Abstract
Purpose
Immune checkpoint inhibitors (ICIs) have been approved for various types of cancer; however, they cause a broad spectrum of immune-related adverse events (irAEs). The association between the development of irAEs and the clinical benefit remains uncertain. We aimed to evaluate the association of irAEs and the treatment efficacy in real-world practice.
Patients and Methods
We conducted a retrospective study on patients with recurrent or metastatic non-small-cell lung cancer, malignant melanoma, renal cell carcinoma, or gastric cancer who received anti-PD-1/PD-L1 antibodies (nivolumab, pembrolizumab, or atezolizumab) at the Keio University Hospital between September 2014 and January 2019. We recorded treatment-related AEs from medical records and graded them using the Common Terminology Criteria for Adverse Events version 4. We performed an overall survival (OS) analysis using a Cox proportional hazards model and the shared frailty model.
Results
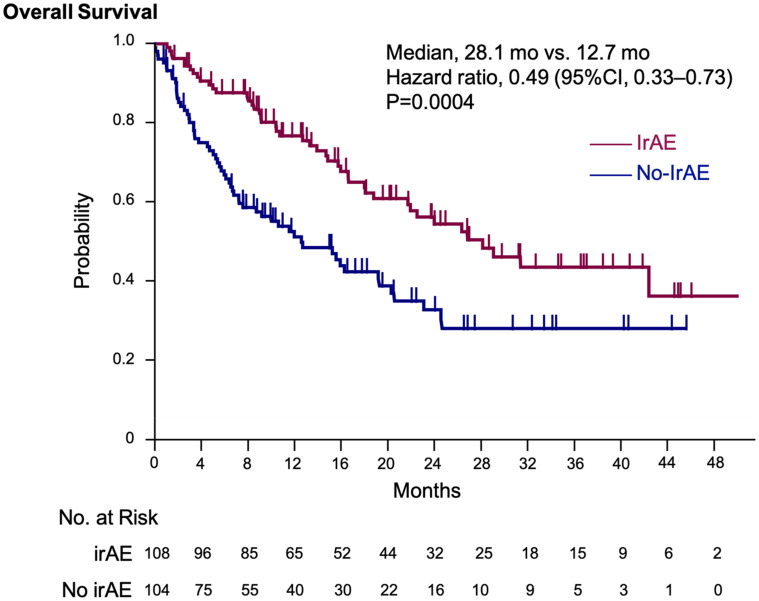
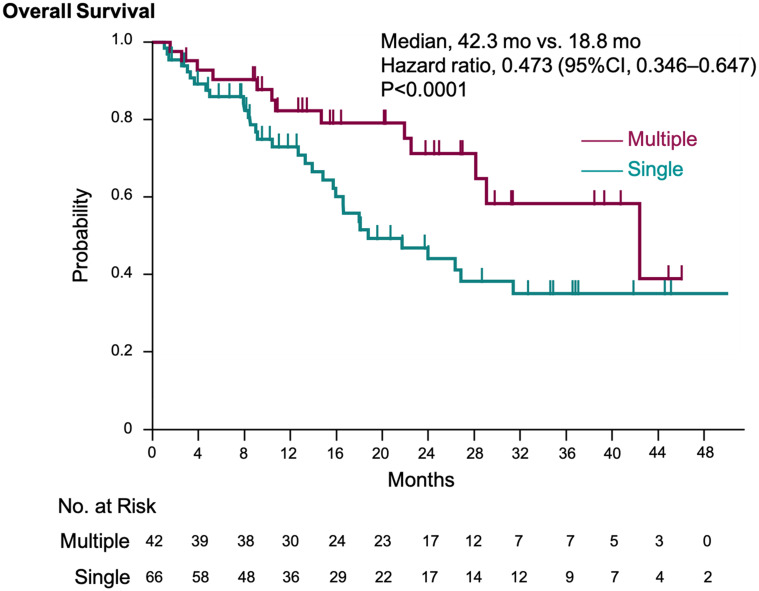
Of 212 patients eligible for this study, 108 experienced irAEs and 42 developed multiple irAEs. The median OS was significantly longer in the irAEs than in the no-irAE group (28.1 months vs 12.7 months; hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.33–0.73; P = 0.0004). Moreover, the OS of patients with multiple irAEs was significantly longer than that of patients with a single irAE (42.3 months vs 18.8 months; HR, 0.473; 95% CI, 0.346–0.647; P < 0.0001).
Conclusion
Our single-center retrospective study revealed a significant tendency associating the development of multiple irAEs with favorable prognoses.
Keywords: immune checkpoint inhibitors, programmed cell death 1, prognosis
Introduction
Immune checkpoint inhibitors (ICIs) have shown a drastic benefit against malignant melanomas1,2 and various types of malignant diseases.3–12 ICIs including antibodies to programmed cell death-1 (PD-1), programmed cell death ligand 1 (PD-L1), cytotoxic T-lymphocyte antigen-4 (CTLA-4), and combinations of these13,14 have been approved for non-small-cell lung cancer (NSCLC), malignant melanoma (MM), renal cell carcinoma (RCC), gastric cancer (GC), and other types of cancers in Japan.
These ICIs are known to cause specific immune-related adverse events (irAEs).15 IrAEs occur in a variety of organs, causing signs and symptoms such as interstitial pneumonia, enterocolitis, hypothyroidism, liver dysfunction, skin rash, vitiligo, hypophysitis, type 1 diabetes, renal dysfunction, neurological disorders, or myocarditis. ICIs are often discontinued due to severe irAEs that may lead to life-threatening conditions. Although the mechanisms of irAEs remain to be elucidated, the enhancement of systemic T-cell activity by ICIs causes a loss of immune tolerance in various organs, resulting in irAEs.16,17
Some retrospective studies have reported that the development of irAEs is associated with clinical benefits such as better treatment responses or prognoses for certain malignancies.18–24 However, these retrospective data were affected by an observation time bias due to higher irAEs risks by longer treatment times in responders. Theoretically unbridle T-cell activity causes severe irAEs, which may also enhance antitumor immune reactions. If the development of irAEs reflects a systemic immune activation, not only the occurrence of irAEs but also the number of irAEs in each patient should be associated with antitumor ICI effects. Therefore, we conducted a retrospective analysis focused on the association between the number of irAEs and the clinical outcomes.
Patients and Methods
Study Design
We retrospectively assessed the efficacy and toxicity of anti-PD-1/PD-L1 antibody, nivolumab, pembrolizumab, and atezolizumab, in patients with four major ICI-approved cancer types: NSCLC, MM, RCC, or GC between September 2014 and January 2019 at the Keio University Hospital in Japan. We excluded patients treated with anti-PD1/PD-L1 antibody during clinical trials or under off-label use, and also patients with prior or concurrent treatment with an anti-CTLA-4 antibody. We collected baseline characteristics, clinical outcomes, and irAEs from clinical records. IrAEs were defined as events occurring during PD-1/PD-L1 treatment and events occurring after ICI treatment, including pneumonitis, diarrhea/colitis, hepatitis, rash, neurological disorders, or endocrine abnormalities, diagnosed as being irAEs by the attending doctor. We graded irAEs using the Common Terminology Criteria for Adverse Events version 4.0. The Keio University Hospital Institutional Ethics Committee approved this study. Informed consent was obtained in the form of opt-out on the website.
Statistical Analysis
Patient characteristics were compared between the two groups (irAEs and no-irAE groups, and multiple irAEs and single irAE groups): the ages and median durations of ICI administration were compared using t-test for difference in means and all other variables were compared using chi-square and Fisher’s exact test. We defined overall survival (OS) as the time from the initiation of immunotherapy to death from any cause, and we measured time to treatment failure (TTF) as the time from the first administration of ICIs to the time of treatment discontinuation for any reason, including disease progression, adverse events, patient preference, or death. Probabilities of survival were estimated using the Kaplan–Meier method and the Log-rank test. We used the Cox proportional hazard regression model to calculate hazard ratios (HRs). To consider the differences of cancer types, we included a category of cancer type as a covariate in each Cox proportional hazard regression model. A shared frailty model was used to calculate HR associated with the number of irAEs. All P values were based on a 2-sided hypothesis, and those less than 0.05 were considered statistically significant. We performed all statistical analyses using the JMP version 14.2.0 software (SAS Institute, Cary, NC, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics and irAEs
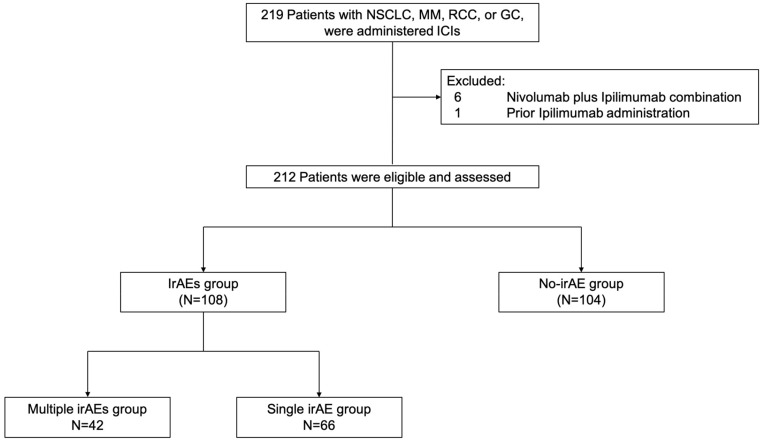
A total of 219 patients were treated with anti-PD-1/PD-L1 antibody monotherapy between September 2014 and January 2019 in the real-world practice (Figure 1). We excluded six patients with MM or RCC who received a combination of nivolumab and ipilimumab and one patient with MM who received ipilimumab prior to PD-1 antibody monotherapy because we could not rule out the possibility of their irAE being due to ipilimumab. Thus, we analyzed data from 212 patients with NSCLC (n = 112), MM (n = 37), RCC (n = 34), or GC (n = 29). The median follow-up time was 11.3 months (range, 0.04–53.0) as of July 31, 2019, the analysis cutoff date.
Figure 1.
Flow diagram of patient selection and analysis according to the development of irAEs.
Abbreviations: GC, gastric cancer; ICIs, immune checkpoint inhibitors; irAEs, immune-related adverse events; MM, malignant melanoma; NSCLC, non-small-cell lung cancer; RCC, renal cell carcinoma.
We found 163 irAEs in 108 patients during the treatment or after ICIs discontinuation. The most commonly observed irAEs were included increased alanine transaminase (ALT) (n = 43), rash (n = 31), diarrhea (n = 26), pneumonitis (n = 23), and hypothyroidism (n = 21). We found irAEs with grade ≥3 in 36 patients (16%). The baseline characteristics of patients with any irAE (irAEs group) were similar to those of patients without irAEs (no-irAE group) (Table 1). The duration of ICI administration in the irAEs group was significantly longer than that in the no-irAE group (median, 5.0 months vs 2.7 months, P = 0.021).
Table 1.
Baseline Characteristics of Patients in the Study
| Characteristics (n = 212) | irAEs (n = 108) |
No-irAE (n = 104) |
P value* |
|---|---|---|---|
| Age, years | |||
| Median (range) | 66 (34–94) | 69 (30–92) | 0.64 |
| Male gender, n (%) | 71 (66) | 75 (72) | 0.31 |
| ECOG performance status, n (%) | |||
| 0 | 36 (33) | 31 (30) | 0.13 |
| 1 | 64 (59) | 54 (52) | |
| ≥2 | 8 (8) | 19 (18) | |
| Treatment line, n (%) | |||
| 1st line | 22 (20) | 16 (15) | 0.79 |
| 2nd line | 40 (37) | 34 (33) | |
| Salvage-line | 46 (43) | 54 (52) | |
| Cancer types, n (%) | |||
| Non-small-cell lung cancer | 63 (58) | 49 (47) | 0.32 |
| Malignant melanoma | 18 (17) | 19 (18) | |
| Renal cell cancer | 16 (15) | 18 (17) | |
| Gastric cancer | 11 (10) | 18 (17) | |
| Types of ICIs, n (%) | |||
| Nivolumab | 83 (77) | 90 (87) | 0.05 |
| Pembrolizumab | 23 (21) | 10 (10) | |
| Atezolizumab | 2 (19) | 4 (4) | |
| Reason for discontinuation of ICIs, n (%) | |||
| IrAEs | 40 (37) | 0 | <0.0001 |
| Progression of disease | 43 (40) | 80 (77) | |
| Ongoing ICIs | 21 (19) | 15 (14) | |
| Others | 4 (24) | 9 (9) | |
| Median duration of ICI administration, months (range) | 5.0 (0.04–46) |
2.7 (0.04–44) |
0.02 |
| ICIs administration over 60 days, n (%) | 76 (70) | 57 (55) | 0.02 |
Notes: *A t-test for difference in means was used to compare ages and median durations of ICI administration; all other variables were compared using Chi-Square and Fisher’s Exact tests.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibitors; irAEs, immune-related adverse events.
Of the 108 patients who experienced irAEs, 32 patients received corticosteroids for the treatment of irAEs including pneumonitis (n = 14), adrenal insufficiency (n = 8), diarrhea (n = 3), neurological disorders (n = 3), increased ALT (n = 3), and nephritis (n = 1).
Association of irAEs with Treatment Efficacy
The median OS was significantly longer in the irAEs than in the no-irAE group (28.1 months vs 12.7 months; HR, 0.49; 95% confidence interval [CI], 0.33–0.73; P = 0.0004) (Figure 2). The TTF was also significantly longer in the irAEs group than in the no-irAE group (median TTF, 5.2 months vs 2.7 months; HR, 0.49; 95% CI, 0.49–0.90; P = 0.0098).
Figure 2.
Kaplan–Meier estimation of overall survival. The Kaplan–Meier estimates of overall survival in the irAEs and no-irAE groups. We adjusted the hazard ratio for death according to the primary tumor type.
Abbreviation:irAE, immune-related adverse events.
In the subgroup of patients who had received ICIs over 60 days, OS in the irAEs group (n = 76) tended to be longer than that in the no-irAE group (n = 57) (median OS, 42.3 months vs 23.1 months; HR, 0.63; 95% CI, 0.35–1.11; P = 0.114). In addition, even in patients who had received ICIs for less than 60 days, the median OS was significantly longer in those in the irAEs group (n = 32) than in those in the no-irAE group (n = 47) (13.3 months vs 3.4 months; HR, 0.46; 95% CI, 0.25–0.84; P = 0.012).
Excluding those who died within 2.7 months (median duration of ICIs administration in non-irAE group), 43 patients experienced single irAEs and 12 had multiple irAEs in the first 2.7 months after initiation of ICIs. Of the 104 patients in the no-irAE group, 82 patients lived at least 2.7 months. We analyzed survival in the group with irAEs within 2.7 months (n = 55) and the no-irAE group (n = 82). Although not statistically significant, the OS in the irAEs group was more favorable than the no-irAE group (29.0 months vs 19.7 months; HR, 0.66; 95% CI, 0.39–1.12; P = 0.12) (Supplementary Figure 1).
Regarding the association of steroid usage with prognosis in irAEs group, the OS in patients without corticosteroid treatment was favorable compared with that in patients with immunosuppressive therapy (median OS, 29.0 months vs 16.6 months; HR, 0.63; 95% CI, 0.34–1.16; P = 0.14).
Association Between the Number of irAEs in Each Patient and Treatment Efficacy
Of the 108 patients who experienced any irAE, we found 42 with multiple irAEs (median number of irAEs, 2; range, 2–4). We evaluated the efficacy and safety of ICIs according to the number of irAEs. Table 2 lists characteristics of patients in the multiple irAEs group (n = 42) and the single irAE group (n = 66). We found no significant difference in terms of the baseline characteristics between the two groups. The median durations of ICI administration were 8.6 months (range, 0.04–46.0) in the multiple irAEs group and 4.0 months (range, 0.04–44.5) in the single irAE group (P = 0.13).
Table 2.
Baseline Characteristics of the Patients Grouped by the Number of irAEs
| Characteristic | Multiple (n = 42) |
Single (n = 66) |
P value* |
|---|---|---|---|
| Age at administration, years | |||
| Median (Range) | 66 (34–86) | 67 (36–94) | 0.39 |
| Male gender, n (%) | 23 (55) | 48 (73) | 0.06 |
| ECOG performance status, n (%) | |||
| 0–1 | 40 (95) | 60 (91) | 0.51 |
| ≥2 | 2 (5) | 6 (9) | |
| Treatment line, n (%) | |||
| 1st line | 10 (24) | 12 (18) | 0.62 |
| 2nd line or later | 32 (76) | 54 (82) | |
| Cancer types, n (%) | |||
| Non-small-cell lung cancer | 25 (60) | 38 (58) | 0.82 |
| Malignant melanoma | 8 (19) | 10 (15) | |
| Renal cell cancer | 6 (14) | 10 (15) | |
| Gastric cancer | 3 (7) | 8 (12) | |
| Type of ICIs, n (%) | |||
| Nivolumab | 30 (71) | 53 (80) | 0.56 |
| Pembrolizumab | 11 (26) | 12 (18) | |
| Atezolizumab | 1 (2) | 1 (2) | |
| Median duration of ICI administration, months (range) | 8.6 (0.04–46.0) |
4.0 (0.04–44.5) |
0.13 |
Notes: *A t-test for difference in means was used to compare ages and median durations of ICI administration; all other variables were compared using Chi-Square and Fisher’s Exact tests.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibitors: irAEs, immune-related adverse events.
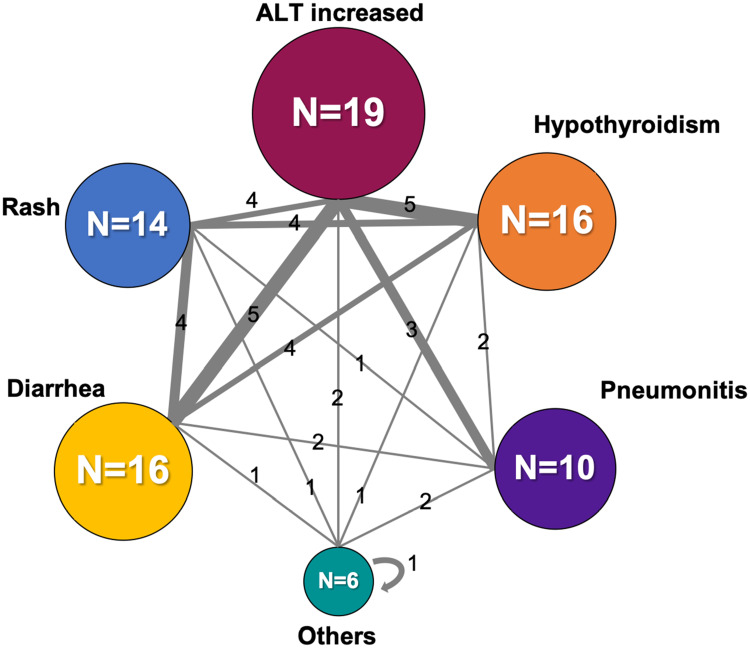
Table 3 shows the number of irAEs in the multiple irAEs group and in the single irAE group. We found no specific tendency in the multiple irAEs group. The most commonly observed grade ≥3 irAEs in the multiple irAEs group (those with incidence ≥5%) were pneumonitis (21%), increased ALT (12%), and adrenal insufficiency (7%); and that in the single irAE group was pneumonitis (6%). The most frequently observed combinations of first and second irAEs were increased ALT and diarrhea (n = 5), and increased ALT and hypothyroidism (n = 5) (Figure 3).
Table 3.
Summary of Adverse Events
| Variables | Multiple irAEs (n = 42) | Single irAE (n = 66) | ||||
|---|---|---|---|---|---|---|
| Any Grade | Grade≥3 | Cortico-Steroids Therapy | Any Grade | Grade≥3 | Cortico-Steroids Therapy | |
| Increased ALT | 23 (55%) | 5 (12%) | 2 (5%) | 20 (30%) | 2 (3%) | 1 (2%) |
| Rash | 16 (38%) | 0 | 0 | 15 (23%) | 1 (2%) | 0 |
| Diarrhea | 16 (38%) | 2 (5%) | 1 (2%) | 10 (15%) | 2 (3%) | 2 (3%) |
| Pneumonitis | 15 (36%) | 9 (21%) | 9 (21%) | 8 (12%) | 4 (6%) | 5 (8%) |
| Hypothyroidism | 17 (40%) | 1 (2%) | 0 | 4 (6%) | 0 | 0 |
| Adrenal insufficiency | 5 (12%) | 3 (7%) | 5 (12%) | 3 (5%) | 2 (3%) | 3 (5%) |
| Neurological disorders | 3 (7%) | 1 (2%) | 1 (2%) | 3 (5%) | 2 (3%) | 2 (3%) |
| Othersa | 2 (5%) | 2 (5%) | 0 | 3 (5%) | 0 | 1 (2%) |
Notes: aOther irAEs include grade 2 arthritis, 2 Grade 2 nephritis in the single irAE group, grade 3 Sjögren syndrome, and grade 3 thrombocytopenia in the multiple irAEs group.
Abbreviations: ALT, alanine aminotransferase; irAEs, immune-related adverse events.
Figure 3.
First and second irAE combination profiles in the multiple irAEs group. Numbers may not match the number of irAEs listed in Table 2 because some patients developed three or more irAEs. Others included adrenal insufficiency, neurological disorders, myocarditis, and thrombocytopenia.
Abbreviations: ALT, alanine transaminase; irAE, immune-related adverse events.
The median time-to-onset of the first irAE was 2.7 months in the single irAE group (range, 0.04–29.8) and 1.5 months in the multiple irAEs group (range, 0.04–32.4) (P = 0.46, Supplementary Figure 2). The median time-to-onset of the second irAEs was 3.8 months in the multiple irAEs group (range, 0.07–34.4), which was significantly longer than that of the first irAE in the single irAE group (P = 0.014).
Patients with multiple irAEs had significantly longer OS compared with patients with a single irAE (median OS, 42.3 months vs 18.8 months; HR 0.473; 95% CI, 0.346–0.647; P < 0.0001) (Figure 4). The TTF in the multiple irAEs group (although not significantly different) was longer than that in the single irAE group (median TTF, 9.5 months vs 4.0 months; HR, 0.69; 95% CI, 0.43–1.10; P = 0.12) (Supplementary Figure 3).
Figure 4.
Kaplan–Meier estimation of overall survival in multiple irAEs and single irAE groups. The Kaplan–Meier estimates of overall survival in the multiple irAEs and single irAE groups. We adjusted the hazard ratio for death according to the primary tumor type.
Abbreviation: irAE, immune-related adverse events.
Discussion
This retrospective study shows a unique insight into the association between the number of irAEs in each patient and the efficacy of ICIs. The frequency of irAEs in our hospital was comparable to those in clinical trials.3–6,25 The incidence of grade ≥3 irAEs was higher than that reported in clinical trials. One of the reasons for this difference would be that a higher number of patients who were at a high risk of developing severe irAEs such as slight ground-glass opacity in their lungs before treatment initiation with ICIs were included in this real-world study. Patients with any irAE showed longer OS than those without irAEs as reported.26–28 In addition, we revealed that OS was more favorable in patients who had developed irAEs in the 2.7 months after the initiation of ICIs (median duration of ICIs administration in no-irAE group) compared with the no-irAE group.
Interestingly, our study revealed that the development of multiple irAEs was associated with longer survivals than single irAE in patients with various types of malignancies. To assess our hypothesis that higher systemic immune activation by ICIs induces multiple irAEs and better antitumor effects, we focused on the association between the number of irAEs and the antitumor response. As we expected, the OS was significantly better in patients with multiple irAEs than in those with a single irAE. Most previous studies assessed only the association of irAE development with prognosis and not the association of the number of irAEs with prognosis. One report in NSCLC showed that patients with multiple irAEs had longer survival than those with only one irAE.26 However, this report analyzed the HRs using a Cox proportional hazard regression model, which is not appropriate for estimating HR associated with the number of irAEs. The occurrence of different irAEs should be considered as recurrent events. In our study, we resolved this problem by estimating the HR using a shared frailty model, which includes multiple irAEs as recurrent events. Although it is difficult to exclude the possibility that longer treatment durations increase the chance of the second irAEs, our results suggest that the development of multiple irAEs may be a predictive biomarker for improved prognoses. Whether patients develop multiple irAEs or not could reflect an association between the efficacy of ICIs and the degree of immune activation by them. Patients who develop single irAE are probably predisposed to the development of the specific irAE, but the irAE does not reflect the systemic immune activation strength after the administration of ICIs. On the other hand, the development of multiple irAEs may represent a high systemic immune activation induced by ICIs. ICIs target not only tumor-specific T cells but also other T cells, and may cause the unintended activation of non-tumor-specific T cells, resulting in irAEs in a variety of organs.17 Recent studies suggest the another possible mechanism underlying irAEs is that T cells that target antigens common to both tumors and healthy tissue are activated by ICIs, which produces both irAEs and antitumor efficacy.29,30 Of note, we found no specific irAE type being associated with longer survival, contrary to some reported results.31,32 Thus, more clinical evidence is required to elucidate the association mechanisms between irAEs and ICIs efficacy.
Since irAEs are often severe, responsible for ICIs discontinuation, and often life-threatening if not properly managed, strategies to detect the development of irAEs in the early stages and appropriate interventions to counteract them are necessary to strengthen the efficacy and benefit of ICIs. However, no biomarkers for predicting the efficacy of ICIs and the development of irAEs exist.33,34 Our results on the association between the presence of multiple irAEs and better prognoses suggest that maintaining ICIs therapy in patients who develop irAEs should be a priority.
We are aware of some limitations of our study due to its retrospective nature. First, we could not define irAEs diagnostic criteria, and some irAEs were diagnosed by exclusion, which could have led to inaccuracies. Second, grade 1 to 2 irAEs could have been underestimated or overlooked in cases in which medical records lacked concrete descriptions; however, we believe most severe irAEs were detected without omission. Third, since our analysis included four cancer types, the ICI administration regimens varied. To account for this, we evaluated HRs for death adjusting for cancer types. Fourth, although some biomarkers, such as PD-L1 expression, tumor mutation burden, and microsatellite instability, have been reported to be associated with the efficacy of ICIs, we could not assess these biomarkers in our study owing to a lack of tissue samples in the majority of the patients. In addition, various clinical factors, such as post-ICI treatment or ICI discontinuation by irAE, would affect prognosis. However, it was difficult to adjust these factors in our statistical analysis. Finally, the observation period was relatively short for evaluating prognosis. As these factors must be considered as potential bias in this study, our results should be interpreted with caution. Additional prospective studies with large numbers of patients and biomarker analysis are needed to validate our findings.
Conclusion
The development of multiple irAEs may be intimately connected with increased survival. Further research on a larger population is warranted to confirm our results and to elucidate the mechanisms of this association.
Acknowledgments
We are deeply indebted to the patients who participated in this study and to their families and care-takers.
Funding Statement
We have no funding to declare.
Disclosure
Dr. Sukawa is affiliated to the department funded by Ono Pharmaceutical Co. Ltd. and has received honoraria from Ono Pharmaceutical Co. Ltd. He also received honoraria from Bayer, Bristol Myer Squibb, and Chugai Pharmaceutical. Dr. Mizuno has received honoraria from Ono Pharmaceutical Co. Ltd. and Bristol-Myers Squibb outside the submitted work. Dr. Suzuki has received honoraria from Astra Zeneca, Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb, MSD Co. Ltd., and Chugai Pharmaceutical Co. Ltd., outside the submitted work. Dr. Funakoshi has received honoraria from Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb, and MSD Co. Ltd., and grants from Ono Pharmaceutical Co. Ltd., outside the submitted work. He also reports non-financial support from Chugai Pharmaceutical Co., Ltd. Dr. Hamamoto has received honoraria from Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb, and Chugai Pharmaceutical Co. Ltd., outside the submitted work. Dr. Kanai has received honoraria from Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb, MSD Co. Ltd., and Chugai Pharmaceutical Co. Ltd, and has received designated donations from Ono Pharmaceutical Co. Ltd., and Chugai Pharmaceutical Co. Ltd., outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi: 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 4.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, Phase 2 trial. Lancet Oncol. 2017;18(3):312–322. doi: 10.1016/S1470-2045(17)30065-7 [DOI] [PubMed] [Google Scholar]
- 8.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. doi: 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. doi: 10.1016/S1470-2045(16)30364-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517.doi: 10.1016/S1470-2045(19)30626-6 [DOI] [PubMed] [Google Scholar]
- 13.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol. 2015;33(18):2092–2099. doi: 10.1200/JCO.2014.60.0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–2385. doi: 10.1093/annonc/mdx286 [DOI] [PubMed] [Google Scholar]
- 17.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 18.Haratani K, Hayashi H, Chiba Y, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018;4(3):374–378. doi: 10.1001/jamaoncol.2017.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toi Y, Sugawara S, Kawashima Y, et al. Association of Immune-Related Adverse Events with Clinical Benefit in Patients with Advanced Non-Small-Cell Lung Cancer Treated with Nivolumab. Oncologist. 2018;23(11):1358–1365. doi: 10.1634/theoncologist.2017-0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Judd J, Zibelman M, Handorf E, et al. Immune-Related Adverse Events as a Biomarker in Non-Melanoma Patients Treated with Programmed Cell Death 1 Inhibitors. Oncologist. 2017;22(10):1232–1237. doi: 10.1634/theoncologist.2017-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii T, Colen RR, Bilen MA, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD Anderson Cancer Center experience. Invest New Drugs. 2018;36(4):638–646. doi: 10.1007/s10637-017-0534-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–74. doi: 10.1016/j.lungcan.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 23.Maeda T, Yoshino K, Nagai K, et al. Development of endocrine immune-related adverse events and improved survival in advanced melanoma patients treated with nivolumab monotherapy. Eur J Cancer. 2019;115:13–16. doi: 10.1016/j.ejca.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 24.Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781. doi: 10.1200/JCO.2014.57.4756 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Zhou S, Yang F, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019;5(7):1008–1019. doi: 10.1001/jamaoncol.2019.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricciuti B, Genova C, De Giglio A, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479–485. doi: 10.1007/s00432-018-2805-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eggermont AMM, Kicinski M, Blank CU, et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2020;6(4):519–527. doi: 10.1001/jamaoncol.2019.5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogado J, Sanchez-Torres JM, Romero-Laorden N, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer. 2019;109:21–27. doi: 10.1016/j.ejca.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 29.Berner F, Bomze D, Diem S, et al. Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol. 2019;5(7):1043–1047. doi: 10.1001/jamaoncol.2019.0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamauchi I, Yasoda A, Matsumoto S, et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS One. 2019;14(5):e0216954. doi: 10.1371/journal.pone.0216954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hua C, Boussemart L, Mateus C, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol. 2016;152(1):45–51. doi: 10.1001/jamadermatol.2015.2707 [DOI] [PubMed] [Google Scholar]
- 33.Manson G, Norwood J, Marabelle A, Kohrt H, Houot R. Biomarkers associated with checkpoint inhibitors. Ann Oncol. 2016;27(7):1199–1206. doi: 10.1093/annonc/mdw181 [DOI] [PubMed] [Google Scholar]
- 34.Fujii T, Naing A, Rolfo C, Hajjar J. Biomarkers of response to immune checkpoint blockade in cancer treatment. Crit Rev Oncol Hematol. 2018;130:108–120. doi: 10.1016/j.critrevonc.2018.07.010 [DOI] [PubMed] [Google Scholar]