Abstract
Objective
To provide a comprehensive review of vancomycin dosing in patients with hematologic malignancies or neutropenia.
Methods
PubMed, Embase and the Cochrane Library were searched through April 2, 2020. Original studies relevant to vancomycin dosing regimen in adults with hematologic malignancies or neutropenia were included. No restriction was applied in study design and language. A descriptive analysis was performed.
Results
Twenty-three studies were included eventually, of which eighteen were case series studies, four were cohort studies and another one was a randomized controlled trial. Five case series studies made a clinical audit of conventional vancomycin dosing in patients with malignancies or neutropenia, showing that the proportion of patients with sub-therapeutic trough levels remained high, ranging from 32% to 88%. Seven case series studies and four cohort studies demonstrated that vancomycin clearance (CLva) tended to be higher in patients with hematologic malignancies or neutropenia, whereas volume of distribution (V) seemed to be comparable to the control group. Five studies proposed individualized initial dosing regimen per the pharmacokinetic changes; however, no prospective validation has been conducted in clinical setting. Additionally, four case series studies suggested that the correlation between vancomycin clearance and estimated creatinine clearance was relatively poor, bringing a great challenge to proper dosing strategy. A randomized controlled trial stated that therapeutic drug monitoring (TDM) of vancomycin could decrease the incidence of nephrotoxicity in immunocompromised febrile patients with hematologic malignancies.
Conclusion
The available evidence indicates that conventional vancomycin dosing leads to suboptimal concentration in patients with hematologic malignancy or neutropenia. TDM accompanied by pharmacokinetic interpretation can decrease the risk of nephrotoxicity. The individualization of the initial dosing regimen and mechanisms of augmented clearance require further research.
Keywords: vancomycin, hematologic malignancy, neutropenia, pharmacokinetics, evidence-based practice
Introduction
A proper dosing regimen is the cornerstone of antimicrobial therapy, which has a great impact on treatment outcome, development of drug resistance as well as dose-dependent toxicity. Traditionally, the use of reduced doses in patients with renal impairment has been widely accepted. Dosage adjustments for patients with renal failure have been listed in labels of various medications and relevant clinical guidelines.1,2 However, more and more studies have underlined the existence of augmented renal clearance (ARC), especially in critically ill patients,3 patients with brain injury4 and neurosurgery,5 which could result in antibiotics’ sub-therapeutic concentrations and poorer outcomes. In this case, an assumption could be made that dosing regimens should be optimized according to the degree of increase in renal function, similar to the downward dose adjustments in patients with renal dysfunction.
Risk of infections will increase in patients with neutropenia, which occurs frequently after chemotherapy for cancer, especially hematologic malignancies.6 Therefore, the administration of optimal antibiotics was recommended in clinically or microbiologically documented infections.7 Additionally, patients with hematologic malignancies or neutropenia have been reported to have enhanced renal clearance,8,9 which would affect the systematic exposure of antibiotics predominately excreted through urine, including the commonly used anti-pseudomonas beta-lactams, aminoglycosides and vancomycin. Hence, the optimization of dosing regimens’ might also be required under the circumstance.
To our knowledge, vancomycin is one of the most well-studied antibiotics with respect to therapeutic drug monitoring (TDM).2,10,11 In spite of the potential changes in pharmacokinetic parameters and possible clinical failure proposed in patients with hematologic malignancies or neutropenia,8,9 neither increased dosing regimen nor TDM of vancomycin has been recommended in these patients, implying that the evidence was insufficient or the integration of evidence into practice should be strengthened. Notably, no comprehensive review has been conducted on this issue.
The objective of this study was to gain an in-depth understanding of the current status of vancomycin dosing regimen, pharmacokinetics and optimization of vancomycin dosing in patients with hematologic malignancies or neutropenia, which could be of great value for clinical practice and identifying knowledge gaps for future research.
Methods
We conducted this systematic review using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12
Data Sources and Searches
PubMed, Embase and the Cochrane Library were searched from their respective inception to July 26th., 2018. A complementary search was also performed to identify the most recent articles (published before April 2, 2020). The search terms included hematologic malignancy, neutropenia and vancomycin. Both mesh terms and text words were used. The search strategy is detailed in Tables S1–S3. Reference lists of the retrieved articles and related reviews were also examined manually for additional studies.
Eligibility Criteria
All records that comprised of adult patients with hematologic malignancies or neutropenia were included. When the proportion of hematologic malignancies or neutropenia was greater than 80% in one individual arm, the arm could be assumed to be patients with hematologic malignancies or neutropenia, respectively. Furthermore, all the patients were required to receive intravenous vancomycin. Outcomes should involve at least one of the followings: vancomycin serum concentration, pharmacokinetic (PK) parameters, vancomycin dosing, clinical response and nephrotoxicity. The exclusion criteria were as follows: (1) insufficient clinical data; (2) study types were cases, reviews or editorials; (3) the analysis was not relevant to vancomycin dosing regimen; (4) duplicate publication. No restriction was applied in language.
Study Selection
Two reviewers (N. H. and W. L.) screened titles and abstracts per the eligibility criteria to identify potential publications independently at first. Then, the full text was assessed for final inclusion. Any disagreement was resolved by discussion between the 2 reviewers or by consulting a third reviewer (S. Z.).
Data Extraction
A pre-specified data form was used to extract the following information: study characteristics (the first author’s name, year of publication, study design, country, sample size), patients’ baseline characteristics (characteristics of patients included, proportion of patients with neutropenia, gender, age, weight, renal function), vancomycin dosing, timing of vancomycin serum concentration sampling, outcomes of interest. The data extraction was performed by one reviewer (N. H.) and checked by another reviewer (W. L.). Discrepancies were addressed by discussion between two reviewers or consultation with the third reviewer (S. Z.) if necessary.
Quality Assessment
The methodological quality of each included study was assessed by 2 reviewers (N. H. and X. L.) independently, and disagreements were resolved by discussion. The potential risk of bias in the randomized controlled trials was assessed using Cochrane risk of bias.13 The quality of cohort studies was assessed per the Newcastle-Ottawa Scale (NOS) scale.14 Concerning case series studies, we used National Institutes of Health (NIH) Quality Assessment Tool (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). As no validated tool for pharmacokinetic studies was available, we used the ClinPK Statement, a reporting guideline for clinical pharmacokinetic studies to assess their quality.15
Data Analysis
To summarize all the information concerning vancomycin dosing in patients with hematologic malignancies or neutropenia, a descriptive analysis was performed.
Results
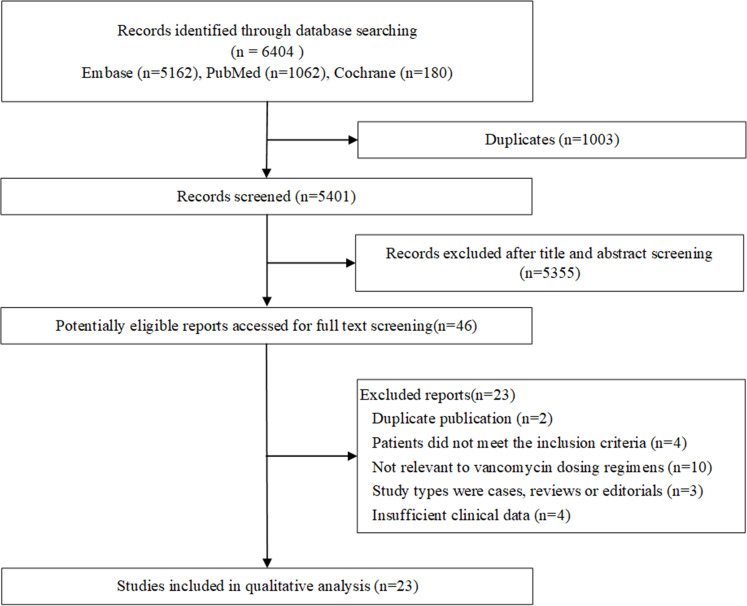
Of the 6404 potentially relevant published reports identified, 46 reports proved potentially eligible after duplicates removed and abstracts screened. On full-text screening, 23 studies were ultimately included in the systematic review (Figure 1). The list of the excluded studies in the process of full-text screening is detailed in Table S4.
Figure 1.
Flow chart of study selection.
The basic characteristics of the included studies can be found in Table S5. All the 23 studies16–38 were published in English, of which three16,18,19 were conference abstracts. One study33 was a simulation study without an actual clinical data. Two studies20,32 adopted the same set of data with different analyses methods. Therefore, twenty-one sets of clinical data were finally included. In 18 studies,17,18,20,22-31,34–38 vancomycin was infused intermittently, and 2 studies adopted continuous infusion,19,21 whereas the remaining 1 conference abstract did not report the specific dosing regimen.16 For single-arm studies, nine studies included neutropenic hematologic patients consecutively,16,18,20,21,26,28,31,34,35 while 6 studies included patients with hematologic malignancies without distinguishing neutropenia from non-neutropenia.17,19,22,27,29,38 Additionally, another two studies included hospitalized patients and took neutropenia as a risk factor.30,36 Concerning comparative cohort studies, there were two studies comparing neutropenic patients with non-neutropenic patients,23,25 and another 2 studies focused on the difference between hematologic malignant patients and control groups.24,37
Concerning the quality assessment of these included studies, 4 studies were not assessed, of which 316,18,19 were conference abstracts and one33 was a simulation pharmacokinetic study. The detailed results of quality assessment are shown in Tables S6–S9. Overall, included studies were of adequate quality.
All the included studies were classified according to their objectives as follows:
Clinical Audit of Vancomycin Dosing
Seven studies16–22 aimed to make a clinical audit of conventional vancomycin dosing in patients with hematologic malignancies or neutropenia. The characteristics and summary of results in each study are listed in Table 1. Six studies reported the proportion of patients with sub-therapeutic concentrations in routine clinical care, and five of which reported value ranging from 32% to 88%. However, Vazin et al22 did not report the specific vancomycin dosing, yielding result (3.6%) that differed significantly from other 5 studies. Furthermore, Vermis et al19 stated that to attain therapeutic vancomycin levels, vancomycin maintenance dose (41.7 mg/kg/d vs. 32.7 mg/kg/d) was significantly higher when ARC (estimated CLCR greater than 120 mL/min) was present in hematologic malignant patients. Overall, vancomycin concentrations following conventional dosage were insufficient in patients with hematologic malignancies or neutropenia.
Table 1.
Studies with Clinical Audit of Vancomycin Dosing
| Author (Year) | Country | Study Design | Characteristics of Patients Included | Patients with Neutropenia (%) | Sample Size | Age (Years) | Gender (M/F) | Weight (kg) | Renal Function | Vancomycin Dosing Regimen | Timing of Serum Vancomycin | Summary of Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hochart 201121 | France | Single-center retrospective study | Acute myeloid leukemia patients with febrile neutropenia | 100% | 54 (67 vancomycin treatment courses, VTCs) | 50 ± 13.6 | 27/27 | 73 ± 18.1 | 107.5 ± 35.4 mL/min | Continuous infusion; loading dose: 15.5 ± 3.3 mg/kg; maintenance dose: 35.4 ± 6.9 mg/kg/d |
At 24 hours for patients with a loading dose and 48 hours for patients without any loading dose. | ▪ the target serum level for continuous infusion was greater than 20 mg/L, and only 6 (12%) cases achieved the target |
| O’Donnell 201116 (conference abstract) | UK | Single-center retrospective study | Bone marrow transplant patients who experienced an episode of febrile neutropenia | 100% | 12 | NR | NR | NR | NR | NR | At 24 hours, then twice weekly. | ▪ 32% of trough levels were subtherapeutic (< 5 mg/L). |
| Donovan 201218 (conference abstract) | United states | Retrospective study | Neutropenic adult patients | 100% | 198 | NR | NR | NR | NR | Intermittent infusion; 15 – 20 mg/kg/dose and administration times are determined by renal function | NR | ▪ 25.3% of patients achieved therapeutic trough concentrations (15 – 20 mg/L) |
| Vazin 201222 | Iran | Prospective study | Patients in a hematology-oncology ward who received at least 3 successive doses of vancomycin and had serum vancomycin concentrations at steady state | 88% | 58 | 36.58 ± 14.33 | 44/14 | 68.05 ± 12.61 | 57/58 (98.2%) a | Intermittent infusion; Correcting dosage based on creatinine clearance was given to 10 (17.23%) of the patients |
Blood samples were taken from the patients who received vancomycin for 3 consecutive days, and just before the administration of the next dose. | ▪ vancomycin trough serum concentration range was 15.59 ± 13.02 mg/L ▪ subtherapeutic trough level (< 10 mg/L) was detected in 3.6% of patients ▪ 53.3% had a level above the maximum therapeutic concentration |
| Ghehi 201320 | Iran | Single-center prospective study | Adults receiving vancomycin for neutropenic fever after HSCT | 100% | 46 | 32.9 ± 12.45 | 30/16 | 74.8 ± 16.6 | 102.5 ± 35.33 mL/min | Intermittent infusion; 31.9 (±10.5) mg/kg/d | Within 30 minutes prior to the fourth dose | ▪ 25 (54.3%) patients had trough concentrations of <10 mg/L ▪ 6 patients (13%) had trough levels of < 5 mg/L |
| Luo 201417 | Canada | Single-center prospective study | Leukemia/bone marrow transplant outpatients (at least two doses of vancomycin) | 42% | 48 | 54.5b | 24/24 | NR | 77 μmol/Lb | Intermittent infusion; once-daily (2073 ± 338 mg/d) |
NR | ▪ 10 (21%) patients had therapeutic vancomycin trough concentrations (i.e., greater than 10 mg/L) |
| Vermis 201419 (conference abstract) |
Belgium | Single-center retrospective study | Patients with hematologic malignancies | 72% | 96 (112 VTCs) | NR | NR | NR | NR | Continuous infusion; loading dose: 15 mg/kg, maintenance dose: 30 mg/kg/d |
NR | ▪ ARCc was observed in 73 VTC with an average renal clearance of 147.0 mL/min versus 79.0 mL/min. ▪ Therapeutic vancomycin levels (20 mg/L) were obtained on day 5 (median) with an average vancomycin maintenance dose of 41.7 mg/kg/day when ARC was present versus 32.7 mg/kg/day on day 3. |
Notes: aProportion of patients with normal renal function; bmedian; cARC was defined as calculated creatinine clearance exceeding 120 mL/min (Cockcroft–Gault formula).
Abbreviations: NR, not reported; HSCT, hematopoietic stem cell transplantation; VTC, vancomycin treatment course; ARC, augmented renal clearance.
The Potential Change in Pharmacokinetic Parameters
Comparative Studies Between Patients with Hematologic Malignancies or Neutropenia and Control Groups
Two studies23,25 were comparative cohort studies between neutropenic patients and non-neutropenic patients, and another 2 studies24,37 focused on the difference between patients with hematologic malignancies and the control group. The characteristics of the four studies are summarized in Table 2.
Table 2.
The Characteristic of Comparative Studies
| Author (Year) | Country | Study Design | Characteristics of Patients Included | Sample Size | Grouping | Gender (M/F) | Age (Years) | Weight (kg) | Patients with Hematologic Malignancies/Neutropenia n(%) | Renal Function | Vancomycin Dosing Regimen | Timing of Serum Vancomycin | Determination of Pharmacokinetic Parameters | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Group | Control Group | Study Group | Control Group | Study Group | Control Group | Study Group | Control Group | Study Group | Control Group | ||||||||||
| Neutropenic patients vs non-neutropenic patients | |||||||||||||||||||
| Haeseker 201425 | Netherlands | Single-center prospective study | Adults received vancomycin intravenously and had at least two plasma samples | 171 | Neutropenia (< 500/mm3): n=56 | Non-neutropenia: n=115 | 104/67 | 55 ± 13 | 61 ± 14 | NR | NR | 55/56 (98.2%)a | 13/115 (11.3%)a | 113 ± 57 mL/min | 107 ± 78 mL/min | Intermittent infusion; an initial loading dose of 15 mg/kg + dose individualization based on TDM and renal function. |
Two plasma samples (peak and trough concentration) | Maximum a posterior (MAP) Bayesian estimation (MW/Pharm 3.60, Mediware, the Netherlands) | CLva, V |
| 68 (a subset of patients with hematologic malignancies) | Neutropenia (< 500/mm3): n=55 | Non-neutropenia: n=13 | NR | NR | NR | NR | NR | 55 (100%)a | 13 (100%)a | 114 ± 57 mL/min | 111 ± 58 mL/min | ||||||||
| Choi 201723 | Korea | Single-center retrospective study | Adults receiving routine TDM of vancomycin (trough and peak). | 1307 | Neutropenia (< 500/mm3) n=162 | Non-neutropenia: n=1145 | 728/579 | 54 (37–65)# | 56 (45–64)# | 62.0 (56.0–70.0)# | 60.0 (53.0–68.7)# | 135 (83.3%)a | 184 (16.1%)a | 0.6 (0.5–0.8) # mg/dL | 0.7 (0.5–0.9) # mg/dL | Intermittent infusion; 1000 mg vancomycin every 12 h | Steady-state serum vancomycin concentration (after at least the fourth dose) | Posterior Bayesian estimation (Abbott’s PKS software) | Serum trough vancomycin concentration at steady state, t1/2 |
| Patients with hematologic malignancies vs non-cancer patients | |||||||||||||||||||
| Al-Kofide 200924 | Saudi Arabia | Single-center retrospective study | Adults receiving vancomycin therapy | 31 | Cancer patients (proportion of patients with hematologic malignancies was 88.9%): n=18 | Patients without cancer: n=13 | NR | 48.5 ± 20.2 | 43.4 ± 22.1 | 66.7 ± 17.1 | 68.9 ± 14 | NRb | NRb | 105.4 ± 62.3 mL/min | 87.2 ± 27.5 mL/min | Intermittent infusion; Initial vancomycin dosing regimens were chosen by attending physicians | Peak and trough vancomycin serum concentration (after the third dose or at steady state) | Pharmacokinetic equations | CLva, V, t1/2 |
| Izumisawa 201937 | Japan | Retrospective cohort study | Adults receiving > 3 days of vancomycin therapy | 522 | Hematologic malignancy patients: n=261 | Non-malignancy patients: n=261 | 321/201 | 65.6 ± 13.6 | 67.2 ± 16.9 | 55.0 ± 10.3 | 56.2 ± 13.1 | 1.47 ± 2.46 × 103/μLc | 7.80 ± 4.66 × 103/μLc | 77.0 ± 29.2 mL/min | 74.1 ± 35.6 mL/min | Intermittent infusion; Initial dosing was not pre-specified |
After ≧ 3 days following the start of administration | Bayesian estimation using TDM software Ver 3.3 | Trough concentration, CLva, Vss, t1/2 |
Notes: #Median (interquartile range); aproportion of patients with hematologic malignancy; bproportion of patients with neutropenia; cthe absolute count of neutrophils.
Abbreviations: NR, not reported; V, volume of distribution; Vss, volume of distribution at steady state; CLva, vancomycin clearance; t1/2, half-life; TDM, therapeutic drug monitoring.
Although both neutropenic patients and the control group applied the standard dosage and consistent sampling time in Choi et al,23 the median serum vancomycin concentration was lower in neutropenic patients than the control group (9.1 mg/L vs. 12.1 mg/L, P < 0.0001). Multiple logistic regression analysis still revealed a significant association between sub-therapeutic vancomycin concentration (trough serum concentration <10 mg/L) and neutropenia (odds ratio [OR]: 1.75; P=0.029).
Additionally, Haeseker et al25 primarily investigated neutropenia and hematologic malignancy’s effect on pharmacokinetic parameters, whereas Al-Kofide et al24 and Izumisawa et al37 focused on the effect of hematologic malignancy. Concerning specific pharmacokinetic parameters, vancomycin clearance (CLva) was higher in patients with hematologic malignancies or neutropenia (Table 3). However, the results for volume of distribution (V) were still conflicting (Table 3). Notably, five patients in Haeseker et al25 received vancomycin in both neutropenic and nonneutropenic period and presented a reversible augmented CLva in the nonneutropenic period (91 ± 26 mL/min vs. 45 ± 10 mL/min, P=0.009).
Table 3.
The Pharmacokinetic Parameters in Comparative Studies
| Author (Year) | Sample Size | Vancomycin Clearance (mL/min) | T1/2 of Vancomycin (h) | V (L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hematologic Malignancies or Neutropenia | Control Group | P value | Hematologic Malignancies or Neutropenia | Control Group | P value | Hematologic Malignancies or Neutropenia | Control Group | P value | ||
| Neutropenic patients vs. non-neutropenic patients | ||||||||||
| Haeseker 201425 | 171 | 67 ± 26 | 50 ± 22 | <0.001 | – | – | – | 62 ± 32 | 56 ± 29 | 0.304 |
| 68 (subset of patients with hematologic malignancies) | 68 ± 26 | 53 ± 16 | 0.024 | – | – | – | 62 ± 32 | 59 ± 18 | 0.691 | |
| Choi 201723 | 1307 | – | – | – | 7.4 h (5.9–10.7 h) # | 8.9 h (6.9 −12.0 h)# | <0.0001 | – | – | – |
| Patients with hematologic malignancies vs. non-cancer patients | ||||||||||
| Al-Kofide 200924 | 31 | 110.1 ± 42 | 71.2 ± 22.2 | 0.005 | 8.6 ± 7.1 | 5.4 ± 1.9 | 0.111 | 70 ± 45 | 31.1 ± 8.3 | 0.002 |
| Izumisawa 201937 | 522 | 0.055 ± 0.017 L/h/kg | 0.051 ± 0.019 L/h/kg | <0.05 | 32.7 ± 13.0 | 37.1 ± 18.8 | <0.05 | 1.81 ± 0.57 L/kg | 1.84 ± 0.62 L/kg | >0.05 |
Notes: –, not reported; #, median (interquartile range).
Development and Validation of PK Models
Although seven studies20,26-29,36,38 calculated vancomycin’s pharmacokinetic parameters in patients with hematologic malignancies or neutropenia, only three studies29,36,38 used non-linear mixed effects modelling. The characteristics and PK parameter of studies included are listed in Table 4, showing a marked difference in CLva from those reported for patients with non-hematologic malignancy and non-neutropenia.39,40 Notably, one study36 included neutropenia as one of the covariates affecting vancomycin clearance, of which vancomycin clearance is increased in patients with neutropenia by 27.7%. Nevertheless, V seemed to be comparable to normal controls without hematologic malignancy and neutropenia.39,40 Additionally, all the PK parameters had great inter-individual variation among patients with hematologic malignancies or neutropenia.
Table 4.
Characteristics and Results of Studies for Developing PK/PPK Models
| Author (Year) | Country | Study Design | Characteristics of Patients Included | Patients with Neutropenia (%) | Sample Size | Number of serum Concentrations | Age | Gender (M/F) | Weight (kg) | Renal Function | Vancomycin Dosing | Timing of Vancomycin Sampling | Pharmacokinetic Modeling Method | Model | Pharmacokinetic Parameters | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V | CL | Ke (h−1) | t1/2 (h) | |||||||||||||||
| Pharmacokinetic analysis | ||||||||||||||||||
| Kureishi 199026 | Canada | Single-center prospective study | Patients with acute leukemia and had absolute granulocyte below 500/mm3 | 100% | 25 | NR | NR | NR | NR | NR | Intermittent infusion; 15 mg/kg q12h | Prior to infusion and at 1 and 3 h post-infusion daily during the first 3 days and every 3 to 7 days thereafter | Equations with two steady-state samples | One-compartment model | 0.61 ± 0.21 L/kg | NR | NR | 5.6 ± 1.8 |
| Le Normand 199428 | France | Single-center prospective study | Patients with hematologic malignancies who were neutropenic (100/mm3) | 100% | 10 | 130 | 36.2 (range: 18–50) | 4/6 | 64.6 ± 10.4 | 141.2 ± 36.2 mL/min | Intermittent infusion; 1000 mg every 12 h |
The first dose: prior to injection, at the end of the infusion, and 11 samples collected until 11 h after the end of the infusion | G-Pharm computer program | Two-compartment model | Vc: 22.9 ± 11.4 L | 158 ± 51 mL/min | NR | 2.94 ± 0.84 |
| Jarkowski 201127 | United states | Single-center prospective study | Acute myeloid leukemia patients receiving vancomycin | NR | 25 | NR | 59.12 ± 16.26 | 17/8 | 86.05 ± 19.42 | 85.72 ± 37.28 mL/min/1.73m2 | Intermittent infusion; 1970.00 ± 605.19 mg/d | Three samples: 1 h, 3–8 h, and 8–24 h post-infusion | Maximum a priori Bayesian estimation using Adapt 5 | Two-compartment model | Vc: 0.23 L/kg Vss: 0.60 L/kg |
0.14 L/h/kg | NR | NR |
| Ghehi 201320 | Iran | Single-center prospective study | Patients with neutropenic fever after HSCT | 100% | 20 | 40 | 29.9 ± 9.5 | NR | 72.5 ± 15.2 (ABW) | 104.7 ± 37.0 mL/min | Intermittent infusion; 31.9 (±10.5) mg/kg/d (69.6%:1g q12h; 17.4%:1g q8h) | First steady-state trough (within 30 minutes prior to the fourth dose), peak concentration, random sample | Equations with two steady-state samples | One-compartment model | 0.60 (0.44–0.76) L/kg | 0.090 (0.071–0.109) L/h/kg 109.7 (82.7–136) mL/min | 0.16 (0.13–0.19) | 4.9 (3.8–6.0) |
| Population pharmacokinetic model | ||||||||||||||||||
| Buelga 200529 | Spain | Single-center retrospective study | Adult inpatients with an underlying hematologic malignancy | 43.7% | 215 | 1004 | 51.5 ± 15.9 | 119/96 | 64.7 ± 11.3 | 89.4 ± 39.2 mL/min | Intermittent infusion | Blood sampling was ordered as required clinically | Nonlinear mixed-effect modeling approach (NONMEM) | One-compartment model | CL (L/h): 1.08 × CLCR (Cockcroft and Gault) (L/h); CVCL: 28.16% V (L) =0.98 ×TBW; CVV:37.15%. |
|||
| Okada 201838 | Japan | Single-center retrospective study | Patients undergoing allo-HSCT who received preventive treatment with vancomycin | NR | 75 | 227 | 49 (range: 17–69) | 49/26 | 59.4 (range: 39.4–104.5) | 113 (range: 47–253) mL/min | Intermittent infusion initial dosage of 1 g/12 hours (if the CLCR was >75 mL/min/1.73 m2). |
Immediately before ad-ministering vancomycin,1 hour after drug administration and at some other points as necessary | Nonlinear mixed-effect modeling approach (NONMEM) | Two-compartment model | Vc (L)= 39.2 × (TBW/59.4)^0.78; CVVc=14.2% CL (L/h) =4.25 × (CLCR/113)^0.70; CVCL=25.2% Vp (L) =56.1; CVVp=66.9% |
|||
| Bury 201936 | The Netherlands | Retrospective matched cohort study | Intravenous vancomycin therapy for ≥ 2 days and at least one available vancomycin concentration | 26.7% | 116 | 742 | 61.4 ± 13.4 | 67/49 | NR | Median 92.7 mL/min | Intermittent infusion The specific dosing was not pre-specified |
NR | Nonlinear mixed-effect modeling (NONMEM) | Two-compartment model | CL(L/h) = 3.22+(1+0.00834 × (CLCR −104)) × 1.277NEUTROPENIA; CVCL=33.0% | |||
Abbreviations: V, volume of distribution; CL, clearance; Ke, elimination rate constant; t1/2, half-life; NR, not reported; ABW, adjusted body weight; TBW, total body weight; CLCR, creatinine clearance; Vc, volume of central compartment; Vss, steady-state volume of distribution; Vp, distribution volume of peripheral compartment.
The Potential Effect of Neutropenia on Creatinine Clearance (CLCR)
Four studies25,28,30,31 reported the potential effect of neutropenia on CLCR. Hirai et al30 conducted a single-center retrospective study in 292 patients with normal serum creatinine concentration, and demonstrated that febrile neutropenia was an independent risk factor of ARC (OR: 2.76; 95% CI: 1.11–6.67; P = 0.0254). However, Haeseker et al25 showed that the estimated CLCR was not significantly different between patients with neutropenia and non-neutropenia (Table 2).
Three studies evaluated the correlation between CLva and estimated CLCR solely. Soto et al31 included 45 neutropenic (<1000/mm3) hematologic patients and demonstrated that the correlation coefficient between CLva (106 ± 37 mL/min) and estimated CLCR (84.7 ± 32 mL/min) was 0.42. Le Normand et al28 illustrated a poor correlation in neutropenic patients (100/mm3) as well (n = 10, r = 0.281). According to Hirai et al,30 the non-ARC patients showed a significant correlation between CLCR ad CLva (r = 0.8726, P < 0.0001); however, no such relationship was observed in patients with ARC (r = 0.1029, P = 0.4866).
Above all, although CLCR possibly has an increase in patients with neutropenia, estimated CLCR itself could not identify the specific patients with ARC, which brought difficulty to the prediction of CLva.
Optimization of Initial Vancomycin Dosing Regimen
Six studies24,25,32,33,36,38 were relevant to the optimization of initial vancomycin dosing regimen. Taghizadeh-Ghehi et al32 evaluated the applicability of the most cited vancomycin one-compartment models developed in common patients using data from their recent study.20 They demonstrated that none of the seven pharmacokinetic models performed well to calculate initial vancomycin dosage in Iranian patients underwent hematopoietic stem cell transplantation. Using a published population pharmacokinetic (PPK) model29 in patients with hematologic malignancies, Fernandez et al33 performed Monte Carlo simulation to calculate vancomycin dosages required in the specific subpopulation. When standard vancomycin dosing (2000 mg/d) was given, cumulative fraction of response (CFR) for S. aureus was 90.4%, 47.3% and 31.2% for CLCR values of <60, 60–120 and >120 mL/min, respectively. If a CFR of 80% was considered to be clinically appropriate, vancomycin doses of 3000 and 4000 mg/d for a CLCR 60–120 and >120 mL/min should be used. Okada et al38 also proposed a vancomycin dosing nomogram in patients undergoing allogeneic hematopoietic stem-cell transplantation based on PPK model and Monte Carlo simulation. Suggested vancomycin dosing is 1g per 12 hours when CLCR ranging from 75 to 90 mL/min, 0.75 g per 8 hours when CLCR ranging from 90 to 120 mL/min, 1g per 8 hours when CLCR ranging from 120 to 175 mL/min, and 1.25 g per 8 hours for CLCR greater than 175 mL/min. Based on individualized pharmacokinetic parameters calculated by AI-Kofide et al,24 the actual dosing regimen for cancer patients should be 60 mg/kg/day, which doubled the required dose for the general population (30 mg/kg/d). Haeseker et al25 demonstrated that to achieve the same AUC24, the mean dosage in patients with neutropenia was significantly higher than the control group (2017 ± 720 vs 1521 ± 727 mg, P < 0.001). In this case, they concluded that the daily dose should be increased with 33% in patients with neutropenia (from 15 mg/kg twice daily to 13 mg/kg three times daily). Similarly, another study36 suggested a 25% increase for vancomycin dosing in neutropenic patients. However, the dosing algorithms aforementioned were inconsistent to some extent and have not been validated in the prospective clinical setting. Hence, no simple upward dose adjustment can be put up with great validity and the initial dosing recommendation still remains investigational.
Evaluation and Implementation of Vancomycin TDM
Two aspects of TDM have been explored before, including the target trough concentration and the evaluation of TDM-guided vancomycin therapy. Suzuki et al35 retrospectively included 63 febrile neutropenic patients with hematologic malignancies and investigated the association of first trough concentration at steady state with clinical efficacy and nephrotoxicity. They proposed that the cut-off value of vancomycin trough concentration should be around 11.5 mg/L in these patients.
To assess the effectiveness and safety of vancomycin TDM and pharmacokinetic interpretation, Fernandez et al34 performed a prospective randomized study in 70 immunocompromised febrile patients with hematologic malignancies. Although there was no significant difference in clinical response rate and duration of fever between TDM-guided group (n=37) and control group (n=33), the incidence of nephrotoxicity significantly decreased (13.5% vs. 42.4%, P < 0.05).
Discussion
Brief Summary of the Systematic Review
Several descriptive studies demonstrated that their routine vancomycin dosing was inadequate for effective antimicrobial therapy. Regarding the alterations in PK parameters, studies showed that CLva tended to be higher in patients with hematologic malignancies or during febrile neutropenia, whereas V seemed to be comparable to the control groups. Although several pharmacokinetic models have been developed and a few dosing regimens have been proposed, there is still no consensus on initial vancomycin dosing in patients with hematologic malignancies or neutropenia. The available evidence indicates that TDM and optimal pharmacokinetic interpretation can help in decreasing the risk of nephrotoxicity.
Implications for Clinical Practice
In view that standard dosing is inadequate for some patients with hematologic malignancies or neutropenia, improper dosing should be considered as a possible reason when clinical improvement was not achieved in these patients with suspected or documented Gram-positive infection. Therefore, optimization of dosing regimen must be considered in both initial dosing and dose adjustment. However, it still remains a question of how to identify the patients with ARC accurately, which makes the individualization of initial dosing difficultly. For example, Soto et al31 and Le Normand et al28 demonstrated that the correlation between estimated CLCR and CLva was poor. Haeseker et al41 also showed that CLva algorithms based on estimated CLCR were unsuitable in these patients. Additionally, Taghizadeh-Ghehi et al32 demonstrated that none of the seven most cited vancomycin one-compartment models performed well to calculate initial dosage. In this case, TDM of vancomycin could be valuable in patients with hematologic malignancies or neutropenia. Without performing TDM, the extremely high dosing could not be administered. Furthermore, previous studies demonstrated that pharmacokinetic dosing programs using measured vancomycin serum levels could predict vancomycin levels with acceptable accuracy and precision.42 Therefore, we recommend, when possible, TDM-guided therapy to optimize vancomycin therapy in patients with hematologic malignancy or neutropenia. Above all, the systematic review underlines the necessity to perform vancomycin TDM in patients with hematologic malignancies or neutropenia, which might be overlooked previously.
Implications for Further Research
According to the comprehensive systematic review, several knowledge gaps have been identified, and are summarized as follows:
The mechanism of the altered PK parameters warrants investigation, which could help us judge whether the phenomenon was deceptive or not.
Two scenarios should be considered to clarify the mechanism of the altered PK parameters. On the one hand is the further research in clinical settings. First, most of the studies did not distinguish whether the change in pharmacokinetic parameters was due to hematologic malignancy or neutropenia. The effect of hematologic malignancy can be complicated by neutropenia and vice versa. Only Haeseker et al25 demonstrated that the augmented clearance was associated with neutropenia rather than hematologic malignancies with a limited sample size. As previous studies showed vancomycin clearance was higher in patients with hematologic malignancies than solid tumors43 and the difference between solid malignancies and control groups was attenuated,44 we assume that the phenomenon might be explained by different proportions of patients with neutropenia. In other words, it is the neutropenia that affects pharmacokinetic changes per se. Conventional doses of vancomycin may not offer adequate systematic exposure in febrile neutropenic patients rather than hematologic malignancy without neutropenia. Further studies are needed to elucidate the exact effect between hematologic malignancies and neutropenia. Second, studies focused on non-neutropenic immunocompromised states are limited currently. Whether the pharmacokinetic changes exist in non-neutropenic immunocompromised states can help in interpreting the mechanism. Third, none of the studies evaluated the change in measured CLCR, which might also be helpful for understanding the mechanism and identifying patients with ARC.
On the other hand, the physiological mechanism responsible for ARC has not been well-defined, which can be investigated using in vitro studies and animal models. Several assumptions have been put up, including: (1) possible changes in renal function and urine flow can be induced by cancer, systematic inflammation and increased intravenous fluid; (2) tubular secretion apart from glomerular filtration; (3) non-renal elimination of vancomycin, such as hepatic conjugation; (4) cancer and neutropenia could enhance vascular permeability, which would induce increased vancomycin extravasation and low serum concentrations.20,23-25,28,29 The above assumptions require further exploration.
No PPK model has been developed in patients with hematologic malignancies and concomitant neutropenia, which could help with determining individualized initial dose.
The optimization of vancomycin dosing in patients with hematologic malignancies or neutropenia requires further research. For example, prospective validation of vancomycin initial dosing regimens, stages of enhanced CLCR and a consensus of initial dosing strategies are urgently needed worldwide.
Few studies on the PKs of vancomycin in patients with hematologic malignancies or neutropenia reported clinical outcomes. Taking safety endpoints as an example, some studies illustrated that patients with hematologic malignancies may be vulnerable to nephrotoxicity.45,46 In this case, the target trough concentration for these patients might be different from other patients and require further research. Indeed, it should be noted that the establishment of the relationship between accelerated vancomycin elimination and outcomes of clinical effectiveness is difficult due to the complexity of these patients.
Strengths and Limitations
To our knowledge, this is the first comprehensive review concerning vancomycin dosing optimization in patients with hematologic malignancy or neutropenia. Additionally, the characteristics and the key results of each individual study are presented in Tables 1–4, which can provide specific details for physicians, pharmacists as well as researchers. However, as the available evidence to date was limited and diverse, no quantitative analysis was performed. Clinical heterogeneity existed across studies. For example, patients included had different types of hematologic malignancies and no consistent definition of neutropenia has been applied among studies. Nevertheless, we consider that this systematic review maps the relevant literature on this topic, allowing us to pay attention to the optimization of vancomycin dosing in patients with hematologic malignancy or neutropenia.
Conclusion
The available evidence indicates that conventional vancomycin dosing leads to suboptimal concentration in patients with hematologic malignancy or neutropenia. TDM accompanied by pharmacokinetic interpretation can decrease the risk of nephrotoxicity. The individualization of the initial dosing regimen and mechanisms of augmented clearance require further research.
Acknowledgments
Thanks for Xiaotong Li’s help for risk of bias assessment during the revision process.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Matzke GR, Aronoff GR, Atkinson AJ, et al. Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80(11):1122–1137. doi: 10.1038/ki.2011.322 [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto K, Takesue Y, Ohmagari N, et al. Practice guidelines for therapeutic drug monitoring of vancomycin: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother. 2013;19(3):365–380. doi: 10.1007/s10156-013-0599-4 [DOI] [PubMed] [Google Scholar]
- 3.Bilbao-Meseguer I, Rodriguez-Gascon A, Barrasa H, Isla A, Solinis MA. Augmented renal clearance in critically Ill patients: a systematic review. Clin Pharmacokinet. 2018;57(9):1107–1121. doi: 10.1007/s40262-018-0636-7 [DOI] [PubMed] [Google Scholar]
- 4.Udy AA, Jarrett P, Lassig-Smith M, et al. Augmented renal clearance in traumatic brain injury: a single-center observational study of atrial natriuretic peptide, cardiac output, and creatinine clearance. J Neurotrauma. 2017;34(1):137–144. doi: 10.1089/neu.2015.4328 [DOI] [PubMed] [Google Scholar]
- 5.Kim AJ, Lee JY, Choi SA, Shin WG. Comparison of the pharmacokinetics of vancomycin in neurosurgical and non-neurosurgical patients. Int J Antimicrob Agents. 2016;48(4):381–387. doi: 10.1016/j.ijantimicag.2016.06.022 [DOI] [PubMed] [Google Scholar]
- 6.Keng MK, Sekeres MA. Febrile neutropenia in hematologic malignancies. Curr Hematol Malig Rep. 2013;8(4):370–378. doi: 10.1007/s11899-013-0171-4 [DOI] [PubMed] [Google Scholar]
- 7.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–e93. [DOI] [PubMed] [Google Scholar]
- 8.Goulenok T, Fantin B. Antimicrobial treatment of febrile neutropenia: pharmacokinetic-pharmacodynamic considerations. Clin Pharmacokinet. 2013;52(10):869–883. doi: 10.1007/s40262-013-0086-1 [DOI] [PubMed] [Google Scholar]
- 9.Lortholary O, Lefort A, Tod M, Chomat AM, Darras-Joly C, Cordonnier C. Pharmacodynamics and pharmacokinetics of antibacterial drugs in the management of febrile neutropenia. Lancet Infect Dis. 2008;8(10):612–620. doi: 10.1016/S1473-3099(08)70228-7 [DOI] [PubMed] [Google Scholar]
- 10.Ye ZK, Chen YL, Chen K, et al. Therapeutic drug monitoring of vancomycin: a guideline of the division of therapeutic drug monitoring, chinese pharmacological society. J Antimicrob Chemother. 2016;71(11):3020–3025. doi: 10.1093/jac/dkw254 [DOI] [PubMed] [Google Scholar]
- 11.Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434 [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Cochrane Collaboration; 2011:1–8. [Google Scholar]
- 14.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed May11, 2020.
- 15.Kanji S, Hayes M, Ling A, et al. Reporting guidelines for clinical pharmacokinetic studies: the ClinPK statement. Clin Pharmacokinet. 2015;54(7):783–795. doi: 10.1007/s40262-015-0236-8 [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell R. Audit of BMT antibiotic febrile neutropenia guidelines. Bone Marrow Transplant. 2011;46:S433–S434. [Google Scholar]
- 17.Luo C, Hussaini T, Lacaria K, Yeung J, Lau TT, Broady RC. Evaluation of a Once-Daily Vancomycin regimen in an outpatient leukemia/bone marrow transplant clinic (OD-VANCO Study). Can J Hosp Pharm. 2014;67(4):280–285. doi: 10.4212/cjhp.v67i4.1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donovan KA, Sklenicka J. Is a standard vancomycin dosing protocol appropriate to achieve therapeutic trough levels in adult neutropenic patients? Pharmacotherapy. 2012;32(10):e237–e238. [Google Scholar]
- 19.Vermis K, Steel E, Vandenbroucke J. Prevalence of augmented renal clearance in haematological patients and the impact on vancomycin dosing. J Oncol Pharm Pract. 2014;20(3):7. [Google Scholar]
- 20.Ghehi MT, Rezaee S, Hayatshahi A, et al. Vancomycin pharmacokinetic parameters in patients undergoing Hematopoietic Stem Cell Transplantation (HSCT). Int J Hematol Oncol Stem Cell Res. 2013;7(4):1–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Hochart C, Berthon C, Corm S, et al. Vancomycin serum concentration during febrile neutropenia in patients with acute myeloid leukemia. Med Mal Infect. 2011;41(12):652–656. doi: 10.1016/j.medmal.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 22.Vazin A, Japoni A, Shahbazi S, Davarpanah MA. Vancomycin utilization evaluation at hematology-oncology ward of a teaching hospital in iran. Iran J Pharm Res. 2012;11(1):163–170. [PMC free article] [PubMed] [Google Scholar]
- 23.Choi MH, Choe YH, Lee SG, Jeong SH, Kim JH. Neutropenia is independently associated with sub-therapeutic serum concentration of vancomycin. Clin Chim Acta. 2017;465:106–111. doi: 10.1016/j.cca.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 24.Al-Kofide H, Zaghloul I, Al-Naim L. Pharmacokinetics of vancomycin in adult cancer patients. J Oncol Pharm Pract. 2010;16(4):245–250. doi: 10.1177/1078155209355847 [DOI] [PubMed] [Google Scholar]
- 25.Haeseker MB, Croes S, Neef C, Bruggeman CA, Stolk LM, Verbon A. Vancomycin dosing in neutropenic patients. PLoS One. 2014;9(11):e112008. doi: 10.1371/journal.pone.0112008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kureishi A, Jewesson PJ, Bartlett KH, Cole CD, Chow AW. Application of a modified bioassay for monitoring serum teicoplanin and vancomycin in febrile neutropenic patients. Antimicrob Agents Chemother. 1990;34(9):1642–1647. doi: 10.1128/AAC.34.9.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarkowski IA, Forrest A, Sweeney RP, et al. Characterization of vancomycin pharmacokinetics in the adult acute myeloid leukemia population. J Oncol Pharm Pract. 2012;18(1):91–96. doi: 10.1177/1078155211402107 [DOI] [PubMed] [Google Scholar]
- 28.Le Normand Y, Milpied N, Kergueris MF, Harousseau JL. Pharmacokinetic parameters of vancomycin for therapeutic regimens in neutropenic adult patients. Int J Biomed Comput. 1994;36(1–2):121–125. doi: 10.1016/0020-7101(94)90102-3 [DOI] [PubMed] [Google Scholar]
- 29.Buelga DS,Fernandez de Gatta MM, Herrera EV, Dominguez-Gil A, Garcia MJ. Population pharmacokinetic analysis of vancomycin in patients with hematological malignancies. Antimicrob Agents Chemother. 2005;49(12):4934–4941. doi: 10.1128/AAC.49.12.4934-4941.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirai K, Ishii H, Shimoshikiryo T, et al. Augmented renal clearance in patients with febrile neutropenia is associated with increased risk for subtherapeutic concentrations of vancomycin. Ther Drug Monit. 2016;38(6):706–710. doi: 10.1097/FTD.0000000000000346 [DOI] [PubMed] [Google Scholar]
- 31.Soto J, Alsar MJ, Chantal P, Sacristan JA. Correlation of vancomycin clearance and creatinine clearance: unreliability for predicting initial dosing in neutropenic haematological patients. J Antimicrob Chemother. 1993;32(6):920–922. doi: 10.1093/jac/32.6.920 [DOI] [PubMed] [Google Scholar]
- 32.Taghizadeh-Ghehi M, Rezaee S, Gholami K, Hadjibabaie M. Predictive performance of vancomycin population pharmacokinetic models in Iranian patients underwent hematopoietic stem cell transplantation. J Res Pharm Pract. 2015;4(3):129–134. doi: 10.4103/2279-042X.162357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez de Gatta MM, Santos BD, Sanchez NA, Dominguez-Gil A, Garcia MJ. Vancomycin dosage optimization in patients with malignant haematological disease by pharmacokinetic/pharmacodynamic analysis. Clin Pharmacokinet. 2009;48(4):273–280. doi: 10.2165/00003088-200948040-00005 [DOI] [PubMed] [Google Scholar]
- 34.Fernandez de Gatta MM, Calvo MV, Hernandez JM, Caballero D, San MJ, Dominguez-Gil A. Cost-effectiveness analysis of serum vancomycin concentration monitoring in patients with hematologic malignancies. Clin Pharmacol Ther. 1996;60(3):332–340. doi: 10.1016/S0009-9236(96)90060-0 [DOI] [PubMed] [Google Scholar]
- 35.Suzuki Y, Tokimatsu I, Morinaga Y, et al. A retrospective analysis to estimate target trough concentration of vancomycin for febrile neutropenia in patients with hematological malignancy. Clin Chim Acta. 2015;440:183–187. doi: 10.1016/j.cca.2014.11.027 [DOI] [PubMed] [Google Scholar]
- 36.Bury D, Ter Heine R, van de Garde E, et al. The effect of neutropenia on the clinical pharmacokinetics of vancomycin in adults. Eur J Clin Pharmacol. 2019;75(7):921–928. doi: 10.1007/s00228-019-02657-6 [DOI] [PubMed] [Google Scholar]
- 37.Izumisawa T, Kaneko T, Soma M, et al. Augmented renal clearance of vancomycin in hematologic malignancy patients. Biol Pharm Bull. 2019;42(12):2089–2094. doi: 10.1248/bpb.b19-00652 [DOI] [PubMed] [Google Scholar]
- 38.Okada A, Kariya M, Irie K, et al. Population pharmacokinetics of vancomycin in patients undergoing allogeneic hematopoietic stem-cell transplantation. J Clin Pharmacol. 2018;58(9):1140–1149. doi: 10.1002/jcph.1106 [DOI] [PubMed] [Google Scholar]
- 39.Matzke GR, Zhanel GG, Guay DR. Clinical pharmacokinetics of vancomycin. Clin Pharmacokinet. 1986;11(4):257–282. doi: 10.2165/00003088-198611040-00001 [DOI] [PubMed] [Google Scholar]
- 40.Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25(4):433–437. doi: 10.1128/AAC.25.4.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haeseker M, Croes S, Neef C, Bruggeman C, Stolk L, Verbon A. Evaluation of vancomycin prediction methods based on estimated creatinine clearance or trough levels. Ther Drug Monit. 2016;38(1):120–126. doi: 10.1097/FTD.0000000000000250 [DOI] [PubMed] [Google Scholar]
- 42.Fernandez de Gatta MM, Fruns I, Dominguez-Gil A. Individualizing vancomycin dosing regimens: an evaluation of two pharmacokinetic dosing programs in critically ill patients. Pharmacotherapy. 1994;14(2):196–201. [PubMed] [Google Scholar]
- 43.Krivoy N, Peleg S, Postovsky S, Ben AM. Pharmacokinetic analysis of vancomycin in steady state in pediatric cancer patients. Pediatr Hematol Oncol. 1998;15(4):333–338. doi: 10.3109/08880019809014017 [DOI] [PubMed] [Google Scholar]
- 44.Omote S, Yano Y, Hashida T, et al. A retrospective analysis of vancomycin pharmacokinetics in Japanese cancer and non-cancer patients based on routine trough monitoring data. Biol Pharm Bull. 2009;32(1):99–104. doi: 10.1248/bpb.32.99 [DOI] [PubMed] [Google Scholar]
- 45.Toro JJ, Diaz-Duque AE, Gass MJ, Haile DJ, Gushiken F. Acute renal injury and vancomycin levels in patients undergoing autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23(3):S139. doi: 10.1016/j.bbmt.2016.12.269 [DOI] [Google Scholar]
- 46.Mae H, Ooi J, Takahashi S, et al. Early renal injury after myeloablative cord blood transplantation in adults. Leuk Lymphoma. 2008;49(3):538–542. doi: 10.1080/10428190701824577 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed May11, 2020.