Abstract
Purpose
To analyze the effects on microaneurysm (MA) and perifoveal perfusion in nonproliferative diabetic retinopathy (NPDR) patients with macular edema (ME) after early intensive treatment using intravitreal ranibizumab (IVR) injections.
Patients and Methods
Prospectively, 25 eyes of 25 type 2 diabetes mellitus patients with ME were included between August 2016 and February 2019. For 6 months, patients were administered 0.5-mg IVR injections monthly. Ocular evaluation, including best-corrected visual acuity (BCVA; using the Early Treatment Diabetic Retinopathy Study chart), central retinal thickness (CRT; using optical coherence tomography), fundus photography, and fluorescein angiography, was performed for all participants. Results obtained at baseline were compared to those observed after 6 months.
Results
Mean BCVA increased significantly from 67.6±3.29 letters at baseline to 76.36±1.61 letters after 6 months (P=0.002) of IVR therapy. CRT decreased significantly from 479.12±16.66 µm at baseline to 369.12±13.02 µm at 6 months. Similarly, the total number of MAs decreased significantly from 5.68±3.41 to 1.60±1.73 (P<0.0001). MA turnover, calculated by adding the MA formation rate to the MA disappearance rate (both calculated as MA number/month) also decreased significantly from 6.88±3.83 to 1.92±1.75 after treatment (P<0.0001). Perifoveal non-perfused area decreased from 2.517±0.456 mm2 at baseline to 2.495±0.293 mm2 at 6 months, but the results were not statistically significant (P=0.954).
Conclusion
Treatment with early intensive IVR therapy in NPDR patients with ME not only improved BCVA and CRT but also decreased MA turnover. However, in the study period of 6 months, IVR therapy did not show significant improvement in perifoveal non-perfused area.
Keywords: nonproliferative diabetic retinopathy, macular edema, ranibizumab, early intensive treatment, microaneurysm
Introduction
Increased prevalence of diabetes mellitus has roused interest in its ophthalmic sequelae such as diabetic retinopathy (DR) and diabetic macular edema (DME).1 DR and DME are major causes of visual impairment in millions of patients worldwide,2 and there are numerous ongoing studies on the efficacy of various treatment modalities for these conditions. While multiple causes of retinopathy have been implicated, vascular endothelial growth factor (VEGF) seems to play an important role in the pathogenesis of these conditions.3,4 DR, in both proliferative and nonproliferative stages, shows an increase in VEGF production,5 which causes structural changes in the retinal vessels, leading to subsequent increase in vessel permeability and angiogenesis, and ultimately, visual impairment.6
Panretinal laser photocoagulation (PRP) and focal laser treatment have played important roles in preventing the progression of DR and in alleviating DME. However, PRP can cause permanent impairment of peripheral vision, night blindness, and deterioration of DME. Further, it cannot be performed in certain patients with concomitant cataract or vitreous hemorrhage.7 Focal laser treatment can also be utilized in selected patients only.8 To address these shortcomings, anti-VEGF therapy has been proposed for the management of both DR and DME.9 Recently, intravitreal, injectable, anti-VEGF antibody has been reported to delay the progression and ameliorate the severity of both DR and DME.10–13
Results of multi-center, randomized-controlled clinical trials such as RIDE, RISE, and RESOLVE studies have shown the efficacy of intravitreal ranibizumab (IVR) injection monotherapy in the treatment of DME, based on markers such as best-corrected visual acuity (BCVA) and central retinal thickness (CRT).14–17 Furthermore, many recent studies have shown that the formation and disappearance of microaneurysms (MAs), as well as the extent of perifoveal non-perfused area, can also be used as markers to predict the progression and prognosis of DME.18–25
In this prospective study, we evaluated the efficacy of IVR therapy by assessing the response to monthly administration of IVR injections for 6 months in nonproliferative diabetic retinopathy (NPDR) patients with DME. Changes in BCVA and CRT, as compared to the baseline, were evaluated monthly, for 6 months. Accompanying markers such as changes in total number of MAs, MA turnover, and extent of perifoveal non-perfused area were also analyzed and compared to their pre-treatment values.
Materials and Methods
Study Design
This was an interventional, prospective, single-center study conducted at Wonkwang University Hospital from August 2016 to February 2019.
All patients included in the study provided signed, informed consents prior to enrollment. The consent statement included the purpose and method of the study as well as information on reasonable expected benefits along with potential risks and side effects.
The study protocol was approved by the Institutional Review Board of the Wonkwang University Hospital (Approval no: WKUH 201,602-CTDG-005). The clinical trial has been registered on ClinicalTrials.gov (ID: NCT02834663). This study abided by the principles defined in the Declaration of Helsinki throughout the trial.
Method
All patients underwent the following baseline ophthalmological examinations and investigations: i) Assessment of BCVA using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart, ii) measurement of intraocular pressure, iii) optical coherence tomography (OCT) (Spectralis OCT®: Heidelberg engineering, Heidelberg, Germany), iv) fundus photography (Kowa nonmyd 7®: Kowa American Corporation, Kowa, USA), and v) fluorescein angiography (FA) (SpectralisHRA2®: Heidelberg engineering, Heidelberg, Germany).
Patients were administered 0.5-mg IVR injections monthly for 6 months. At each visit, the above-mentioned ophthalmological tests (except FA) were repeated. FA was performed at baseline and was repeated after 3 and 6 months of treatment.
Enrollment Criteria
NPDR patients aged >40 years, having type 2 diabetes mellitus, with BCVA of the study eye being ≥20/200 (Snellen equivalent using ETDRS testing), and having CRT of ≥300 µm on OCT were included in this study. Patients with proliferative diabetic retinopathy (PDR), previous history of vitreoretinal surgery, post-cataract operation status (≤4 months before participation in this study), uncontrolled hypertension, or glaucoma were excluded from the study. Patients who had received prior treatment with anti-VEGF drugs, intraocular corticosteroids, and/or retinal laser application were also excluded. If both eyes met the study inclusion criteria, only the more severely affected eye was selected for treatment, based on BCVA.
Assessment of Treatment Efficacy
Patients were evaluated for changes in BCVA and CRT of the treated eye, from the start of the study to 6 months, until trial completion.
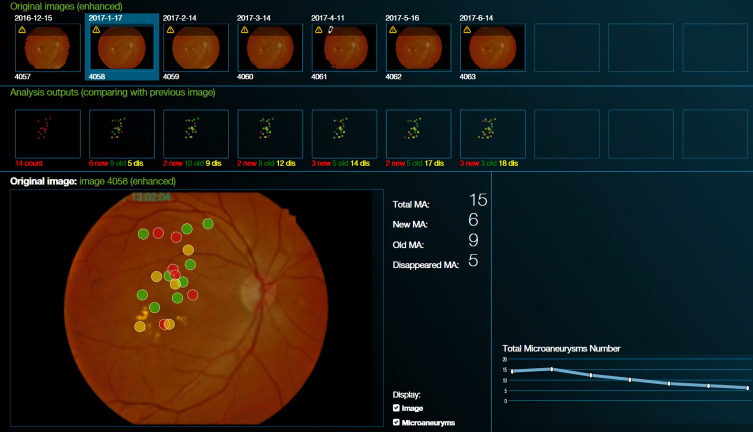
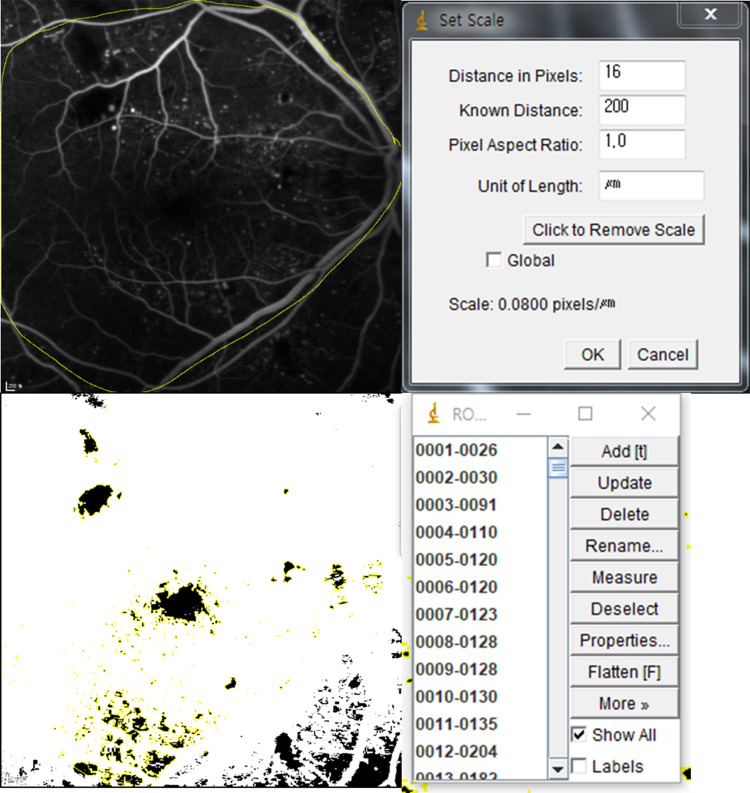
The MAs and perifoveal non-perfused areas in individual retinas were evaluated at 6 months using fundus photography and FA imaging. The Retmarker (version 1.0.2 by Retmarker Ltd, Coimbra, Portugal) software was used for automatic measurement and analysis of changes in number and extent of MAs on fundus photographs and to calculate the total number and turnover of MAs. It includes a co-registration algorithm that allows comparison within the same retinal location between different visits for the same eye. MA turnover was calculated by adding the MA formation rate (number of new MAs detected/month) to the MA disappearance rate (number of MAs resolved/month) (Figure 1).20–22 Perifoveal non-perfused area was analyzed using the arteriovenous phase in the FA image (from each of the three examinations) when perifoveal capillary network was best visualized, that is, approximately 30 seconds after injection, and was estimated by connecting the blood vessels instead of the branches of superior and inferior temporal venules within 30 degrees. Subsequently, the ImageJ software (version 1.25a 23/04/2018 by ImageJ, USA) was used for scaling each image by 16 pixels, down to 200 µm. Thereafter, the contrast and sensitivity of all photographs were converted to the maximum values to compare the perfused and non-perfused areas. The raw red-green-blue (RGB) images were then converted to 8-bit images, with the threshold set for optimal visualization of the non-perfused area. Thus, FA images that needed analysis were converted to raw RGB images, the program automatically converted the thresholds, and one retina specialist assessed the image for accuracy. The same threshold was applied to all images of the same patient. Subsequently, particle analysis was performed to calculate the total perifoveal non-perfused area (Figure 2).
Figure 1.
Comparison of new and disappeared microaneurysms on fundus photograph for detecting diabetic retinopathy using Retmarker (version 1.0.2 by Retmarker Ltd, Coimbra, Portugal) automated software.
Figure 2.
Comparison of changes in non-perfused retinal area estimated using perifoveal fluorescein angiography. The angiography image is analyzed using ImageJ software (version 1.52a 23/04/2018 by ImageJ, National Institutes of Health, Wayne Rasband, Bethesda, Maryland, USA), which is an automated system used for accurate image analysis (distance in pixels: 16, known distance: 200µm, pixel aspect ratio: 1.0).
Statistical Analysis
The SPSS Statistics 18 (IBM, Armonk, NY, USA) software was used for data analysis. The repeated measures analysis of variance with Bonferroni correction was used to analyze continuous outcome measures, including BCVA, CRT, total number of MAs, MA formation rate, MA disappearance rate, MA turnover, and perifoveal non-perfused area, before and after completion of 6 months of IVR therapy. A P-value <0.05 was considered statistically significant.
Results
Baseline Characteristics
Totally, 25 eyes of 25 patients (13 males, 12 females; mean age, 63.80±11.45 years; range, 41–80 years) were included in the study. Baseline characteristics of all study patients are summarized in Table 1.
Table 1.
Baseline Characteristics of All Study Patients
| Variable | Data |
|---|---|
| Total (eye, %) | 25 |
| Male | 13 (52%) |
| Female | 12 (48%) |
| Age (years) | |
| Mean ± SD | 63.80 ± 11.45 |
| Range | 41–80 |
| Duration of DM (year) | 12.04 ± 7.475 |
| HbA1c (%) | |
| Mean ± SD | 8.27 ± 2.11 |
| Range | 5.9–13 |
| BCVA (ETDRS letters) | |
| Mean ± SD | 67.60 ± 3.29 |
| Range | 10–84 |
| CRT (µm) | |
| Mean ± SD | 479.12 ± 16.66 |
| Range | 300–624 |
| Total microaneurysms (number) | |
| Mean ± SD Range |
5.68 ± 3.41 13.00 |
| Perifoveal non-perfused area (mm2) | |
| Mean ± SD | 2.517 ± 0.456 |
| Range | 0.41–10.86 |
Note: The values are presented as mean±SD and range unless otherwise indicated.
Abbreviations: SD, standard deviation; BCVA, best-corrected visual acuity; CRT, central retinal thickness; DM, diabetic mellitus; ETDRS, Early Treatment Diabetic Retinopathy Study; HbA1c, glycosylated hemoglobin.
BCVA & CRT Outcomes
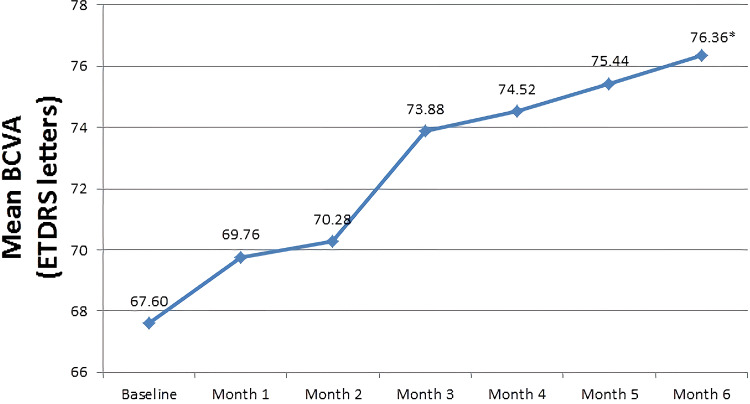
The mean BCVA (ETDRS letters) at baseline was 67.6±3.29 letters, which significantly increased to 76.36±1.61 letters after administration of six monthly IVR injections (P<0.0001) (Figure 3).
Figure 3.
Mean best-corrected visual acuity (using Early Treatment Diabetic Retinopathy Study chart) over the six-month study period. *Statistically significance is P < 0.05 with baseline.
Abbreviations: BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study.
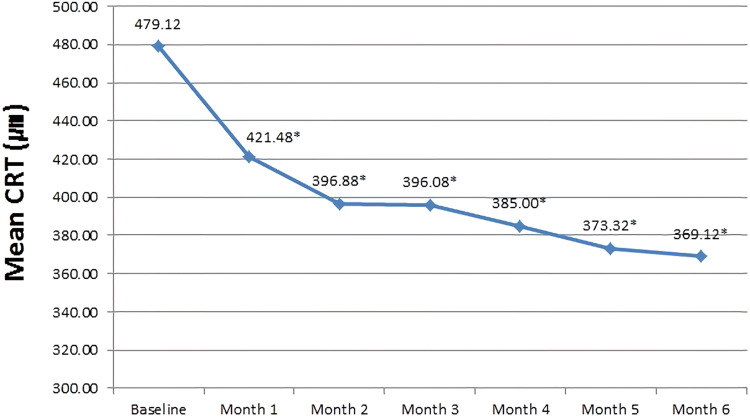
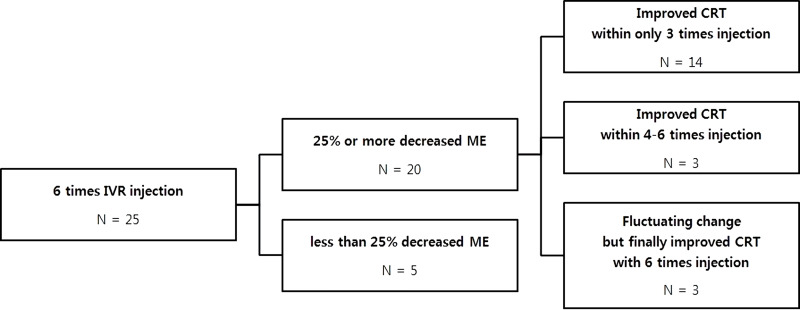
The associated mean CRT measured at baseline was 479.12±16.66 µm, which significantly decreased to 369.12±13.02 µm after completion of the treatment regimen (P<0.0001) (Figure 4). Considering the definition of improved CRT as a ≥25% reduction in macular edema, 20/25 eyes included in the study demonstrated ≥25% reduction in macular edema. Of the 20 eyes, 14 showed improvement in CRT with administration of ≤3 IVR injections, and 3 eyes demonstrated decrease in CRT after administration of ≥4 IVR doses. The remaining 3 eyes showed fluctuating decreases and increases in CRT during the course of treatment, but finally showed definitive improvement after administration of all 6 IVR injections (Figure 5).
Figure 4.
Mean central retinal thickness over the six-month study period. *Statistically significance is P < 0.05 with baseline.
Abbreviation: CRT, central retinal thickness.
Figure 5.
A flow chart showing relationship between numbers of injection and improvement in central retinal thickness.
Abbreviations: IVR, intravitreal ranibizumab; ME, macular edema; CRT, central retinal thickness.
Changes in MA Turnover and Perifoveal Non-Perfused Area
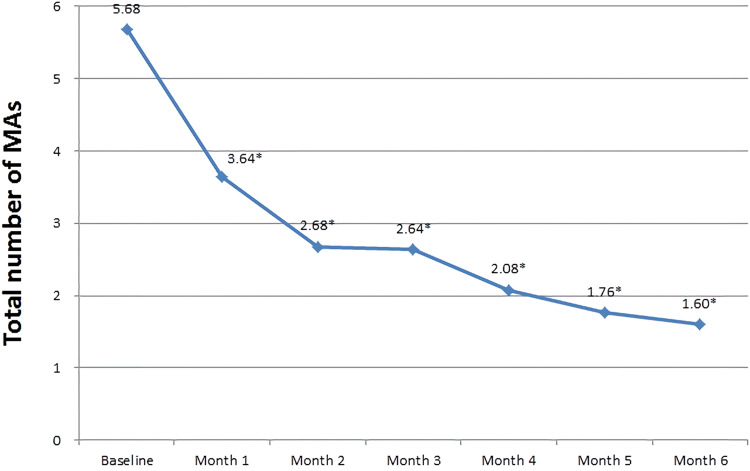
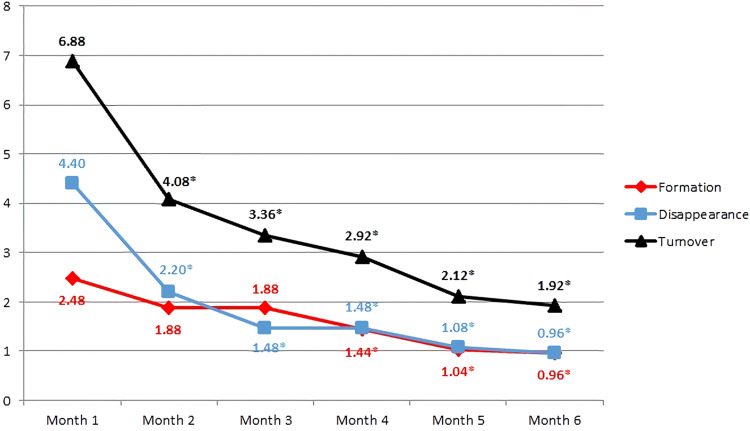
The total mean number of MAs before IVR injection was 5.68±3.41, which significantly decreased to 1.60±1.73 after administration of six monthly IVR injections (P<0.0001) (Figure 6). The MA turnover after the first IVR injection was 6.88±3.83, which significantly reduced to 1.92±1.75 after 6 months (P<0.0001) (Figure 7).
Figure 6.
Mean total number of microaneurysms over the six-month study period. *Statistically significance is P < 0.05 with baseline.
Abbreviation: MA, microaneurysm.
Figure 7.
Mean turnover, rate of formation, and rate of disappearance of microaneurysms over the six-month study period. *Statistically significance is P < 0.05 with baseline.
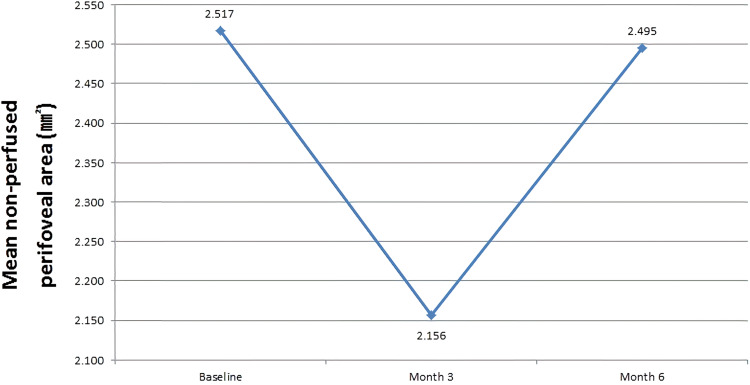
The mean perifoveal non-perfused area measured across three FA tests was 2.517±0.456 mm2 at baseline, which after administration of three, monthly IVR injections, decreased to 2.156±0.387 mm2 and after six, monthly IVR injections, increased to 2.495±0.293 mm2. Compared to baseline, after administration of six, monthly IVR injections, perifoveal non-perfused area showed a mild decrease, though the difference was not statistically significant (P=0.221) (Figure 8) (Table 2).
Figure 8.
Changes in extent of mean perifoveal non-perfused area over the six-month study period.
Table 2.
Comparative Analysis of Prognostic Ophthalmic Markers of Diabetic Retinopathy
| Before Injection | After 6 IVR Doses | P value | |
|---|---|---|---|
| BCVA (ETDRS letters) | 67.60 ± 3.29 | 76.36 ± 1.61 | <0.0001* |
| CRT (µm) | 479.12 ± 16.66 | 369.12 ± 13.02 | <0.0001* |
| Total number of MAs | 5.68 ± 3.41 | 1.60 ± 1.73 | <0.0001* |
| MA formation rate (MA number/month) |
2.48 ± 1.98 | 0.96 ± 1.02 | <0.0001* |
| MA disappearance rate (MA number/month) |
4.40 ± 2.66 | 0.96 ± 1.09 | <0.0001* |
| MA turnover | 6.88 ± 3.83 | 1.92 ± 1.75 | <0.0001* |
| Perifoveal non-perfused area (mm2) | 2.517 ± 0.456 | 2.495 ± 0.293 | 0.221 |
Notes: MA formation rate, MA disappearance rate, MA turnover are defined as before injection and after first injection. The values are presented as mean±SD and range unless otherwise indicated. Statistically significance is p < 0.05 with *Repeated measures analysis of variance.
Abbreviations: IVR, intravitreal ranibizumab; BCVA, best-corrected visual acuity; CRT, central retinal thickness; MA, microaneurysm; ETDRS, Early Treatment Diabetic Retinopathy Study.
Safety Outcomes
No ocular or systemic adverse events associated with IVR injections were observed in any participant during the study period.
Discussion
In this study, we prospectively analyzed the outcomes following administration of monthly IVR injections for 6 months in NPDR patients with DME. The patients showed visual improvement by 8.76 letters, after completing the study-treatment protocol, as compared to baseline as well as demonstrated a 105.8-µm reduction in CRT. These results were similar to those of other studies that analyzed the use of anti-VEGF drugs for DR.14–17 Further, we found that 80% patients showed improvements in CRT after administration of monthly IVR injections for 6 months. An interesting finding was that among these patients, 70% showed improvement in CRT with administration of ≤3 IVR injections and 30% demonstrated improvements in CRT after administration of ≤6 IVR injections. In other words, we imply that during the treatment of NPDR patients with DME, administration of all 6 injections may not be necessary and in some cases, 3 injections could prove to be quite effective.
MAs develop in the early stages of DR, and the total number as well as turnover rate are known to play an important role in identifying and defining the disease cycle. Furthermore, MAs can be a predictive factor for the occurrence of complications that may cause visual impairment by worsening retinopathy or ME.18,21,26 Formation and disappearance of MAs is a dynamic activity, of which MA disappearance occurs mostly due to platelet and fibrin thrombi, is an irreversible process, and can indicate the occurrence of retinal capillary occlusion and vascular damage. However, it could result in the restructuring of retinal vasculature and capillary-flow bypass.27,28 Therefore, not only the formation of MAs but also their disappearance should be considered important during the evaluation of MA turnover.
MA counts and MA turnover are good markers for DR progression, and high MA turnover is a risk factor for the development of clinically significant macular edema (CSME) requiring photocoagulation. Further, MA turnover has been shown to be more reliable than MA counts.18,20 Leicht et al,29 reported that high MA turnover can result in a higher risk of developing CSME and worsening DR without DME. They also suggested that anti-VEGF therapy using IVR could delay the progression to diabetic fundus and reverse existing fundus changes to restore normality. Limited research exists regarding the effects of VEGF inhibitors such as ranibizumab on MA turnover in NPDR patients with DME, and no prospective study has evaluated the efficacy of intensive treatment using ranibizumab, which has been performed in our study. This is the strength of our study. In this study, we prospectively observed that the total number, formation rate, disappearance rate, and overall turnover of MAs, each reduced significantly after administration of six monthly IVR injections. Our results showed that these MA-related parameters could be used as appropriate markers for evaluating the progression of retinopathy and that IVR injections could delay the progression of DR.
Hyperglycemia in diabetes induces high glucose concentration within the retina, eventually damaging the endothelium and pericytes of the retinal vessels. These structural damages lead to retinal ischemia and destruction of the blood-retinal barrier, causing the development of MAs, further decreasing retinal perfusion. Increased VEGF level is known to play an important role in mediating this process.30 However, physiologic levels of VEGF are important in maintaining retinal microvascular circulation during ischemic states.24 Thus, although the mechanism of action of an anti-VEGF antibody such as ranibizumab on retinal vessels in DME is not yet elucidated, it may have a detrimental effect on retinal circulation.
Capillary loss is known to be a risk factor for DR progression. In a subanalysis of the RESTORE (extension) study, all treatment arms (ranibizumab monotherapy, ranibizumab plus laser combination therapy, laser monotherapy) showed increased capillary loss, but no difference in capillary loss around fovea was reported. This shows that IVR injections do not increase the risk of aggravating central capillary perfusion.24 Findings of the RISE and RIDE trials conducted by Ip et al,12,13 showed that administration of IVR injections can reduce the risk of progression and severity of DR and that capillary loss within the macula is an important factor for the progression to PDR. However, IVR injections for DME lead to improved vision on their own accord, regardless of the absence of macular capillary perfusion. This suggests that the presence of retinal non-perfusion may not significantly correlate with improving vision, despite being an important risk factor for predicting the severity and progression of DR, and early intervention may be indicated for achieving maximal therapeutic effects. Additionally, Bonnin et al,25 showed that 3 monthly injections of anti-VEGF agents improved BCVA and CRT and reduced grades of DR severity, such as regression of red dots (hemorrhages or MAs) were observed on color photographs, but no improvement in vessel perfusion in capillary non-perfusion area was reported. Similarly, in this study, despite a decrease in perifoveal non-perfused area after 3 months of treatment and an increase after 6 months, a significant improvement in vision was observed after early intensive treatment.
A retrospective analysis of FA images from the RISE and RIDE trials performed by Campochiaro et al,30 over a period of 3 years revealed that the area of retinal non-perfusion increased from baseline over time in groups of patients who were administered IVR in concentrations of 0.3 mg, 0.5 mg, and sham (including patients crossed over to treatment with ranibizumab). However, slower changes were evident in the IVR injection group, implying that IVR injections retarded the progression of non-perfusion faster, compared with sham. Furthermore, in patients treated using IVR injections, retinal non-perfusion area decreased paradoxically until 12 months. Karst et al,24 analyzed data from the RESTORE study for 3 years and showed that capillary loss decreased at 6 months and increased after 6 months on FA with IVR monotherapy. Although our result was not statistically significant and the study was conducted in a short period, we observed a decrease in perifoveal non-perfused area after six monthly IVR injections, similar to the results obtained by Campochiaro et al and Karst et al.
Limitations of the current study include the small sample size (n=25 eyes), short study-duration of 6 months, and the lack of a control group. However, this study is unique, as it prospectively analyzed not only the improvement in BCVA and reduction in CRT but also changes in both MA turnover and perifoveal non-perfused area in NPDR patients with DME, following IVR therapy. Furthermore, this study employed RetmakerDR and ImageJ software programs to assess the occurrence of changes within the same retinal location, despite differing timings of examinations ensuring consistency and continuity of findings and enabling a more accurate analysis of the examination results.
Conclusion
After administration of early, intensive IVR injections, BCVA improved and CRT decreased in NPDR patients with ME. Further, they decreased the MA turnover as compared to baseline. However, the extent of perifoveal non-perfused area showed a mild overall decrease as compared to baseline, after 6 months. Thus, our data suggest that early intensive IVR injections can delay DR progression, but over a 6-month period, IVR therapy did not show a significant improvement in perifoveal non-perfused area. Hence, further randomized-controlled studies of longer duration are indicated to understand the impact of newer treatment strategies on the progression and prognosis of DR.
Acknowledgments
This study was presented at the 122nd Annual Meeting of the Korean Ophthalmological Society (Seoul, Korea) on November 2, 2019. Financial support for the study was received from Novartis, Korea.
Data Sharing Statement
Available from the corresponding author on reasonable request.
Ethics and Approval
All procedures performed in this study were approved by the Institutional Review Board of the Wonkwang University Hospital and conducted according to the principles of the Declaration of Helsinki. Prior to the procedure, written informed consent was obtained from all patients. Also, potential risks and side effects were explained.
Disclosure
The authors report no conflicts of interest with respect to this work.
References
- 1.De Barros Garcia JMB, Isaac DLC, Avila M. Diabetic retinopathy and OCT angiography: clinical findings and future perspectives. Int J Retina Vitreous. 2017;3:14. doi: 10.1186/s40942-017-0062-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy RK, Pieramici DJ, Gune S, et al. Efficacy of ranibizumab in eyes with diabetic macular edema and macular nonperfusion in RIDE and RISE. Ophthalmology. 2018;125:1568–1574. doi: 10.1016/j.ophtha.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 3.Simunovic MP, Maberley DA. Anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy: A systematic review and meta-analysis. Retina. 2015;35:1931–1942. doi: 10.1097/IAE.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 4.Figueira J, Silva R, Henriques J, et al. Ranibizumab for high-risk proliferative diabetic retinopathy: an exploratory randomized controlled trial. Ophthalmologica. 2016;235:34–41. doi: 10.1159/000442026 [DOI] [PubMed] [Google Scholar]
- 5.Ishida S, Usui T, Yamashiro K, et al. VEFG164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44:2155–2162. doi: 10.1167/iovs.02-0807 [DOI] [PubMed] [Google Scholar]
- 6.Funatsu H, Yamashita H, Noma H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243:3–8. [DOI] [PubMed] [Google Scholar]
- 7.Chandra S, Sheth J, Anantharaman G, Gopalakrishnan M. Ranibizumab-induced retinal reperfusion and regression of neovascularization in diabetic retinopathy: an angiographic illustration. Am J Ophthalmol Case Rep. 2018;4(9):41–44. doi: 10.1016/j.ajoc.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SN, Chhablani J, Chan CK, et al. Characterization of microaneurysm closure after focal laser photocoagulation in diabetic macular edema. Am J Ophthalmol. 2013;155:905–912. doi: 10.1016/j.ajo.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Writing committee for the Diabetic Retinopathy Clinical Research Network. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. J Am Med Assoc. 2015;314:2137–2146. doi: 10.1001/jama.2015.15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203. doi: 10.1056/NEJMoa1414264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart MW. A review of ranibizumab for the treatment of diabetic retinopathy. Ophthalmol Ther. 2017;6:33–47. doi: 10.1007/s40123-017-0083-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130:1145–1152. doi: 10.1001/archophthalmol.2012.1043 [DOI] [PubMed] [Google Scholar]
- 13.Ip MS, Domalpally A, Sun JK, Ehrlich JS. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology. 2015;122:367–374. doi: 10.1016/j.ophtha.2014.08.048 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 Phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 15.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–2022. doi: 10.1016/j.ophtha.2013.02.034 [DOI] [PubMed] [Google Scholar]
- 16.Boyer DS, Nguyen QD, Brown DM, Basu K, Ehrlich JS. Outcomes with as-needed ranibizumab after initial monthly therapy: long-term outcomes of the phase III RIDE and RISE trials. Ophthalmology. 2015;122:2504–2513. doi: 10.1016/j.ophtha.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 17.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter Phase II study. Diabetes Care. 2010;33:2399–2405. doi: 10.2337/dc10-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunes S, Pires I, Rosa A, Duarte L, Bernardes R, Cunha-Vaz J. Microaneurysm turnover is a biomarker for diabetic retinopathy progression to clinically significant macular edema: findings for type 2 diabetics with nonproliferative retinopathy. Ophthalmologica. 2009;223:292–297. doi: 10.1159/000213639 [DOI] [PubMed] [Google Scholar]
- 19.Sjølie AK, Klein R, Porta M, et al. Retinal microaneurysm count predicts progression and regression of diabetic retinopathy. Post-hoc results from the DIRECT Programme. Diabet Med. 2011;28:345–351. doi: 10.1111/j.1464-5491.2010.03210.x [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro ML, Nunes SG, Cunha-Vaz JG. Microaneurysm turnover at the macula predicts risk of development of clinically significant macular edema in persons with mild nonproliferative diabetic retinopathy. Diabetes Care. 2013;36:1254–1259. doi: 10.2337/dc12-1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pappuru RKR, Ribeiro L, Lobo C, Alves D, Cunha-Vaz J. Microaneurysm turnover is a predictor of diabetic retinopathy progression. Br J Ophthalmol. 2019;103:222–226. doi: 10.1136/bjophthalmol-2018-311887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haritoglou C, Kernt M, Neubauer A, et al. Microaneurysm formation rate as a predictive marker for progression to clinically significant macular edema in nonproliferative diabetic retinopathy. Retina. 2014;34:157–164. doi: 10.1097/IAE.0b013e318295f6de [DOI] [PubMed] [Google Scholar]
- 23.Krawitz BD, Phillips E, Bavier RD, et al. Parafoveal nonperfusion analysis in diabetic retinopathy using optical coherence tomography angiography. Transl Vis Sci Technol. 2018;7:4. doi: 10.1167/tvst.7.4.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karst SG, Deak GG, Gerendas BS, et al. Association of changes in macular perfusion with ranibizumab treatment for diabetic macular edema: a subanalysis of the RESTORE (Extension) study. JAMA Ophthalmol. 2018;136:315–321. doi: 10.1001/jamaophthalmol.2017.6135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnin S, Dupas B, Lavia C, et al. Anti-vascular endothelial growth factor therapy can improve diabetic retinopathy score without change in retinal perfusion. Retina. 2019;39:426–434. doi: 10.1097/IAE.0000000000002422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horii T, Murakami T, Nishijima K, Sakamoto A, Ota M, Yoshimura N. Optical coherence tomographic characteristics of microaneurysms in diabetic retinopathy. Am J Ophthalmol. 2010;150:840–848. doi: 10.1016/j.ajo.2010.06.015 [DOI] [PubMed] [Google Scholar]
- 27.Boeri D, Maiello M, Lorenzi M. Increased prevalence of microthromboses in retinal capillaries of diabetic individuals. Diabetes. 2001;50:1432–1439. doi: 10.2337/diabetes.50.6.1432 [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro L, Nunes S, Cunha-Vaz J. Microaneurysm turnover in the macula is a biomarker development of clinically significant macular edema in type 2 diabetes. Current Biomarker Findings. 2013;3:11–15. [Google Scholar]
- 29.Leicht SF, Kernt M, Neubauer A, et al. Microaneurysm turnover in diabetic retinopathy assessed by automated RetmarkerDR image analysis - potential role as biomarker of response to ranibizumab treatment. Ophthalmologica. 2014;231:198–203. doi: 10.1159/000357505 [DOI] [PubMed] [Google Scholar]
- 30.Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014;121:1783–1789. doi: 10.1016/j.ophtha.2014.03.021 [DOI] [PubMed] [Google Scholar]