population. Therefore, the cancer survivorship follow-up programme needs to be active in meeting emerging needs related to the rising number of survivors.
To sum up, significant progress in the cure rate was observed over the 30-year period. Despite the encouraging results, 20% of children are still suffering from unbeatable cancer. Given the rarity and heterogeneity of paediatric malignancies, childhood cancer needs to be a priority for the Lithuanian health system to ensure sustainable development and increment of cure rates that currently exceed 80% for several diseases (21, 22).
CONCLUSIONS
Tremendous progress in the survival rates of children treated at Vilnius University Hospital Santaros Klinikos was observed over the 30-year study period: the 5-year overall survival increased from 37.3% to 70.5%. Reduction of treatment-related mortality and the disease recurrence rate reflected positive socioeconomic changes in the country. Late mortality beyond five years compromises the treatment outcome in a minor part of survivors. Collaborative international clinical and research efforts (e.g., enrolment of children in cancer-specific academic clinical trials, harmonized monitoring of the quality of life) are crucial for ensuring the best cure and care.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Jelena Rascon, Giedrė Smailytė
References
- Li H, Yu G Dong C Jia Z An J Duan H et al.. PedMap: a pediatric diseases map generated from clinical big data from Hangzhou, China. Sci Rep. 2019; 9(1): 17867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassal G Fitzgerald E Schrappe M Arnold F Kowalczyk J Walker D et al.. Challenges for children and adolescents with cancer in Europe: the SIOP-Europe agenda. Pediatr Blood Cancer. 2014; 61(9): 1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017; 67(1): 7–30. [DOI] [PubMed] [Google Scholar]
- Gatta G Botta L Rossi S Aareleid T Bielska-Lasota M Clavel J et al.. Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5 – a population-based study. Lancet Oncol. 2014; 15(1): 35–47. [DOI] [PubMed] [Google Scholar]
- Gatta G Peris-Bonet R Visser O Stiller C Marcos-Gragera R Sanchez MJ et al.. Geographical variability in survival of European children with central nervous system tumours. Eur J Cancer. 2017; 82: 137–48. [DOI] [PubMed] [Google Scholar]
- Bonaventure A Harewood R Stiller CA Gatta G Clavel J Stefan DC et al.. Worldwide comparison of survival from childhood leukaemia for 1995–2009, by subtype, age, and sex (CONCORD-2): a population-based study of individual data for 89 828 children from 198 registries in 53 countries. Lancet Haematol. 2017; 4(5): e202–E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard-Jones K Lewison G Camporesi S Vassal G Ladenstein R Benoit Y et al.. The state of research into children with cancer across Europe: new policies for a new decade. Ecancermedicalscience. 2011; 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paapsi K, Magi M, Mikkel S, Saks K, Aareleid T, Innos K. The impact of under-reporting of cases on the estimates of childhood cancer incidence and survival in Estonia. Eur J Cancer Prev. 2017; 26 Joining forces for better cancer registration in Europe: 147–52. [DOI] [PubMed] [Google Scholar]
- Kairiene I, Pasauliene R, Lipunova N, Vaitkeviciene G, Rageliene L, Rascon J. Improved outcome of childhood acute myeloid leukemia in an Eastern European country: Lithuanian experience. Eur J Pediatr. 2017; 176(10): 1329–37. [DOI] [PubMed] [Google Scholar]
- Vaitkeviciene G, Matuzeviciene R, Stoskus M, Zvirblis T, Rageliene L, Schmiegelow K. Cure rates of childhood acute lymphoblastic leukemia in Lithuania and the benefit of joining international treatment protocol. Medicina. 2014; 50(1): 28–36. [DOI] [PubMed] [Google Scholar]
- Jakutis G, Rageliene L, Rascon J. Survival of children treated for Ewing sarcoma in Lithuania: a single centre experience. Acta medica Lituanica. 2017; 24(4): 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juskaite A, Tamuliene I, Rascon J. Results of neuroblastoma treatment in Lithuania: a single centre experience. Acta Med Litu. 2017; 24(2): 128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancelyte M, Nemaniene R, Rageliene L, Rascon J. Wilms tumour in children: 18 years of experience at Vilnius University Hospital Santaros Klinikos, Lithuania. Acta Med Litu. 2019; 26(2): 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann F, Feychting M, Mogensen H, Schmiegelow K, Zeeb H. Social inequalities along the childhood cancer continuum: an overview of evidence and a conceptual framework to identify underlying mechanisms and pathways. Front Public Health. 2019; 7: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CG, Howard SC, Bouffet E, Pritchard-Jones K. Science and health for all children with cancer. Science. 2019; 363(6432): 1182–6. [DOI] [PubMed] [Google Scholar]
- Rutkauskiene G, Labanauskas L. Treatment of patients of high-risk group of medulloblastoma with the adjuvant lomustine, cisplatin, and vincristine chemotherapy. Medicina. 2005; 41(12): 1026–34. [PubMed] [Google Scholar]
- Dorffel W, Riepenhausen M, Luders H, Bramswig J. Late effects following treatment of Hodgkin lymphoma during childhood and adolescence. Results of the hodgkin lymphoma late effects research project. Klin Padiatr. 2016; 228(6–07): 286–93. [DOI] [PubMed] [Google Scholar]
- Ng AK, Kenney LB, Gilbert ES, Travis LB. Secondary malignancies across the age spectrum. Semin Radiat Oncol. 2010; 20(1): 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C, Nichols HB. Trends in late mortality among adolescent and young adult (AYA) cancer survivors. J Natl Cancer Inst. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger KC Mertens AC Sklar CA Kawashima T Hudson MM Meadows AT et al.. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006; 355(15): 1572–82. [DOI] [PubMed] [Google Scholar]
- Cools J. Improvements in the survival of children and adolescents with acute lymphoblastic leukemia. Haematologica. 2012; 97(5): 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard-Colin V Brugieres L Reiter A Cairo MS Gross TG Woessmann W et al.. Non-Hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol. 2015; 33(27): 2963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]