Abstract
American Indian/Alaska Native (AI/AN) and Canadian Indigenous people are disproportionally affected by hepatitis C virus (HCV) infection yet are frequently underrepresented in epidemiologic studies and surveys often used to inform public health efforts. We performed a systematic review of published and unpublished literature and summarized our findings on HCV prevalence in these Indigenous populations. We found a disparity of epidemiologic literature of HCV prevalence among AI/AN in the United States and Indigenous people in Canada. The limited data available, which date from 1995, demonstrate a wide range of HCV prevalence in AI/AN (1.49%–67.60%) and Indigenous populations (2.28%–90.24%). The highest HCV prevalence in both countries was reported in studies that either included or specifically targeted people who inject drugs. Lower prevalence was reported in studies of general Indigenous populations, although in Canada, the lowest prevalence was up to 3-fold higher in Aboriginal people compared with general population estimates. The disparity of available data on HCV prevalence and need for consistent and enhanced HCV surveillance and reporting among Indigenous people are highlighted. HCV affects Indigenous peoples to a greater degree than the general population; thus we recommend tribal and community leaders be engaged in enhanced surveillance efforts and that funds benefitting all Indigenous persons be expanded to help prevent and cover health care expenses to help stop this epidemic.
Keywords: American Indian, Canadian Aboriginal, epidemic, hepatitis C virus, indigenous, prevalence, systematic review
Abbreviations
- AI/AN
American Indian/Alaska Native
- HCV
hepatitis C virus
- anti-HCV
hepatitis C virus antibody
- IHS
Indian Health Service
- PWID
people who inject drugs
INTRODUCTION
In the United States and Canada, Indigenous peoples (i.e., American Indian/Alaskan Native (AI/AN) and Canadian Indigenous people, respectively) are disproportionately affected by hepatitis C virus (HCV) infection. It is estimated that in the United States, between 3.4 million and 6 million people and between 220,000 and 245,000 people in Canada are currently living with HCV, of whom approximately 3% in each country may be Indigenous (1–5). In National Notifiable Diseases Surveillance System reports released by the US Centers for Disease Control and Prevention, AI/AN people consistently have had the highest reported per capita rates of incident acute HCV infection (1.8 in 100,000) and HCV-related mortality (12.95 in 100,000) compared with other races in the United States since 2002 (6). The National Notifiable Diseases Surveillance System data source provides critical indicators of public health data but is limited by the voluntary, nonsystematic nature of disease notification by states and health jurisdictions reporting to the Centers for Disease Control and Prevention. At the same time, AI/AN people are not adequately represented in other public health surveillance systems such as the National Health and Nutrition Examination Survey. This is problematic because the National Health and Nutrition Examination Survey and other nationwide reporting efforts commonly are used to inform the need for prevention and treatment resource allocation for HCV. Although Indigenous people represent a small percentage of the total US (0.9 AI/AN persons (1.7%) alone or in combination with other races, respectively) and Canadian populations (4.8% Indigenous people, including First Nations, Metis, and Inuit), these populations are frequently underrepresented for similar reasons in other epidemiologic studies (7, 8). For example, the majority of HCV studies are conducted in large urban areas or academic centers, where AI/AN and Indigenous populations are not commonly represented. In many studies, data on these populations are often merged with the “other” category of racial or ethnic minorities or omitted altogether because of small numbers and to preserve confidentiality.
HCV incidence has been increasing in association with injection drug use and has been associated with the surging opioid epidemic (9–11). Assessing HCV risk in people who inject drugs (PWID) is important to provide indications of new risk patterns. Incident HCV infection rates are highest in PWID in developing countries (12), and given that the highest rates of newly identified HCV are in AI/AN populations (13), it is reasonable to expect high rates of HCV in AI/AN PWID as well. However, neither injection drug use nor HCV among PWID has been well characterized among AI/AN or Indigenous people in North America.
HCV disease prevalence is not likely to be uniform across the United States or Canada and can differ by sex, race/ethnicity, and between communities, which may also be associated with residency in urban versus rural areas with differential exposure and testing rates (14–16). This is an important consideration because there are 566 US federally recognized tribes spread out across 35 states and, in Canada, over 634 First Nations governments, as well as Metis and Inuit groups, each with unique identities, cultures, and customs (17, 18). Although not every community may be represented in studies of HCV, knowledge of HCV prevalence among AI/AN and Indigenous people and PWID has the potential to inform the need for screening and treatment programs in various communities. This study was designed to evaluate and summarize existing data on HCV prevalence in AI/AN and Indigenous populations and in PWID in these populations, and inform gaps in knowledge.
METHODS
We reviewed studies examining HCV infection in AI/AN and Indigenous people and summarized the results to ascertain the prevalence of HCV in this and AI/AN and Indigenous PWID populations. Prevalence here is defined as exposure to HCV via presence of anti-HCV antibodies, whereas chronic HCV infection is defined as having anti-HCV antibodies and being positive for HCV RNA.
Information sources
During June 2017, we searched the PubMed database using multiple search strategies. The most productive search strategies included 1) “Inuits”(Mesh)) AND “Hepatitis C”(Mesh); 2) “Alaska Natives”(Mesh)) AND “Hepatitis C”(Mesh); and 3) “Indians, North American”(Mesh)) AND “Hepatitis C”(Mesh). We performed a separate search of Journal of Health Disparities Research and Practice, which was not completely indexed in PubMed, using the term “Hepatitis C.” We also searched online conference proceedings and abstracts from the following: Conference on Retroviruses and Opportunistic Infections (2014–2017); The Liver Meeting: American Association for the Study of Liver Diseases Annual Conference (2016); International Symposium on Hepatitis C Virus and Related Viruses; The Viral Hepatitis Congress (2012–2015); World Indigenous Peoples’ Conference on Viral Hepatitis (2014); and International Conference on Viral Hepatitis (2011–2016). Abstracts were not available for the World Hepatitis Summit or the International Symposium on Hepatitis Care in Substance Users. Websites including the Native Health Database website (19), the Indian Health Service website (20), and the IHS Primary Care Provider (21) were searched. Conference proceedings and abstracts were searched using keywords “hepatitis C,” “American Indian,” “Alaskan Native,” “AI/AN,” “Indigenous,” “Aboriginal,” “Inuit,” “First Nations,” “Native American,” and “Metis.” The aforementioned websites were also searched using the term “hepatitis C.” These conference searches were performed through September 2017.
Study selection and eligibility criteria
Titles and abstracts of studies were reviewed to identify potential articles of interest on the basis of inclusion and exclusion criteria. Full texts of articles were then reviewed to verify inclusion. Literature was included if an article 1) reported an Indigenous demographic variable (e.g., AI/AN, Aboriginal/First Nations race); 2) reported HCV prevalence for Indigenous people or reported proportion of HCV-infected Indigenous people as defined by presence of anti-HCV or HCV RNA; and 3) intravenous or injection drug use in Indigenous people and associated HCV prevalence in this population were reported. Literature was excluded if 1) no primary data were reported (e.g., review or opinion articles); 2) HCV was self-reported and not ascertained through laboratory tests; 3) Indigenous people were combined with other race groups (e.g., AI/AN with Pacific Islander); and 4) there were multiple publications from the same study sample.
Data collection process
Data were abstracted by the investigators to a Excel data form (Microsoft, Redmond, CA). For missing data elements, attempts were made to contact study authors to obtain pertinent data.
Data items
Variables for which data were sought included study name, study location, study dates, study design, recruitment setting, Indigenous persons sample size, the number of persons with anti-HCV antibodies, HCV prevalence for Indigenous (AI/AN in the United States and Indigenous Canadian) populations, and sample characteristics (i.e., sex, age, number reporting injection drug use). When available, HCV prevalence was calculated as N(Indigenous people with anti-HCV antibodies)/N(Indigenous population) and reported as a percentage along with 95% confidence intervals.
Risk of bias in individual studies
Risk of bias in individual studies may occur as selection bias, performance bias, reporting bias, and as bias for outcome measures. Some of these biases are addressed during study screening for inclusion or exclusion. Separate tables of HCV prevalence in PWID-exclusive populations or in those reporting injection drug use as a potential risk factor were constructed to demonstrate the impact of injection drug use within these studies.
Synthesis of results
HCV prevalence was stratified by country (United States or Canada) and used for all data analyses, including construction of forest plots, and assessment of study heterogeneity. Four forest plots of HCV prevalence were created: 1) among AI/AN people in the United States; 2) among Indigenous people in Canada; 3) among AI/AN PWID in the United States; and 4) among Indigenous PWID in Canada. R statistical software (R Foundation for Statistical Computing, Vienna, Austria) along with “metaphor,” “meta,” and “weightr” statistical packages was used to assess data heterogeneity of proportional data (e.g., I2, Cochran’s Q). Data on HCV prevalence in PWID were further abstracted from included studies when sufficient data were available.
Risk of bias across studies
We intended to assess publication bias using funnel plots for proportional data and Egger regression test, as appropriate, given the known limitations of these methods (e.g., inadequate number of trials or studies, vulnerability to sampling bias) (22).
RESULTS
A total of 68 articles were identified during our search period and after removal of duplicates (Figure 1) (23). Of these, 17 studies of HCV in AI/AN and Indigenous populations were included in our review and stratified by country. Overall, 10 and 7 studies were conducted in the United States and Canada, respectively.
Figure 1.
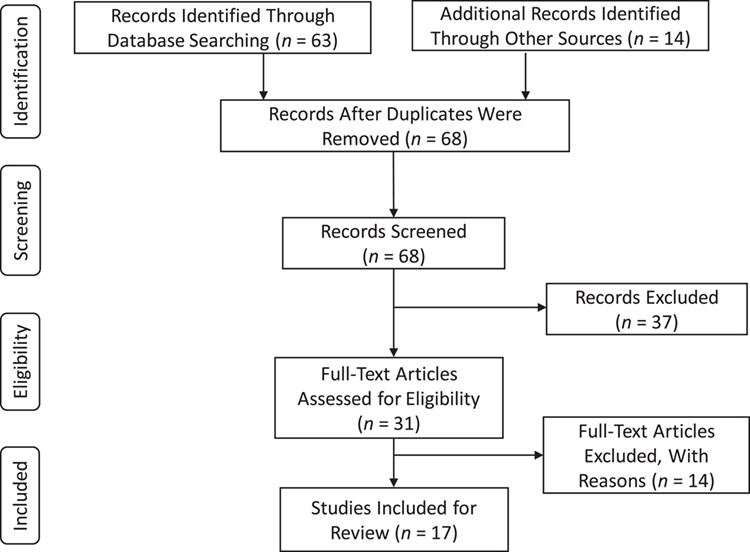
Four-phase flow diagram showing the systematic review stages based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement. Summary of excluded records or articles: 37 records did not meet eligibility criteria during the initial screening of titles and abstracts. In the next phase, 14 more records were excluded for which prevalence was not reported or could not be determined using the reported data: 5 did not have sufficient information on sample size; 3 were review articles; 3 used data from the same study, for which the most recent was chosen for inclusion in the review; 1 reported data from persons positive for hepatitis C virus only; 1 reported incidence; 1 was an opinion article and contained no data.
HCV in AI/AN in the United States
In the United States, studies on HCV in AI/AN populations were primarily conducted in medical clinics including IHS, Cherokee Nation Health Services, and a treatment facility for drug dependence serving the AI/AN community (Table 1) (24–33). HCV prevalence ranged widely, from 1.49% to 67.60% (Figure 2). HCV prevalence was the highest (67.57%) in a study in which specifically PWID were surveyed, although the sample size of AI/AN persons included was small (n = 37) (24). The second highest HCV prevalence (36.34%) was reported in a study of AI/AN persons with a history of chronic liver disease, whereas the lowest prevalence (1.49%) was reported in a large HCV screening study conducted at an IHS clinic (27). In a single study of AI/AN women receiving prenatal care, HCV prevalence was 3.05% (25). In 2 studies, authors reported additional testing for viral RNA, indicating chronic infection. In a study by Neumeister et al. (26), 21 of 28 anti-HCV–positive individuals had chronic infection, whereas Scott et al. (32) reported 2 people with chronic infections out of 5 exposed individuals. Analyses revealed substantial heterogeneity (Q (degrees of freedom = 10) = 1,368.9234; P < 0.0001) for which 99.27% of the total variance existed between studies (I2). Publication bias was not assessed, because there were inadequate numbers of studies to properly evaluate using a funnel plot or more advanced regression-based assessments.
Table 1.
Study Characteristics and Anti–Hepatitis C Virus Prevalence in American Indian/Alaska Native People in the United States, 1995–2017
| First Author, Year (Reference No.) | Location | Years | Study Design | Recruitment Setting | Sample Size, No. | Anti-HCV, No. | Age, years | Sex, No. | HCV Prevalence, % | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ||||||||||
| Samuel, 2001 (24) | New Mexico | 1995–1997 | Cross-sectionala | Mobile outreach | 37 | 25 | ≥15 | NR | NR | 67.57 | 52.48, 82.65 |
| Wilson, 2004 (25) | Arizona | 1999–2000 | Record review | IHS clinic | 426 | 13 | 15–44 | 0 | 426 | 3.05 | 1.42, 4.69 |
| Neumeister, 2007 (26) | Nebraska | NR | Screening study | IHS clinic | 243 | 28 | ≥19 | 82 | 161 | 11.52 | 7.49, 15.51 |
| Bialek, 2008 (27) | Arizona | 2000–2002 | Record review | IHS clinic | 1,496 | 239 | ≥18 | 744 | 752 | 15.98 | 14.12, 17.83 |
| California | 2002–2003 | Record review | IHS clinic | 344 | 125 | ≥18 | 183 | 161 | 36.34 | 31.25, 41.42 | |
| Huffman, 2011 (28) | Washington | 2003–2007 | Screening study | Chemical dependency treatment facility | 322 | 91 | ≥18 | NR | NR | 28.26 | 23.34, 33.18 |
| Tohme, 2013 (29) | United States (NR) | 2009–2010 | Cross-sectional | Community sample | 1,379 | 87 | ≥18 | 701 | 678 | 6.31 | 5.03, 7.59 |
| Dutler, 2014 (30) | United States (NR) | NR | Screening study | IHS clinic | 2,016 | 30 | 18–72 | NR | NR | 1.49 | 0.96, 2.02 |
| Casberg, 2015 (31) | Oregon | 2012–2013 | Screening study | IHS clinic | 329 | 16 | Born between 1945 and 1965 | NR | NR | 4.86 | 2.54, 7.19 |
| Scott, 2015 (32) | Oregon | 2011 | Cross-sectional | IHS clinic | 71 | 5 | 18–75 | 26 | 45 | 7.04 | 1.09, 12.99 |
| Mera, 2017 (33) | Oklahoma | 2015–2017 | Screening study | Cherokee Nation Health Services | 31,399 | 1,076 | 20–69 | NR | NR | 3.43 | 3.23, 3.63 |
Abbreviation: NR, not reported.
a Study only included people who inject drugs.
Figure 2.
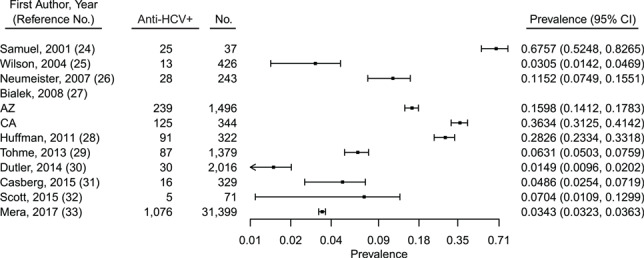
Overall prevalence of HCV antibody (anti-HCV+) among American Indian/Alaska Native people in the United States, 1995–2017. AZ, Arizona; CA, California; CI, confidence interval; HCV, hepatitis C virus.
Four of the 10 included studies contained data on HCV prevalence from studies that included participants who self-reported injection drug use (24, 26, 28, 29). These data were abstracted and summarized in Figure 3. HCV prevalence was highest among self-reported PWID and ranged from 25.68% to 67.60%, which significantly exceeded estimates observed in studies of non-PWID AI/AN persons without a history of chronic liver disease (1.49%–7.04%).
Figure 3.
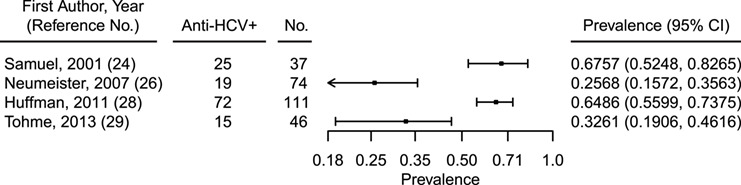
Prevalence of HCV antibody (anti-HCV+) among American Indian/Alaska Native people who inject drugs in the United States, 1995–2017. CI, confidence interval; HCV, hepatitis C virus.
HCV in indigenous people in Canada
Of 7 studies of HCV in Indigenous people in Canada (Table 2), prevalence ranged from 2.28% to 90.24% (Figure 4) (34–40). Five of these studies were conducted in either PWID or populations that included PWID. In an all-female study of sex workers, of whom 65% reported ever having injected drugs, HCV prevalence was 43.60% (40). Chronic infection was not reported in these studies. Meta-analyses of the data were not pursued, because of substantial heterogeneity (Q (degrees of freedom = 6) =1,943.9797; P < 0.0001), for which 99.69% of the total variance existed between studies (I2). Publication bias was also not assessed, because of the low number of studies. The highest HCV prevalence (58.89%–90.24%) was reported in studies conducted in PWID populations or when injection drug use was self-reported (Figure 5). These included the Vancouver Injection Drug Users Study, the Cedar Project, and the Scientific Evaluation of Supervised Injecting study, which was conducted at a safe injecting facility in Vancouver (35, 36, 40).
Table 2.
Study Characteristics and Anti–Hepatitis C Virus Prevalence in Canadian Aboriginal People, 1995–2017
| First Author, Year (Reference No.) | Location | Years | Study Design | Recruitment Setting | Sample Size, No. | Anti-HCV, No. | Age, years | Sex, No. | HCV Prevalence, % | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ||||||||||
| Minuk, 2003 (34) | Manitoba | 1999 | Cross-sectional | Community sample | 307 | 7 | All ages | NR | NR | 2.28 | 0.61, 3.94 |
| Wood, 2005 (35) | British Columbia | 2003–2004 | Cross-sectionala | Safe injecting facility | 123 | 111 | NR | NR | NR | 90.24 | 85.00, 95.49 |
| Dawood, 2006 (37) | Manitoba | 1995–2003 | Cross-sectional | Serum specimens | 16,152 | 563 | NR | 5,676 | 10,476 | 3.49 | 3.20, 3.77 |
| Miller, 2006 (36) | British Columbia | 1996–2003 | Prospective cohorta | Community sample | 80 | 53 | ≤24 | 28 | 52 | 66.25 | 55.89–76.61 |
| Wylie, 2006 (38) | Manitoba | 2003–2004 | Cross-sectionala | Community sample | 237 | 141 | ≥15 | NR | NR | 59.49 | 53.24, 65.74 |
| Mehrabadi, 2008 (39) | British Columbia | 2003–2005 | Prospective cohort | Community sample | 250 | 109 | 14–30 | 0 | 250 | 43.60 | 37.45, 49.75 |
| Cedar Project Partnership, 2008 (40) | British Columbia | 2003–2005 | Cross-sectional | Community sample | 518 | 177 | 14–30 | 268 | 250 | 34.17 | 30.09, 38.25 |
Abbreviation: NR, not reported.
a Study only included people who inject drugs.
Figure 4.
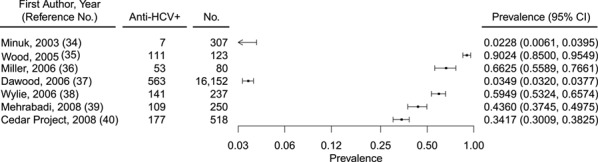
Overall prevalence of HCV antibody (anti-HCV+) among American Indian/Alaska Native people in Canada, 1995–2017. CI, confidence interval; HCV, hepatitis C virus.
Figure 5.
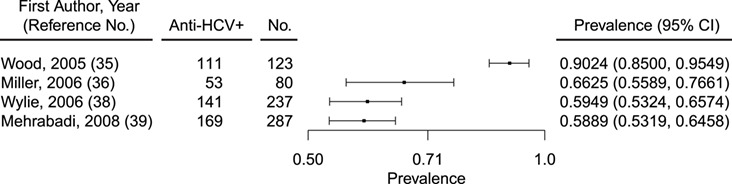
Prevalence of HCV antibody (anti-HCV+) among American Indian/Alaska Native people who inject drugs in Canada, 1995–2017. CI, confidence interval; HCV, hepatitis C virus.
DISCUSSION
AI/AN people in the United States and Indigenous people in Canada are disproportionally affected by HCV yet are frequently underrepresented in epidemiologic studies and surveys often used to inform public health efforts. To address this issue, we performed a systematic review of published and unpublished literature and summarized our findings on HCV prevalence in these populations.
We found that HCV prevalence in AI/AN and Indigenous populations varied grossly overall and when stratified by country. In 10 US studies conducted between 1995 and 2017, the lowest estimated prevalence was 1.49% and the highest was 67.60%. The vast majority of these studies were conducted in IHS clinics or within health systems that specifically served AI/AN populations. In 7 Canadian studies, the majority conducted in injecting populations, the minimum HCV prevalence was 2.28% and the maximum was 90.24%. As expected, the highest HCV prevalence reported from each country was derived from a study specifically targeting PWID, given that injection drug use is the primary risk factor for incident HCV infection (24).
The findings of our review are important because they demonstrate the need for consistent and enhanced HCV surveillance and reporting among Indigenous people. Studies of prevalence, including of persons with anti-HCV antibodies to monitor exposure and those with HCV RNA to monitor burden of active infection, are key in monitoring disease epidemics and play important roles in informing public health initiatives, prevention, as well as the need for and access to treatment. Because HCV is mostly asymptomatic, testing is often overlooked and, as a result, at least 20% of Canadians and 50% of people in the United States are unaware of their infection status. (5–7). Furthermore, only a minority of acutely infected individuals display symptoms associated with liver dysfunction (e.g., jaundice, nausea, fatigue, abdominal pain) (41, 42). The deficit in testing and knowledge of infection creates a significant public health concern, because persons with undiagnosed HCV can continue to actively transmit the infection despite available curative treatment.
Surveillance reports continue to show an increase in HCV overall and specifically among US AI/AN and Canadian Indigenous people (6). Previously, it had been estimated that 1.3% of the US population was currently living with HCV; however, this estimate did not include persons living on reservations (1). To improve on this number, Edlin et al. (3) estimated an additional 123,000 AI/AN persons were infected and would have been overlooked in the previous study (1). Incidence of reported acute HCV infection has also jumped from 1.8–3.1 cases per 100,000 population in AI/AN people in recent years (13). In a 2011 country-wide Canadian study, it was estimated that 0.6%–0.7% of the total general population were anti-HCV positive (4). Still, the lowest prevalence found in this review was 3-fold higher in Indigenous people compared with the general population estimate (4). Consequently, the findings of this review support those of previous reports in that this disease affects Indigenous peoples to a greater degree than it does the general population.
In the United States and Canada, the HCV epidemic is concentrated in PWID. Because HCV is a blood-borne disease and is highly infectious, up to 20% of new injectors will become HCV positive within 1 year of engaging in the practice (43). Upward of 80% of new infections are associated with injection drug use (5). Surprisingly, only a single study focused on PWID was identified in the United States using our criteria, whereas the majority of included Canadian studies were performed specifically in PWID in Indigenous populations. The range of prevalence estimates found in PWID in the AI/AN population in the United States (25.68%–67%) was comparable to those of other studies of non-AI/AN PWID in several urban areas in the United States (range, 13.7%–91.9%) (44–47). The range of HCV prevalence in studies of Indigenous PWID in Canada (58.89%–90.24%) was narrower and generally higher compared with the US AI/AN groups but was comparable to that reported in studies of HCV prevalence in PWID in other Canadian non-Indigenous PWID groups (46%–87.6%) (35, 48). Although information on exposure to injection drug use was obtained from participants in several US-based studies, the lack of targeted studies, such as those done in Indigenous PWID in Canada, highlights a disparity in data and knowledge of AI/AN PWID and PWID in the United States in general. Thus, in this review, we provide important information on populations significantly burdened by the disease and for which there are few epidemiologic studies.
This study had several limitations. There was a considerable lack of available data that described HCV prevalence in our populations of interest and only a handful of studies met our inclusion criteria. Although some websites focused on Indigenous populations were included in the search, we focused more on research results and did not include broader public health websites such as the Public Health Agency of Canada, or the US Centers for Disease Control and Prevention in the review, resulting in some missing data. Because of the considerable heterogeneity between studies (e.g., study design, screening criteria), we deemed it inappropriate to conduct a meta-analysis or generate a point estimate. Thus, we were limited to reporting a summary of our findings rather than providing a comparison of HCV prevalence in these populations to national estimates. Furthermore, data presented on Indigenous PWID could be improved upon by abstracting data specifically from studies of HCV in broader PWID populations, including in urban and rural areas. Despite these limitations, we found evidence that HCV is indeed high in AI/AN and Canadian Indigenous populations, especially in PWID. We also highlight the broad range of HCV prevalence due to study design and study population, thus demonstrating the need for systematic and enhanced HCV surveillance nationwide—in urban and rural populations. Furthermore, to truly grasp the impact of HCV in Indigenous populations as a whole, we support the need for tribal collaboration and more research, given that only 17 studies in the last 2 decades met inclusion criteria for this review.
Results from this review have the potential to influence public heath by giving insight on the HCV epidemic in AI/AN and Canadian Indigenous peoples. HCV is no longer a disease without a cure and the World Health Organization has set its sights on eliminating HCV as a public health threat by 2030. One strategy is to target interventions to prevent spread, increase resources, and maximize health care access in populations at high-risk for infection and for those who have a high burden of disease. It is also important to acknowledge that high HCV prevalence in Indigenous people may be due to contributors to health disparities such as poverty, colonization, historical trauma, cultural disconnection, and other health determinants that lead to engagement in high-risk behaviors and activities as well as decreased engagement and use of health care (49). In Canada, significantly more research has been conducted to examine risk and prevalence of blood-borne infections (e.g., HIV, HCV) in Indigenous populations compared with those in the United States. Indigenous cultural status in Canada has been associated with high-risk injection exposures and HIV and HCV infection (50–52). HCV infection and injection drug use are associated with attending or having a parent who attended a residential school, pointing to the deleterious health effects of historical trauma resulting from familial fragmentation and loss of cultural identify (50, 52). Wylie et al. (38) found that risk of HCV and other blood-borne infections was lower in PWID who recently moved to Winnipeg, suggesting a link between urbanization and risk. The Canadian studies add substantially to the body of knowledge about potential reasons for disparities, which is missing from US studies. Additional research with Indigenous populations is needed to identify and address the underlying systemic issues, including poverty, historical trauma, and social inequities that contribute to risk of HCV and other infections like HIV that are disproportionately coprevalent.
Knowing the HCV landscape justifies the need for expanded access to syringe-exchange and opioid-substitution treatment programs that, when used jointly, can substantially reduce the risk of viral transmission by up to 74% (53). It is critical that screening efforts be expanded to increase knowledge of HCV status in AI/AN and Indigenous communities. This review also identified the need to support expanded access to healthcare resources and HCV treatment in limited resource settings such as tribal lands. Although current US guidelines suggest that anyone with HCV should be treated, there are still several obstacles met by patients positive for HCV. Federal funds and Medicaid coverage are not sufficient or sustainable to treat all persons with HCV, given the high expense of new curative drugs and that many insurers still impose nonevidence-based sobriety and liver-fibrosis score restrictions. In 2017, IHS budgeted $3,851 per person compared with the $10,348 per person other federal programs budget for health care expenditures (54). In light of our findings, we recommend researchers and public health practitioners engage with Indigenous leaders to collectively enhance political will to provide resources for surveillance, research, and treatment efforts to benefit all Indigenous persons and help stop this epidemic.
ACKNOWLEDGMENTS
Author affiliations: Division of Epidemiology, Biostatistics, and Preventive Medicine, Department of Internal Medicine, University of New Mexico Health Sciences Center, Albuquerque, New Mexico (Veronica Bruce, Kimberly Page); Health Sciences Library and Informatics Center, University of New Mexico Health Sciences Center, Albuquerque, New Mexico (Jonathan Eldredge); Office of Research, Center for Healthcare Equity in Kidney Disease, University of New Mexico Health Sciences Center, Albuquerque, New Mexico (Yuridia Leyva); Cherokee Nation Health Services, Tahlequah, Oklahoma (Jorge Mera); and Albuquerque Area Southwest Tribal Epidemiology Center, Albuquerque Area Indian Health Board, Inc, Albuquerque, New Mexico (Kevin English).
This research was undertaken as a joint collaboration by the authors to advance research on hepatitis C virus infection.
This work was supported by National Institutes of Health grant UL1TR001449; V.B. was supported by CTSC/NCATS Research Supplement UL1TR001449-02S1 to Promote Diversity in Health-Related Research.
Conflict of interest: none declared.
REFERENCES
- 1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and nutrition examination survey 2003 to 2010. Ann Intern Med. 2014;160(5):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Center for Health Statistics National Health and Nutrition Examination Survey 2009–2010. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2009.
- 3. Edlin BR, Eckhardt BJ, Shu MA, et al. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62(5):1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trubnikov M, Yan P, Archibald C. Estimated prevalence of hepatitis C virus infection in Canada, 2011. Can Commun Dis Rep. 2014;40(19):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Public Health Agency of Canada Hepatitis C in Canada: 2005-2010 surveillance report In: Centre for Communicable Diseases and Infection Control Infectious Disease Prevention and Control Branch. Ottawa, Ontario: Public Health Agency of Canada; 2012. [Google Scholar]
- 6. Centers for Disease Control and Prevention Surveillance for Viral Hepatitis – United States, 2016https://www.cdc.gov/hepatitis/statistics/2016surveillance/commentary.htm#hepatitisC. Accessed September 18, 2018.
- 7. Statistics Canada Census Profile 2016 Census Catalogue Number 98–316-X2016001 2017. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E. Accessed July 15, 2018.
- 8. Norris T, Vines PL, Hoeffel EM. The American Indian and Alaska Native Population: 2010. Washington, DC: United States Census; 2012. (Report Number C2010BR-10)https://www.census.gov/content/dam/Census/library/publications/2012/dec/c2010br-10.pdf. Accessed July 15, 2018. [Google Scholar]
- 9. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged </=30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453–458. [PMC free article] [PubMed] [Google Scholar]
- 10. Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014;59(10):1411–1419. [DOI] [PubMed] [Google Scholar]
- 11. Bruneau J, Roy E, Arruda N, et al. The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction. 2012;107(7):1318–1327. [DOI] [PubMed] [Google Scholar]
- 12. Grebely J, Larney S, Peacock A, et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction. 2019;114(1):150–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention Viral Hepatitis Surveillance. United States, 2016. Atlanta, Georgia: Centers for Disease Control and Prevention; 2016. https://www.cdc.gov/hepatitis/statistics/2016surveillance/pdfs/2016HepSurveillanceRpt.pdf. Accessed October 31, 2018. [Google Scholar]
- 14. Reilley B, Leston J, Hariri S, et al. Birth cohort testing for hepatitis C virus - Indian Health Service 2012–2015. MMWR Morb Mortal Wkly Rep. 2016;65(18):467–469. [DOI] [PubMed] [Google Scholar]
- 15. Hall EW, Rosenberg ES, Sullivan PS. Estimates of state-level chronic hepatitis C virus infection, stratified by race and sex, United States, 2010. BMC Infect Dis. 2018;18(1):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uhanova J, Tate RB, Tataryn DJ, et al. The epidemiology of hepatitis C in a Canadian indigenous population. Can J Gastroenterol. 2013;27(6):336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Conference of State Legislatures Federal and State Recognized Tribes. http://www.ncsl.org/research/state-tribal-institute/list-of-federal-and-state-recognized-tribes.aspx. Accessed June 14, 2018.
- 18. Statistics Canada First Nations People, Métis and Inuit in Canada: Diverse and Growing Populations. https://www150.statcan.gc.ca/n1/pub/89-659-x/89-659-x2018001-eng.htm. Accessed June 13, 2019.
- 19. Native American Health Information Services Native Health Database. University of New Mexico Health Sciences Library and Informatics Center: https://hslic-nhd.health.unm.edu/. Accessed June 14, 2018. [Google Scholar]
- 20. Indian Health Service The Federal Health Program for American Indians and Alaska Natives. https://www.ihs.gov. Accessed June 13, 2019.
- 21. Indian Health Service Indian Health Service Primary Care Provider https://www.ihs.gov/provider/. Accessed June 13, 2019.
- 22. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 24. Samuel MC, Doherty PM, Bulterys M, et al. Association between heroin use, needle sharing and tattoos received in prison with hepatitis B and C positivity among street-recruited injecting drug users in New Mexico, USA. Epidemiol Infect. 2001;127(3):475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson C. Hepatitis C infection and type 2 diabetes in American-Indian women. Diabetes Care. 2004;27(9):2116–2119. [DOI] [PubMed] [Google Scholar]
- 26. Neumeister AS, Pilcher LE, Erickson JM, et al. Hepatitis-C prevalence in an urban native-American clinic: a prospective screening study. J Natl Med Assoc. 2007;99(4):389–392. [PMC free article] [PubMed] [Google Scholar]
- 27. Bialek SR, Redd JT, Lynch A, et al. Chronic liver disease among two American Indian patient populations in the southwestern United States, 2000–2003. J Clin Gastroenterol. 2008;42(7):949–954. [DOI] [PubMed] [Google Scholar]
- 28. Huffman S, Brucker R, Redd JT, et al. Integrating viral hepatitis screening and prevention services into an urban chemical dependency treatment Facility for American Indians and Alaska Natives. J Health Dispar Res Pract. 2011;5(2):32–42. [Google Scholar]
- 29. Tohme RA, Xing J, Liao Y, et al. Hepatitis C testing, infection, and linkage to care among racial and ethnic minorities in the United States, 2009–2010. Am J Public Health. 2013;103(1):112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dutler L, Laughlin A. Hepatitis C prevalence in a native American reservation clinic: impact of a screening and monitoring program. The IHS Primary Care Provider. 2014;39(3):26–32. [Google Scholar]
- 31. Casberg J, Crosby L, Lelonek M. Effect of hepatitis C education on patient knowledge and behavior. The IHS Primary Care Provider. 2015;40(1):1–6. [Google Scholar]
- 32. Scott J, Kowdley KV, Ioannou GN, et al. The prevalence and presumed etiology of elevated aminotransferase levels in a Pacific northwest tribal community. J Health Care Poor Underserved. 2015;26(3):957–966. [DOI] [PubMed] [Google Scholar]
- 33. Mera J, Gahn D, Lyon L, et al. The Road to Hepatitis C Elimination in Cherokee Nation Health Services In: Presented at the International Symposium on Hepatitis Care in Substance Users. Jersey City, NJ; September 15, 2017. [Google Scholar]
- 34. Minuk GY, Uhanova J. Viral hepatitis in the Canadian Inuit and first nations populations. Can J Gastroenterol. 2003;17(12):707–712. [DOI] [PubMed] [Google Scholar]
- 35. Wood E, Kerr T, Stoltz J, et al. Prevalence and correlates of hepatitis C infection among users of North America's first medically supervised safer injection facility. Public Health. 2005;119(12):1111–1115. [DOI] [PubMed] [Google Scholar]
- 36. Miller CL, Strathdee SA, Spittal PM, et al. Elevated rates of HIV infection among young aboriginal injection drug users in a Canadian setting. Harm Reduct J. 2006;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dawood M, Smart G, Wood M, et al. Hepatitis C virus infection among first nation and non-first nation people in Manitoba, Canada: a public health laboratory study. Can J Microbiol. 2006;52(10):999–1005. [DOI] [PubMed] [Google Scholar]
- 38. Wylie JL, Shah L, Jolly AM. Demographic, risk behaviour and personal network variables associated with prevalent hepatitis C, hepatitis B, and HIV infection in injection drug users in Winnipeg, Canada. BMC Public Health. 2006;6:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mehrabadi A, Craib KJ, Patterson K, et al. The Cedar Project: a comparison of HIV-related vulnerabilities amongst young aboriginal women surviving drug use and sex work in two Canadian cities. Int J Drug Policy. 2008;19(2):159–168. [DOI] [PubMed] [Google Scholar]
- 40. Cedar Project Partnership, Mehrabadi A, Paterson K, et al. Gender differences in HIV and hepatitis C related vulnerabilities among aboriginal young people who use street drugs in two Canadian cities. Women Health. 2008;48(3):235–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koretz RL, Abbey H, Coleman E, et al. Non-A, non-B post-transfusion hepatitis. Looking back in the second decade. Ann Intern Med. 1993;119(2):110–115. [DOI] [PubMed] [Google Scholar]
- 42. Marcellin P. Hepatitis C: the clinical spectrum of the disease. J Hepatol. 1999;31(Suppl 1):9–16. [DOI] [PubMed] [Google Scholar]
- 43. Hagan H, Pouget ER, Des Jarlais DC, et al. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168(10):1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Page K, Morris MD, Hahn JA, et al. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Infect Dis. 2013;57(Suppl 2):S32–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boodram B, Golub ET, Ouellet LJ. Socio-behavioral and geographic correlates of prevalent hepatitis C virus infection among young injection drug users in metropolitan Baltimore and Chicago. Drug Alcohol Depend. 2010;111(1–2):136–145. [DOI] [PubMed] [Google Scholar]
- 46. Des Jarlais DC, Arasteh K, Feelemyer J, et al. Hepatitis C virus prevalence and estimated incidence among new injectors during the opioid epidemic in New York City, 2000-2017: protective effects of non-injecting drug use. Drug Alcohol Depend. 2018;192:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tseng FC, O'Brien TR, Zhang M, et al. Seroprevalence of hepatitis C virus and hepatitis B virus among San Francisco injection drug users, 1998 to 2000. Hepatology. 2007;46(3):666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miller CL, Johnston C, Spittal PM, et al. Opportunities for prevention: hepatitis C prevalence and incidence in a cohort of young injection drug users. Hepatology. 2002;36(3):737–742. [DOI] [PubMed] [Google Scholar]
- 49. Sarche M, Tafoya G, Croy CD, et al. American Indian and Alaska native boys: early childhood risk and resilience amidst context and culture. Infant Ment Health J. 2017;38(1):115–127. [DOI] [PubMed] [Google Scholar]
- 50. Lemstra M, Rogers M, Thompson A, et al. Risk indicators associated with injection drug use in the aboriginal population. AIDS Care. 2012;24(11):1416–1424. [DOI] [PubMed] [Google Scholar]
- 51. Wood E, Montaner JS, Li K, et al. Burden of HIV infection among aboriginal injection drug users in Vancouver, British Columbia. Am J Public Health. 2008;98(3):515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Craib KJ, Spittal PM, Patel SH, et al. Prevalence and incidence of hepatitis C virus infection among aboriginal young people who use drugs: results from the Cedar Project. Open Med. 2009;3(4):e220–e227. [PMC free article] [PubMed] [Google Scholar]
- 53. Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane review and meta-analysis. Addiction. 2018;113(3):545–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. National Congress of American Indians Health Care: Reducing Disparities in the Federal Health Care Budget Upholding the Promises, Respecting Tribal Governance: for the Good of the People. Washington, DC: National Congress of American Indians; 2016. http://www.ncai.org/resources/ncai-publications/NCAI-2017-BudgetReport-Layout-FINAL.pdf. 2017 pages 51–59. [Google Scholar]


