Abstract
Herein is presented a case of a 71-year-old woman with mild SARS-CoV-2 respiratory infection who experienced acute myopericarditis diagnosed using clinical, biological, and electrocardiogram data and cardiac magnetic resonance imaging. The presented case highlights the risk of cardiac involvement, even in the absence of severe respiratory COVID-19 infection. The mechanisms involved in acute myocardial injury in SARS-CoV-2 infection are not well known and requires further studies to determine whether it is related to direct myocardial damage by the virus or to a systemic condition.
Résumé
Nous présentons le cas d’une femme de 71 ans qui présentait une infection respiratoire légère causée par le virus SRAS-CoV-2 et qui a subi une myopéricardite aiguë diagnostiquée à partir de données cliniques, biologiques et électrocardiographiques et d’un examen d’imagerie par résonance magnétique cardiaque. Ce cas met en lumière le risque d’atteinte cardiaque chez les patients atteints de COVID-19, même en l’absence d’infection respiratoire grave. On ne connaît pas bien les mécanismes qui participent à l’atteinte myocardique aiguë chez les patients infectés par le virus SRAS-CoV-2, et des recherches plus poussées sont nécessaires pour déterminer si cette atteinte est causée directement par le virus ou si elle est due à un trouble systémique.
We present a case of a 71-year-old woman with mild SARS-CoV-2 respiratory infection who experienced acute myopericarditis diagnosed according to clinical, biological, and electrocardiogram (ECG) data and cardiac magnetic resonance imaging (CMR).
Case
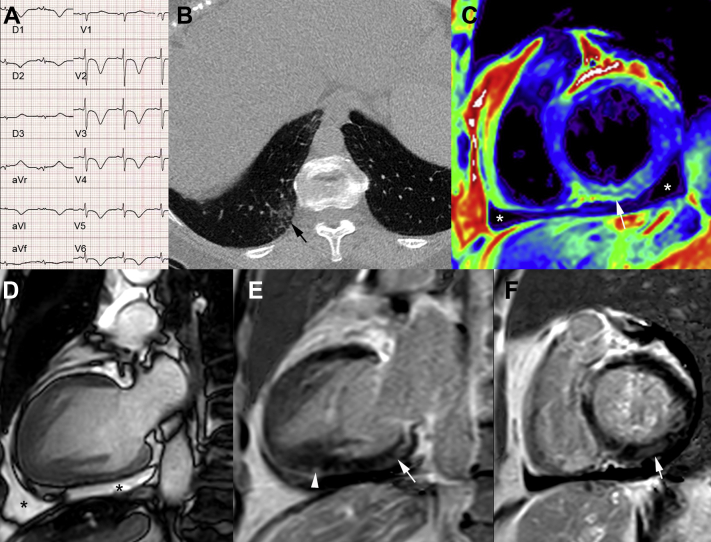
A 71-year-old woman with a history of breast cancer treated with surgery, chemotherapy, radiotherapy, and hormonotherapy and no previous cardiovascular events or echocardiography abnormalities at oncological follow-up presented to the emergency department with a 2-week history of flu-like symptoms, mild fever (38°C), chest pain, and a mild decrease of blood oxygen saturation (91%). She was hemodynamically stable, and had no clinical signs of heart failure. ECG revealed diffuse inverted T waves and elongated QT up to 700 ms (Fig. 1A). She had mild persistent elevations in high-sensitivity troponin T, brain natriuretic peptide, C-reactive protein, and fibrinogen levels (60 ng/L, 474 ng/L, 9 mg/L, and 5.5 g/L, respectively). Pulmonary computed tomography showed mild bilateral peripheral lower pulmonary lobe ground-glass opacities (Fig. 1B). A nasopharyngeal swab was performed, and was positive for SARS-CoV-2 on real time reverse transcriptase polymerase chain reaction assay. She had neither concomitant infections nor autoimmune disease. The patient was admitted to a specialized COVID-19 unit in our institution. Echocardiography showed infero-septal and infero-apical left ventricle (LV) wall hypokinesia, LV ejection fraction of 56% and a moderate pericardial effusion. There was no evidence of coronary disease on coronary angiography. CMR at day 9 showed recovery of LV wall motion, normal LV ejection fraction (61%) and persistence of a mild pericardial effusion (Fig. 1C and D). Short inversion time inversion recovery sequences and T2 map showed elevated myocardial T2 value up to 60 ms (N = 50 ms) suggestive of myocardial edema in the basal inferior LV wall (Fig. 1C).
Figure 1.
Electrocardiogram (ECG), pulmonary computed tomography, and cardiac magnetic resonance imaging (MRI) data in a 71-year-old woman, who presented with mild SARS-CoV-2 respiratory infection and acute myopericarditis. (A) ECG: diffuse inverted T waves and elongated QT. (B) Pulmonary computed tomography image showing mild peripheral right lower pulmonary lobe ground-glass opacities (black arrow). (C) Cardiac MRI short axis T2 map showing inferior myocardial edema (white arrow). (D) Vertical long axis (steady-state free precession MRI sequence) showing pericardial effusion (asterisk). Vertical long axis (E) and short-axis (F) phase-sensitive inversion-recovery sequence showing multiple areas of subepicardial and midwall late gadolinium enhancement in the inferior-basal left ventricular wall (white arrows) and in the inferior apical wall (arrowhead).
Furthermore, myocardial T1 mapping showed focal increased native myocardial T1 up to 1350 ms (N = 1200 ms; CMR 3T) and increased extracellular volume (0.39%).
Cardiac contrast-enhanced magnetic resonance imaging showed multiple areas of inferior subepicardial and mid-wall late gadolinium enhancement (LGE) on the basis of visual analysis. Although LGE volume quantification was not done, LGE intensity was more than 4 SDs higher than reference myocardium (Fig. 1E and F). On the basis of clinical, biological, ECG, and CMR Lake Louise Criteria, and T1 and T2 mapping the diagnosis was consistent with acute myopericarditis. The initial follow-up during hospitalization showed resolution of chest pain but persistence of ECG abnormalities without any specific treatment. Echocardiography at follow-up on day 21 showed disappearance of pericardial effusion, no pericardial thickening, and normal LV wall motion.
Discussion
Myocarditis can be classified on the basis of etiology, clinicopathological, and clinical criteria. Acute myocarditis is a type of myocardial injury that could be of infectious, autoimmine, or toxic origins.1 Acute myocardial injury diagnosis on the basis of elevation of troponin and ECG abnomalities has been reported in patients with COVID-19.2 An increased level of high-sensitivity troponin in 7.2% of overall COVID-19 patients and 22% in those who required care in the intensive care unit has been reported.3 There are few reports of acute myocarditis in COVID-19 patients who underwent CMR,3,4 and only 1 showed myocarditis without symptoms and signs of interstitial pneumonia.4 Herein, we report acute myopericarditis diagnosed using CMR in a patient with mild respiratory COVID-19 infection. Consistent with the literature,3 the present case showed LV regional wall motion abnormalities, which is not a common feature of other forms of non-COVID-19 myocarditis. Another particular feature in this case is that the degree of myocardial edema was quite intense because it was still present on CMR obtained more than 3 weeks after symptom onset. Significant interstitial edema within the myocardium has been reported in a case of acute myocarditis in a COVID-19 patient who underwent endomyocardial biopsy.3 Viral-induced myocarditis results from the interaction of the virus with the host’s immune system. The viral non-COVID-19 myocarditis most commonly is initiated by the introduction of the virus or reactivation of a dormant virus. The viral proliferation in cardiomyocytes can cause direct injury, however, the most important myocardial injury results from the activation of the innate and acquired immunity after viral invasion.1 The pathogenesis of acute myocardial injury in SARS-CoV-2 infection is still poorly known. One potential mechanism is direct myocardial involvement mediated via host protein angiotensin converting enzyme 2, because it is now known that the SARS-CoV-2 virus uses angiotensin converting enzyme 2 as a co-receptor for entry into the lung and brain cells.5 It is unclear if cardiac involvement in SARS-CoV-2 viral infection could be related to direct damage of cardiomyocytes via this process. In non-COVID-19 viral myocarditis the earliest response to the viral infection is the activation of the innate immune system, which induces inflammatory responses via toll-like receptors present on all cells and particularly in the cardiovascular system. The activation of the innate system induces the expansion of T and B lymphocytes.1 The COVID-19-associated endomyocardial biopsy showed diffuse CD3+ T lymphocytic inflammatory infiltrates with huge interstitial edema without the presence of SARS-Cov-2 within the myocardium.3
Severe hypoxia in respiratory COVID-19 infection induces oxidative stress, and myocardial injury can occur as a consequence of increased myocardial oxygen demand. This mechanism of myocardial injury is unlike in the present case because there were neither hemodynamic modifications nor severe hypoxia.
The presented case highlights the risk of cardiac involvement, even in the absence of severe respiratory COVID-19 infection. The mechanisms involved in acute myocardial injury in SARS-CoV-2 are still not well known and require further studies.
Novel Teaching Points.
-
•
The diagnosis of acute myopericarditis in patients with COVID-19 can be challenging.
-
•
Cardiac involvement might be present even in the absence of severe respiratory COVID-19 infection.
-
•
The mechanisms involved in myocardial injury in SARS-CoV-2 are still not well known.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This research has adhered to ethical guidelines.
See page 437 for disclosure information.
References
- 1.Sagar S., Liu P.P., Cooper L.T., Jr. Myocarditis. Lancet. 2012;379:738–747. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sala S., Peretto G., Gramegna M. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–6. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M., Kleine-Wever H., Kruger N. The novel coronavirus 2019 (2019-nCoV) uses the SARS coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry in target cells. Cell. 2020;181:1–10. [Google Scholar]