Highlights
-
•
Coronavirus disease 2019 (COVID-19) may be present with non-specific clinical manifestations, especially in patients with comorbidities.
-
•
We present a case of infective endocarditis concomitant COVID-19, illustrating the management and main outcomes in a developing country.
-
•
Diagnosing and managing a patient with COVID-19 could be challenging, there is not yet an approved medication regimen for COVID-19 infections
-
•
We present a case of infective endocarditis concomitant COVID-19, illustrating the management and main outcomes in a developing country.
Keywords: COVID-19, Blood culture-negative infective endocarditis, SARS-CoV-2
Abstract
Coronavirus disease 2019 (COVID-19) has emerged as a pandemic and public health crisis across the world. With its high infectivity and rapid spread, the severity of the disease is escalating in certain populations, especially in patients with pre-existing cardiovascular disease. In developing countries, infective endocarditis remains a problem in patients with rheumatic heart disease. We report the case of a patient with a diagnosis of infective endocarditis concomitant with COVID-19, including the diagnosis, management, and main outcomes.
Introduction
On March 11, 2020, the World Health Organization (WHO) declared a global pandemic caused by coronavirus disease 2019 (COVID-19). Since then, this disease has affected more than 12 million people in over 200 countries or regions worldwide. Several studies including one meta-analysis have reported cardiovascular involvement due to this infection (Emami et al., 2020). Moreover, it should not be forgotten that the diagnosis of COVID‐19 does not imply the exclusion of other diseases.
The incidence of rheumatic fever (RF) and rheumatic heart disease (RHD) has been decreasing globally since the early 1900s, although these continue to occur predominantly in developing countries, particularly in low socioeconomic settings and those with inadequate education of the population, and more than 15 million cases of RHD have been reported worldwide. Infective endocarditis (IE) continues to be a serious threat to any patient with RHD, and even with the advances made in the treatment of IE, the mortality and morbidity in developing countries remain high (Seckeler and Hoke, 2011). Moreover, the selection of antibiotic therapy for patients with COVID-19 and culture-negative IE should be considered carefully due to possible complications and accompanying disease.
Cardiovascular disease is the most common comorbidity found in COVID-19 patients. The clinical manifestations of IE and COVID-19 are challenging, and both diseases may present with fever, chills, dyspnea, fatigue, cough, and myalgia (Murdoch et al., 2009). However, COVID-19 concomitant with infective endocarditis will be found in developing countries and initial screening will be vague.
We report the case of a patient with COVID-19 who presented with shortness of breath as an example to highlight that infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may accompany various other clinical conditions. Every physician, especially those in developing countries, should be aware and consider echocardiography when evaluating patients with COVID-19.
Case report
A 61-year-old male was referred to the cardiac center with the chief complaint of shortness of breath 2 days before admission, a history of fever (38.4 °C), chest discomfort, and a minor dry cough. He had experienced symptoms including dyspnea on effort and orthopnea 1 year before, but had not undergone a comprehensive medical examination. He had a history of hypertension and a previous smoking history. On March 8, 2020, 2 weeks before he was admitted, he had returned from Saudi Arabia, where he had been to practice worship. On his return, he had felt a bit fatigued, with no other symptoms. On admission, the patient was alert, his blood pressure was 133/68 mmHg, pulse 92 beats per minute, body temperature 37.3 °C, and respiratory rate 26 breaths per minute, and he had an oxygen saturation of 94% using a nasal cannula. Chest auscultation revealed rhonchi at the base of the lungs, four out of six pansystolic murmurs at the apex through the lower left sternal border, and a diastolic murmur two out of four in the right upper sternal border. His extremities revealed Osler nodes and splinter hemorrhages in the index finger.
A blood culture was taken immediately, and 12 hours later his initial laboratory tests reflected leukocytosis, lymphopenia, elevated high sensitivity troponin I (Hs-Trop I, 2736.7 ng/ml), alterations of hepatic function (alanine aminotransferase (ALT) 2826 U/l, aspartate aminotransferase (AST) 1808 U/l), kidney function alteration (estimated glomerular filtration rate (eGFR) 38.7 ml/min), and mild hyponatremia. An electrocardiogram showed sinus rhythm with an ischemic anteroseptal wall, left axis deviation, and left ventricular hypertrophy. A chest X-ray had been performed at another hospital previously and showed a cardiothoracic ratio >50% with lung fields within the normal limit. As this was incompatible with the clinical symptoms, a chest computed tomography (CT) examination was performed. The chest CT showed multilobar ground-glass opacities affecting both superior lobes, the right medial lobe, and the posterior, medial, and lateral segments of both inferior lobes (Figure 1A).
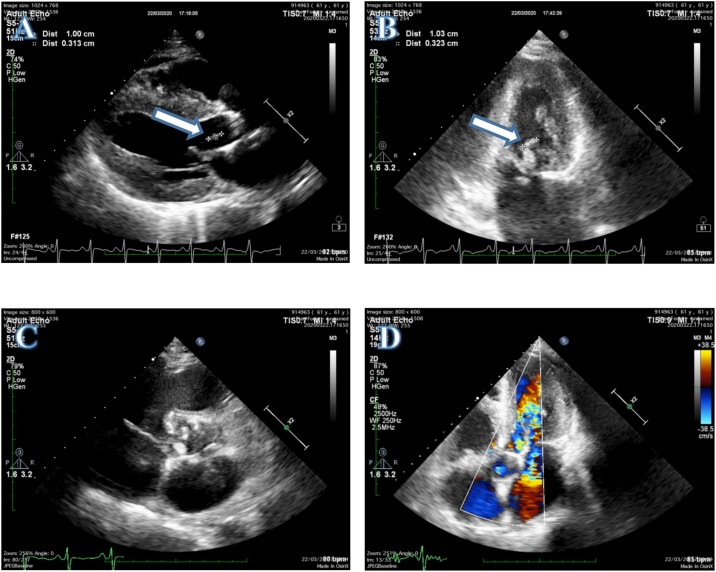
Figure 1.
Transthoracic echocardiogram: (A) PLAX view showing a vegetation in the anterior mitral leaflet (white arrow). (B) Two chamber view confirming a vegetation in the anterior mitral leaflet (white arrow). (C) Calcified aortic cusp of rheumatic heart disease. (D) Five chamber view with Doppler signal showing aorta regurgitation.
Arterial blood gas analysis showed no abnormality, with pH 7.43, PaCO2 38.5 mmHg, PaO2 89.4 mmHg, HCO3 − 21.7 mmol/l, and SaO2 94.6%. A transthoracic echocardiogram (TTE) revealed a flail mitral leaflet with a vegetation measuring 10 mm × 3 mm in size, producing severe mitral regurgitation, dilatation of the left heart and right atrium, and moderate aorta regurgitation. These findings suggested that the patient was suffering from RHD and IE (Figure 2 ) nasopharyngeal swab test reverse-transcription polymerase chain reaction (RT-PCR) assay was performed to confirm COVID-19. While awaiting the results, the patient was quarantined in the isolation room.
Figure 2.
(A) CT image of the chest in the axial and coronal planes obtained during admission, revealing ground-glass opacities in both superior lobes, the right medial lobe, and the posterior, medial, and lateral segments of both inferior lobes. (B) CT image before discharge showing resolution of the infection foci in both lungs.
p. 1824–36. DOI : 10.1001/jama.2020.6019
Based on the available data, the patient was diagnosed with acute decompensated heart failure, possible IE, suspected COVID-19, RHD, acute liver failure, and acute kidney injury. The treatment regimen comprised oxygen via nasal cannula, loop diuretic, fluid rehydration with 1.5 l of 0.9% sodium chloride, moxifloxacin 400 mg, N-acetylcysteine 200 mg every 8 hours, hepatoprotection, and ramipril 2.5 mg every 24 hours. The following day, the swab result came back, confirming that the patient was positive for SARS-CoV-2. It was then decided to start treatment with oseltamivir 75 mg every 12 hours and azithromycin 500 mg daily.
On days 2 and 3 of hospitalization, the patient continued to report a productive cough and fatigue. His C-reactive protein (CRP) level was increased at 18.9 mg/l and procalcitonin was 0.12 ng/ml. Ascorbic acid up to 1 g in a divided dose and N-acetylcysteine 200 mg every 8 hours were added, and ACE inhibitor treatment was withheld. On day 4 of hospitalization, the patient’s oxygenation gradually worsened; arterial blood gas results were pH 7.55, PaCO2 36.8 mmHg, PaO2 77.7 mmHg, HCO3 − 26 mmol/l, and SaO2 90.4%. He was given an 8 l/min non-rebreathing mask to maintain SaO2 above 94%. Blood tests revealed leukocytosis (white blood cell count 17.4 × 109/l), thrombocytopenia (platelets 143 × 109/l), elevated AST (1135 U/l), elevated ALT (1634 U/l), decreased kidney function (eGFR 35 ml/min), and hyponatremia (132 mmol/l). On day 6 of hospitalization, his clinical symptoms began to improve and physical examination vital signs were within normal limits. On days 8–10, physical examinations showed improvements and he started light mobilization. A chest CT obtained on day 10 showed resolution of the abnormalities, with a decrease in ground-glass opacities (Figure 1B). A nasopharyngeal swab re-examination was performed twice, on days 5 and 8 of hospitalization, with both returning negative results. Therefore the patient was discharged on day 10 of hospitalization.
Discussion
As the current world pandemic develops, COVID-19 concomitant with other diseases will be common in daily practice. In the case reported here, the patient presented with shortness of breath, fever, fatigue, and a dry cough. He also had a history of heart failure, which complicated the diagnosis, making it difficult to distinguish clinically between a cardiac and a respiratory etiology (Fried et al., 2020). However, COVID-19 was still suspected in this patient, since some patients develop cardiovascular manifestations such as angina chest pain, shortness of breath, and arrhythmias (Guan et al., 2020).
The TTE results were considered to indicate RHD complicated with IE. IE was established based on the modified Duke criteria, along with three minor criteria (Osler node lesions, splinter hemorrhages, and fever) (Li et al., 2000). The negative culture results in our center, however, probably resulted from the previous administration of antibiotics in the referral hospital. We could not proceed to investigate the microorganism causing IE, as serological testing and PCR using blood or valve biopsies could not be performed during this pandemic era in our center. Moreover, based on the TTE, we found severe mitral regurgitations with jet length ≥2 cm, velocity ≥3 m/s, and moderate aorta regurgitation seen in two views. These TTE findings confirmed that the patient was suffering from RHD (Reméanyi et al., 2012).
Through discussions, a relationship between COVID-19 and IE was refuted, since the process of vegetation is initiated through a transient bacteremia, followed by binding and adherence to the damaged endothelium and encasing in a platelet/fibrin matrix. Thromboplastin released by tissue factor from the damaged endothelium causes platelet aggregation and cleavage of fibrinogen to fibrin(McCormick et al., 2002). We also believe that confirming the role of a virus in endocarditis would be quite complex, and we could not find any reported case directly proving a virus as the causative agent (Fournier et al., 2011).
At present there is limited evidence from the literature on the management of patients with COVID-19 and IE. Concerns about the safety of ACE inhibitors have arisen, as it has been hypothesized that they may increase the severity of COVID-19 in hospitalized patients. We decided to withhold the patient’s ACE inhibitor on day 4 of hospitalization since the patient developed a worsening cough. Once the patient’s status had clinically improved, he was continued on angiotensin receptor blockers (ARBs) until discharge. Data from human studies support treatment with ARBs or ACE inhibitors regardless of the ACE2 protein theory (Sriram and Insel, 2020). Having seen the positive effects of the use of these drugs in patients with hypertension, heart failure, valvular heart disease, and chronic kidney disease, we will continue to prescribe ARBs for our patient.
Oseltamivir, an inhibitor of viral neuraminidase, was given to the patient. A decision to administer oseltamivir for compassionate use was based on the availability at that time. However, in a study conducted by Sanders et al. it was reported that oseltamivir does not have a positive effect in the management of COVID-19 if it is not concomitant with an influenza A or B virus infection (Sanders et al., 2020). Other antiviral drugs could not be prescribed during that time, and oseltamivir was the only available drug.
The case patient also suffered from hypervolume low osmolarity hyponatremia accompanied by an alteration of kidney function, therefore he was administered 3% sodium chloride, but unfortunately he did not respond. It was decided to add tolvaptan, a diuretic that works by binding to V2 receptors, interfering with aquaporin 2 movement to the luminal side of cortical collection channel cells through cAMP activation and inhibiting water reabsorption. This drug has a protective effect on kidney function because it provides a diuretic effect without activating the renin–angiotensin system, which might be beneficial for COVID-19 patients (Okada et al., 2009, Onogawa et al., 2011).
The guidelines for antibiotic administration in culture-negative IE are still unclear. According to the Journal of Antimicrobial Chemotherapy, the antimicrobial protocol for culture-negative IE caused by an unknown microorganism includes four intravenous antimicrobial agents: amoxicillin, vancomycin, gentamicin, and amphotericin B (Menu et al., 2017). The kidney function of this patient was not improved with drug administration and adequate rehydration during hospitalization. As such, giving an aminoglycoside antibiotic such as gentamicin would have aggravated the patient’s kidney function.
In a patient with heart failure and IE related to RHD, surgery is the best option; however, during this pandemic situation it is not possible to perform cardiac surgery in our center. To date, there are no literature reports supporting or rejecting heart valve surgery in COVID-19 patients. In accordance with the guidelines, it was decided to hold any prophylactic antibiotic in our patient, since he was not going to receive any medical procedures in the near future (Habib et al., 2015).
In conclusion, among the reported cases of COVID-19, we report a novel case of COVID-19 concomitant with IE and the management of this patient. The management of IE may be difficult since there are several limitations to normal practice during this pandemic. This case illustrates the potential severity of complications and the challenges in developing standard management for the disease given that much remains unknown about COVID-19 management in special populations. A full routine cardiac examination screening should be performed in patients with COVID-19. We hope that this case will expand knowledge and help clinicians in their approach to other such cases.
Ethical approval
The patient gave permission and informed consent for the publication of this case report.
Funding source
Funding support was provided by the Faculty of Medicine, Hasanuddin University.
Conflict of interest
None declared.
References
- Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: a Systematic Review and Meta-Analysis. Arch Acad Emerg Med [Internet] 2020;8(1):e35. doi: 10.22037/aaem.v8i1.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P.E., Charrel R., Raoult D. Viral endocarditis or simple viral disseminated infection? Clin Infect Dis. 2011;53:p. 1298. doi: 10.1093/cid/cir681. [DOI] [PubMed] [Google Scholar]
- Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E. The Variety of Cardiovascular Presentations of COVID-19. Circulation. 2020;141(23):1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M. Comorbidity and its impact on 1,590 patients with Covid-19 in China: A nationwide analysis. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib G., Lancellotti P., Antunes M.J., Bongiorni M.G., Casalta J.P., Del Zotti F. ESC Guidelines for the management of infective endocarditis. Vol. 36. Eur Heart J. 2015;2015:3075–3123. doi: 10.1093/eurheartj/ehv319. [DOI] [Google Scholar]
- Li J.S., Sexton D.J., Mick N., Nettles R., Fowler V.G., Ryan T. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin Infect Dis. 2000;30(4):633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- McCormick J.K., Tripp T.J., Dunny G.M., Schlievert P.M. Formation of Vegetations during Infective Endocarditis Excludes Binding of Bacterial‐Specific Host Antibodies to Enterococcus faecalis. J Infect Dis. 2002;185(7):994–997. doi: 10.1086/339604. [DOI] [PubMed] [Google Scholar]
- Menu E., Gouriet F., Casalta J.P., Tissot-Dupont H., Vecten M., Saby L. Evaluation of empirical treatment for blood culture-negative endocarditis. J Antimicrob Chemother. 2017;72(1):290–298. doi: 10.1093/jac/dkw362. [DOI] [PubMed] [Google Scholar]
- Murdoch D.R., Corey R.G., Hoen B., Miró M., Fowler V.G., Bayer A.S. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century The international collaboration on Endocarditis-prospective cohort study. Arch Intern Med. 2009;169(5):463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T., Sakaguchi T., Hatamura I., Saji F., Negi S., Otani H. Tolvaptan, a selective oral vasopressin V2 receptor antagonist, ameliorates podocyte injury in puromycin aminonucleoside nephrotic rats. Clin Exp Nephrol. 2009;13(5):438–446. doi: 10.1007/s10157-009-0196-0. [DOI] [PubMed] [Google Scholar]
- Onogawa T., Sakamoto Y., Nakamura S., Nakayama S., Fujiki H., Yamamura Y. Effects of tolvaptan on systemic and renal hemodynamic function in dogs with congestive heart failure. Cardiovasc Drugs Ther. 2011;25(SUPPL. 1) doi: 10.1007/s10557-011-6350-4. [DOI] [PubMed] [Google Scholar]
- Reméanyi B., Wilson N., Steer A., Ferreira B., Kado J., Kumar K. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease-an evidence-based guideline. Nat Rev Cardiol. 2012;9:297–309. doi: 10.1038/nrcardio.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Seckeler M.D., Hoke T.R. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol. 2011;3:67–84. doi: 10.2147/CLEP.S12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K., Insel P.A. Risks of ACE inhibitor and ARB usage in COVID-19: evaluating the evidence. Clin Pharmacol Ther. 2020 doi: 10.1002/cpt.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]