Highlights
-
•
Microorganisms have been use as weapons since pre-historic times.
-
•
Biowarfare is the intentional use of biological agents as weapons in war scenarios.
-
•
Bioterrorism is the intentional use of biological agents against a civilian population.
-
•
Biocrime is the intentional use of biological agents against a specific individual.
-
•
Microbial forensics can be applied to solve cases of BW, BT, and BC.
Keywords: Biocrime, Bioterrorism, Biowarfare, Genetics, Microbial forensics
Abstract
Microbial Forensics is a field that continues to grow in interest and application among the forensic community. This review, divided into two sections, covers several topics associated with this new field. The first section presents a historic overview concerning the use of microorganisms (or its product, i.e. toxins) as harmful biological agents in the context of biological warfare (biowarfare), bioterrorism, and biocrime. Each case is illustrated with the examination of case reports that span from prehistory to the present day.
The second part of the manuscript is devoted to the role of MF and highlights the necessity to prepare for the pressing threat of the harmful use of biological agents as weapons. Preventative actions, developments within the field to ensure a timely and effective response and are discussed herein.
1. Introduction
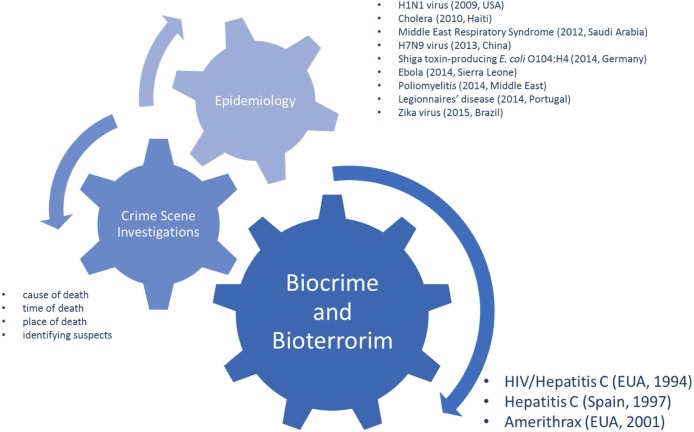
Microbial forensics (MF or forensic microbiology) is a recent and forthrightly expanding multidisciplinary branch of research that lays its foundations upon classical sciences such as forensic genetics, microbiology, epidemiology, medicine, molecular, and evolutionary biology [[1], [2], [3]]. Scientists within the field of MF endeavor to detect, identify, and trace the origin (i.e.: assigning to a source) of life-threatening pathogenic agents (bacteria, viruses, and toxins) [4]. This field has applications in a multitude of forensic casework scenarios, including bioterrorism [5,6], biocrime [7,8], fraud [9,10], outbreaks and transmission of pathogens [11,12], or accidental release of a biological agent, and/or a toxin [13,14]. Traditionally, neither pathogens’ outbreak monitoring nor toxicology is regarded as MF topics. Nonetheless, in our opinion, these two topics are fundamental to MF. The dispersion of pathogens can be either natural or incidental. If incidental, it can be either intentional or due to medical malpractice. Therefore, the application of reliable and robust surveillance protocols for pathogen monitoring would provide valuable information to distinguish between the spontaneous and harmful spread of microorganisms (i.e., related to biowarfare- BW, bioterrorism - BT, or biocrime - BC). Besides the differences between forensic investigations and epidemiological studies in terms of protocols and objectives, it is our opinion that the investigations of the sources of outbreaks fall in the domain of MF due to the shared aim of determining the source of the microorganisms involved (Fig. 1 ) [2,3,12].
Fig. 1.
Application of Microbial Forensics with the indication of the most recent and paradigmatic examples.
As such, one of the priorities in MF is to distinguish between an accidental and an intentional release of a given pathogenic agent. Healthcare personnel should be constantly alert for unusual, unexpected, or unexplained illnesses. According to the United States Centers for Disease Control and Prevention (CDC) (http://emergency.cdc.gov/Documents/Planning/PlanningGuidance.PDF), several diagnostic indicators might point out an infectious disease outbreak associated with the intentional release of a biologic agent. Among them are:
-
i)
an unusual temporal or geographic clustering of illness, such as persons who attended the same public event or gathering or patients presenting with clinical symptoms suggesting an unexplained infectious disease outbreak (e.g., febrile illness, sepsis, pneumonia, respiratory failure, or rash);
-
ii)
an unusual age distribution for common diseases (e.g., an increase of a children-characteristic illness among the adult population);
-
iii)
a large number of cases of acute flaccid paralysis with prominent bulbar palsies, suggestive of a release of botulism toxin.
An intentional release of a given biological agent can constitute either a covert or an overt action. In the case of covert action, this release remains unannounced and concealed, going unnoticed for days or even weeks. The first sign of the spread is the occurrence of sick individuals, that may unknowingly be infecting others. An infected person may search for medical care, possibly at a distance from the release area. Contrarily, in the case of overt action, this release is immediately noticed and may even be announced. Contrarily, in overt actions, public health officials, and healthcare and communication systems are readily informed by perpetrators and overwhelmed by requests for information and treatment. Overt action aims to cause widespread panic.
The use of pathogenic agents as biological weapons (also known as bioweapons), present several advantages over other more conventional weapons (e.g., chemical weapons):
-
i)
microorganisms are relatively inexpensive and can be easily mass-produced;
-
ii)
large quantities of biological agents can be effortlessly concealed in small vials and easily transported;
-
iii)
some agents can become water or airborne resulting in a widespread area of dissemination within a short time frame;
-
iv)
some of these pathogenic agents present person-to-person transmission.
Although plant materials can also be used for similar purposes (e.g., abrin and ricin), this work focuses on topics related to the use of microorganisms as bio-weapons, from exploring the historical perspectives of BW to the most recent applications in BT, and BC.
1.1. Biowarfare
Biowarfare (BW) refers to the intentional use of biological agents (e.g., bacteria, viruses, fungi, and toxins) as weapons in war scenarios [15]. BW agents can be deadlier than other conventional weapon systems as even minute quantities can cause mass casualties and/or fatalities depending on the agent used [16,17].
The intentional use of microorganisms (or their toxins) as weapons is almost as old as humanity itself. Since pre-historic and ancient Greek and Roman times there have been reported examples such as the use of poisoned darts or contaminating water springs and wells with corpses or cadavers. From this early stage, BW has become more sophisticated, leaning towards the capability of being a weapon of mass destruction when associated with an appropriate delivery system, as specialized munitions on the battlefield and for covert use. Such developments are a direct result of advances in both the fields of microbiology and biotechnology [18,19].
As such, the historical evolution of BW can be divided into three distinct periods:
-
i)
from prehistory to 1900: except for a few well-documented cases, before to the foundation of Microbiology as a science - as a direct result of the studies of Louis Pasteur and Robert Koch culminating in the acceptance of the germ theory of disease - it is difficult to clarify if these BW attacks constituted real threats or were a part of political hoaxes;
-
ii)
from 1900 through 1945: this period is characterized by the emergence of small and unsophisticated national BW programs (e.g., Germany, Japan, the Soviet Union, and the United States; in detail below) and the use of biological weapons in both World War I and II;
-
iii)
after 1945: the broader access to biological agents and the progress made in the fields of biotechnology and biochemistry allowed the BW programs to become democratized and accessible even to small groups and individuals. In this period, the lethal potential of BW agents increased due to the developments in genetic engineering (Table 1) [20,21].
Table 1.
Examples of the use of microorganism in biowarfare during the past millennia.
| Date | Examples of the use of microorganism in Biowarfare |
|---|---|
| Pre-historic times | Melanesian tribesman (actual Vanuatu) used arrowheads contaminated with tetanus [20] |
| 14th century BC | The Hittite army send rams infected with tularemia to their enemies [119] |
| 6th century BC (Trojan War) |
Scythian archers infected their arrows by dipping them into decomposing cadavers and human blood containing C. perfringens and C. tetani [120] |
| 1155 | Emperor Barbarossa poisons water wells with human bodies in Tortona, Italy [121] |
| 1340 (Hundred Years War) |
Jean, Duke of Normandy, casted dead horses over the wall into the besieged the castle of Thun l’eveque, captured by the Englishman [122] |
| 1346 | Tartar (Mongol) army catapulted bodies of plague victims over the city walls of Caffa (Feodosia, Ukraine) to attach the Genoese army [123] |
| 1422 | Lithuanian army catapulted corpses of those who died in battle, manure and garbage into the town of Karlstein (Bohemia) [122] |
| 1495 | Spanish sold wine mixed with blood of leprosy patients to their French opponents in Naples (Italy) [121] |
| 1500 | Pizarro offered variola-contaminated clothing to South America native communities [124] |
| 1650 | Polish fire saliva from rabid dogs towards their enemies [121] |
| 1676: Antoine Philips van Leeuwenhoek, commonly referred as "the Father of Microbiology", identifies microorganisms. | |
| 1710 | Russian army catapulted bodies of plague victims into Swedish cities in Reval (Estonia) |
| 1763 (French-Indian War) |
British offered smallpox-contaminated blankets to Native Americans [122] |
| 1776–1781 (American Revolutionary War) |
British attempted to spread smallpox among the continental forces by inoculating civilians fleeing from Boston [122] |
| 1797 | The Napoleonic armies floods the plains around Mantua (Italy) to enhance the spread of malaria [125] |
| 1861–1863 (American Civil War) |
Confederates troops sold yellow fever and smallpox-infected clothing to Union troops [125] |
| Confederates troops contaminate water supplies for the Union forces with animal corpses [121] | |
| End of the 19th century: development of the germ theory of disease and foundation of microbiology by Louis Pasteur (1822–1895) and Robert Koch (1843–1910) | |
| 1914–1918 (World War I) |
German troops sold horses and mules infected with glanders and anthrax to the Allies [126] |
| German troops sold sheeps infected with glanders and anthrax to Russia (in Romania) [126] | |
| German troops sold sheeps infected with glanders and anthrax to the Britain and Indian armies [126] | |
| German troops attempted to spread cholera in Italy and plague in St. Petersburg [126] | |
| 1925: The “Protocol for the Prohibition of the Use in War of Asphyxiating, Poisonous or Other Gases and of Bacteriological Methods of Warfare”, also referred as the “Geneva Protocol”, was signed (38 signatories and 140 parties) | |
| 1939–1945 (World War II) |
Japanese army poisoned water wells in Chinese villages to study cholera and typhus outbreaks [127,128] |
| Japanese inoculated prisoners of war with agents causing gas gangrene, anthrax, meningitis, cholera, dysentery and plague [127,128] | |
| 1972: The “Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on their Destruction”, also referred as the “Biological Weapons Convention” (BWC) was signed (actually has 182 parties) | |
| 2001: The US Patriot Act is signed in, providing Federal and national law enforcement officials with enhanced counter-terrorism capacities. | |
Note: in some of the presented cases (e.g.: plague during the siege of Caffa, smallpox during the French-Indian War and yellow fever during the yellow fever) is difficult to distinguish if the disease spread was due to the intentional release of the microorganisms or if it was due to the limited hygienic conditions during the period or the contact between populations with different immunities.
For both microbiologists and historians it is challenging to distinguish between natural epidemics and deliberate biological attacks (e.g., plague outbreak during the siege of Caffa, malaria outbreak by the Napoleonic armies, yellow fever, and smallpox outbreaks during American Civil War; Table 1 ), mainly due to:
-
i)
the lack of reliable scientific data regarding an alleged bioterrorism attack, especially before the advent of modern microbiology;
-
ii)
the secretive nature and polemical conditions surrounding any putative biological attack, within which the available documents are susceptible to multiple political manipulations, and thus difficult to interpret objectively;
-
iii)
the chronological distance from the ancient reports on these biological attacks, and the possible misunderstanding if they are read through a contemporary lens [21,22].
1.1.1. The German BW program
At the beginning of World War I (WWI), between late 1914 and early 1915, Germany implemented the first documented state BW program. The country was also a pioneer in the dissemination of biological weapons and for launching a true BW campaign, employing different biological pathogens against several neutral countries - including Argentina, Finland (by the time under Russian control), France, Norway, Romania, Russia, Spain, and the United States [20]. At the time, the main aim was to target livestock being shipped from neutral countries to the Allies in the anticipation of gaining a tactical advantage on the battlefield. This relied heavily on the collaboration of covert operatives (e. g., Anton and Carl Dilger, Frederick Hinsch, and Paul Hilken) in seaport cities to disseminate the pathogens [23] (Table 1). An example of such biological sabotage programs included the inoculation of allied horses and mules with glanders (Burkholderia mallei) and anthrax (Bacillus anthracis). However, such efforts did not achieve the expected military consequences.
After WWI, this program continued; it has been suggested that the bacterial agents causing typhoid (Salmonella enterica serovar Typhi), paratyphoid fever (S. enterica serovar Paratyphi), dysentery (Shigella spp.), cholera (Vibrio cholera), plague (Yersinia pestis), glanders (B. mallei), anthrax (B. anthracis), and wound infections (e.g., Staphylococcus aureus, Streptococcus spp., Bacteroides spp., Clostridium spp.) were the most suitable for the German war efforts, mainly when these microorganisms were used for the production of aerosols or the contamination of water sources [24].
During World War II (WWII), in 1941, despite Adolf Hitler’s recurrent and stringent commands against the use of biological weapons, Heinrich Himmler founded an entomological institute, under the cover of the concentration camp at Dachau. Under the supervision of Eduard May, allegedly this institute was devoted to defensive research for Waffen-Schutzstaffel (commonly known as the SS) guards and staff protection against insect-borne diseases, such as malaria (Plasmodium spp.) and louse-vectored typhus (Rickettsia prowazekii). However, according to May’s reports, it has been suggested that the true objectives of this institute were associated with the German BW program, including offensive purposes. Also, in 1943, Hermann Göring ordered the creation of a unit of “cancer research” at a new institute in Nesselstedt (near Poznan, Poland), under the supervision of Kurt Blome. It has been suggested that both Rudolf Mentzel and Erich Schumann were interested in the development of biological weapons for the Third Reich [25].
Besides the Dachau facility, other institutions were involved in biological weapons development for defensive proposes; activities were restricted and few scientists were involved in these studies. Some of this workforce was devoted to the development and production of vaccines (e.g., for the foot and mouth disease virus, plague, and yellow fever) or sera (e.g., anthrax, botulism, and tularemia), while other activities focused on the dissemination of aerosolized pathogenic agents (e.g., aerosols dispersed using aircraft, dissemination using glass containers, and insect-borne dissemination). Field-tests were conducted by emulating non-pathogenic bacteria and aerial spraying of the foot and mouth disease virus [24]. On the 29th of April 1945, American troops liberated Dachau and dissolved the entomological institute [25].
1.1.2. The Japanese BW program
The Japanese BW program began in 1932 under the command of Major General Shiro Ishii with the creation of the first biological weapons research facility at the Tokyo army’s medical school, concealed as part of the Kwantung Army’s Water Purification Department. Although considered as quite primitive in the level of technical sophistication, this program enrolled 3000 military personnel and hundreds of the foremost medical doctors and technicians and included more than 150 research centers, including the infamous Unit 731 (known as the Epidemic Prevention and Water Purification Department, this research center was located in Pingfang, Manchuria - responsible for human experimentation during the Second Sino-Japanese War), scattered throughout the Japanese empire, from Manchuria to Indonesia [26].
Organisms and diseases of interest to the Japanese program were possibly tularemia (Francisella tularensis), glanders (B. mallei), meningitis and other forms of meningococcal disease (Neisseria meningitidis), brucellosis (Brucella spp.), typhoid fever (S. enterica serovar Typhi), dysentery (Shigella spp), cholera (V. cholerae), plague (Y. pestis, disseminated via the Uji bomb), anthrax (B. anthracis, disseminated via the Ha bomb), botulism (Clostridium botulinum; toxin) and smallpox (Variola major) (Table 1) [18,21].
This program remains throughout History as one of the most atrocious by its dimension and the experiments performed on humans. It is estimated that at least 3000 prisoners of war (Chinese, Koreans, Mongolians, Soviets, Americans, British, and Australians) were allegedly used as test subjects in Unit 731. These tests included inoculation with several pathogens, vivisection, weapons tests, and germ warfare attacks [21]. No prisoner left the Japanese death factories alive [27]. As a consequence of the dissemination of pathogens during the Japanese BW program, it is supposed that thousands of civilians perished as a direct consequence of the attacks [27]. Moreover, due to the difficulty in controlling the dissemination, several Japanese soldiers were also victims of these attacks [21].
Immediately after the end of WWII, the Soviets convicted Japanese scientists engaged in these activities for war crimes. However, the United States government covertly granted immunity to these scientists, possibly to gain exclusive access to the work developed and scientific breakthroughs from the BW program [28].
1.1.3. The American BW program
The American BW program was secretly implemented, in 1942, under the auspices of President Franklin Roosevelt after the suspicion of the use of BW agents by Germany and Japan [29]. In 1947, President Harry Truman withdrew the US from the Geneva Protocol with Senate approval and conducted open-air field testing using non-pathogenic bacteria (Bacillus globigii and Serratia marcensces) on naval vessels near the Virginia Coast and San Francisco Bay, along with the dissemination of bacterial aerosols in bus stations and airports. At its peak, this program involved nearly 3400 people and several research and production facilities and was responsible for investigations on more than 30 agents, including bacteria (e.g., B. anthracis, Brucella suis, Coxiella burnetti, Francisella tularensis), toxins (e.g., botulin and staphylococcal enterotoxin B), and viruses (e.g.,Venezuelan equine encephalitis and yellow fever virus) [30].
The program was officially dismantled by President Richard Nixon, in 1969 and all facilities involved were converted for defense purposes only. In 1972, the Biological Weapon and Toxin Convention (BWTC) was signed [30]. However, before this decision, ten different BW agents have been weaponized, sophisticated delivery systems had been developed, and a huge research program was in place in the country [31].
1.1.4. The post-Soviet BW program
The Soviet BW program was initiated in the mid-1920s and was secretly continued until at least the 1990s, even after singing the BWTC, in 1972 [32]. This program was divided into two phases:
-
i)
from its start to 1972 (including the WWII, Russian Civil War, and the beginning of the Cold War): this period was characterized by the research and use of naturally occurring microorganisms;
-
ii)
from 1972 to 1991: during this period, even after the termination of the American BW program, the Soviets increased the level of sophistication of their program, investing in biotechnological developments to create novel or modify existing bacterial and viral strains. It has been hypothesized that many of the BW scientists remain in Russia providing basic know-how to launch a third phase of the program with the support of the current President of the Russian Federation, Vladimir Putin [33]. At its peak, the Soviet BW program included nearly 60,000 scientists and technicians dispersed across more than 50 military and research centers [34].
Around 50 biological agents were developed using either naturally occurring or genetically engineered strains of several bacteria (e.g., B. anthracis, Brucella melitensis, B. mallei, Clostridium perfringens, Clostridium tetani, F. tularensis, and Yersinia pestis), toxins (e.g., botulinum neurotoxin) and viruses (e.g., Marburg virus, smallpox virus, Variola major, and Venezuelan Equine Encephalitis Virus) [34].
After the disintegration of the former Soviet Union, many scientists (e.g., Vladimir A. Pasechnik and Kanatjan Alibekov) deserted and provided international intelligence agencies with details about the ongoing works of Biopreparat (Biological Substance Preparation - the Soviet Union’s major BW agency from the 1970s onwards). This information forced President Boris Yeltsin to admit not only that the country had covertly sustained its BW program (after signing the BWTC) but also that they were responsible for the anthrax outbreak (accidental aerosol release from a military facility) at Sverdlovsk, in 1979 [34].
1.1.5. The Iraqi BW program
The Iraqi BW program was launched, in the early 1980s, by Saddam Hussein. Throughout the Gulf War, the Iraqi Biological Research Centre for Military defense, located at Salman Pak, studied the use of several bacteria (e.g., B. anthracis, B. melitensis, C. botulinum, and C. perfringens), toxins (e.g., aflatoxin, trichothecene), and viruses (e.g., camelpox virus, influenza virus, rotavirus, and West Nile virus) as bioweapons. This research culminated in the mass production of bioweapons either loaded into munitions (200 bombs and 25 ballistic missiles) or stored in spray tanks for later dissemination as aerosols. As a result, both troops on the battlefield and civilian populations in the region of conflict were threatened by the possible use of these weapons [35,36].
The United Nations Special Commission (UNSCOM), mandated to eliminate and prevent the revitalization of the Iraqi BW program, was expelled from Iraq, after seven years of work. As a consequence, Operation Desert Fox began. During its duration, the United States and Great Britain bombarded and destroyed three locations associated with the BW program [35,36].
1.2. Bioterrorism
Contrary to BW, in a BT attack, biological agents are intentionally released against a civilian population [15]. This spread is motivated or justified by ideological objectives (either political or religious) intending to cause panic, mass casualties, or economic loss [37]. The biological agents can be used as they naturally occur or be genetically modified to improve mass dissemination (e.g., higher mortality or resistance to currently available medicines and vaccines) [38]. When facing the possibility of a BT attack is crucial to identify the agent involved, not only to prevent panic among the population but also to control the morbidity and mortality associated with the spread of the agent [39]. The emphasis of MF on BT emerged from the challenges that arose as a result of the infamous Amerithrax mailing attacks in 2001 [40].
According to the CDC Strategic Planning Group (https://www.selectagents.gov/SelectAgentsandToxinsList.html), human health can be threatened by several microorganisms (for a more systemic review, please read Ecker et al. 2005 [41]).
In a BT attack using bacteria as weapons the most frequent diseases are anthrax (Bacillus cereus biovar anthracis - a variant of the B. cereus bacterium that has acquired plasmids similar to those of B. anthracis) [42], glanders (B. mallei) [43], melioidosis (Burkholderia pseudomallei) [43], brucellosis (Brucella spp.) [44], diphtheria (Corynebacterium diphtheria) [45], Q fever (C. burnetii) [46], hemolytic-uremic syndrome (Escherichia coli 0157:H7) [47], tularemia (F. tularensis) [48], salmonellosis (Salmonella spp.) [49], typhus fever (R. prowazekii) [50], Rocky Mountain spotted fever (Rickettsia rickettsii) [50], cholera (V. cholera) [51], plague (Y. pestis) [52]. In an attack using bacterial toxins the most frequent diseases are botulism (C. botulinum, neurotoxins) [53], gangrene (C. perfringens, Epsilon toxin) [54], dysentery (Shigella dysenteriae - Stx1 and Stx2) [55], and food poisoning (Staphylococcus aureus, enterotoxins T-2 toxin and tetrodotoxin) [56].
In a BT attack using viruses as weapons the most frequent diseases are encephalitis (Eastern Equine Encephalitis, Tick-borne encephalitis-complex viruses - Central European tick-borne encephalitis, Far Eastern tick-borne encephalitis, Russian spring and summer encephalitis viruses -, and Venezuelan equine encephalitis virus) [[57], [58], [59]], hemorrhagic fever (Chapare, Crimean-Congo, Ebola, Guanarito, Kyasanur Forest disease, Lassa fever, Lujo, Machupo, Marburg, Omsk, Rift Valley fever, and Sabia virus) [60,61], respiratory syndromes isolated (highly pathogenic strains of 1918 Influenza and Avian Influenza H5N1, and SARS virus) [[62], [63], [64], [65]] or in combination with neurological symptoms (Hendra and Nipah virus) [66], immunodeficiency syndromes (Human immunodeficiency virus - HIV) [67] and rashes (Foot-and-mouth disease [68], Monkeypox [69], Variola major – Smallpox – and Variola minor virus – Alastrim [[70], [71], [72]]). Depending on the effortlessness of transmission, the morbidity and mortality rates associated, and their capability to be mass-produced, these biological agents are classified into three categories (A, B, and C) by the CDC (https://emergency.cdc.gov/agent/agentlist-category.asp) [73,74] (Table 2 ).
Table 2.
Classification of potential bioterrorism agents (bacteria, virus, protozoan and toxins) capable of induce diseases in humans, according to the United States Centre for Disease Control and Prevention (CDC) Strategic Planning Group.
| Category | Definition | Agent and Disease |
|---|---|---|
| A |
|
|
| B |
|
|
| C |
|
|
Adapted from https://emergency.cdc.gov/agent/agentlist-category.asp.
1.2.1. R.I.S.E: (1972. Chicago, Illinois, United States)
In 1972, a group designated R.I.S.E. (the acronym is not fully known but it is believed that R was for Reconstruction, the S for Society, and the E for Extermination; the I remains a mystery [75]) attempted to attack water treatment systems, mainly targeting the Chicago area. This group intended to relaunch a new society more toned with ecological values by killing off the already existing world population. This attempt failed since some of the group recruits denounced the plans to the Chicago Police Department. The attempt failed because some of the new recruits of the groups denounced the plans to the Chicago Police Department. Subsequent analysis, performed by the CDC, concluded that the group possessed viable cultures of S. enterica serovar Typhi, Shigella sonnei, C. botulinum, Neisseria meningitidis, and C. diphtheria.
Allen A. Schwandne (the group leader) and Steve Pera (a biology expert working in collaboration with the group) were arrested and released on bail. Both activists retreated to Cuba; Mr. Schwandner was arrested for counterrevolutionary activities against the Cuban regime, sentenced to six years, and died during imprisonment. In 1974, Mr. Pera voluntarily returned to the United States, negotiated a plea agreement, and sentenced to five years imprisonment [76].
1.2.2. Dark Harvest (1981, Porton Down, England)
In 1981, an activist group auto-denominated “Dark Harvest Commandos” dropped a package at the London-Exeter railway line, near the Porton Down campus, where the Chemical defense Establishment was located. The package contained soil collected from Gruinard Island, a testing site for anthrax spore bombs used by the British government during WWII (1941). With this attack, the group claimed to be returning the “seeds of death” to their source. The analysis performed revealed low concentrations of B. anthracis spores (approx. 10 organisms/gram of soil), in a form that was highly unlikely to cause any harm. As such, following the test, in 1941, the levels of contamination had been decaying by natural means. This group sent a second package addressed to the Conservative Party Conference. This group sent a second package addressed to the Conservative Party Conference. However, in this second incident, no spores were identified in the soil analyzed. In 1986, after the initiative to decontaminate the soil, the activities of this group ceased [77,78].
1.2.3. The Rajneeshees (1984, The Dalles, Oregon, USA)
In 1984, before a forthcoming election in Wasco County, the Rajneeshees – a religious cult – intended to sicken voters so they would be incapable of voting, hence enabling the cult to gain political control of the county seat and government. Salmonella enterica serovar Typhimurium bacteria (strain ATCC 14028) was isolated, cultured, and mass-produced from Bactrol discs – legitimately acquired from VWR Scientific (Seattle, Washington) to be used in the Rajneesh Medical Corporation (RMC) licensed laboratory – and used against inhabitants of The Dalles [76,79].
This remains the largest act of BT in the United States and included three separate incidents. The first incident consisted of contaminating the hand of several commune members. These members then greeted people with a handshake and contaminated doorknobs and urinals at the Wasco County courthouse. However, no casualties were registered as a result of this attack. The second incident, in August, was perpetrated against three county commissioners unfavorable to the Rajneeshees. While visiting the commune, the County commissioners were offered drinking water contaminated with the selected bacteria. Among these representatives, two became sick and one was hospitalized. In the third incident in September of the same year, members of the cult contaminated salad bars, salad dressing, and coffee creamers in local restaurants with the same bacteria. As a consequence of this attack, more than 750 people became sick and 45 were hospitalized [80].
The cult was also studying the use of several other agents (S. enterica serovar Typhi, Enterobacter cloacae, Neisseria gonorrhoeae, S. dysenteriae, hepatitis, and AIDS) and the possibility of contaminating The Dalles’ water supply system using dead rodents (beavers, rats, and mice) [79].
1.2.4. Aum Shinrikyo (1990–1995, Japan)
The Aum Shinrikyo is a religious cult that became widely known for the attack perpetrated in the Tokyo subway system, on the 20th March 1995, using Sarin gas (a deadly nerve agent) poisoning nearly 6000 people. The cult claimed to have between 20,000–40,000 worldwide followers and a net worth of $1.5 billion dollars [81].
Besides this chemical agent, the cult was also involved in BT activities using toxins and microorganisms, such as botulinum toxin, B. anthracis, V. cholera, and C. burnetii while attempted to obtain Ebolavirus [82,83]. To maintain its sufficiency, instead of buying cultures from external suppliers, the cult isolated their own cultures. This fact may explain the several unsuccessful BT attempts executed by the cult. Clostridium botulinum was harvested in the soil from Ishikarigawa Basin during the spring of 1990 [81]. In April 1990, the cult attempted to release botulin toxin, using a vehicle driven around the Japanese Parliament and other government buildings in Tokyo. Three years later, by the occasion of the Crown Prince wedding, the cult sprayed this toxin from a truck circulating the Imperial Palace and other main government buildings in Tokyo. Another two years later, right before the Sarin gas attack in the subway, an attack with botulin toxin was prevented in the subway at Kasumagaseki Station [81]. Also, an anthrax strain presenting high homology to the Sterne 34F2 strain, the most commonly used for cattle and livestock vaccination, was sprayed on the roof of the Aum building in East Tokyo [84].
1.2.5. Amerithrax (2001, USA)
On the 18th September 2001, a week after the attacks on the Twin Towers of the World Trade Center in New York, letters containing spores of B. anthracis were mailed to media companies (ABC News, CBS News, NBC News, the New York Post, and the National Enquirer). These letters held the postmark from Trenton, New Jersey. Only two of these letters were recovered (one addressed to the New York Post and other to Tom Brokaw at NBC).
The second wave of letters began three weeks later, on the 9th October. Two more letters with the same postmark were sent to the Senate offices of the Democratic U.S. Senators Tom Daschle (South Dakota) and Patrick Leahy (Vermont).
As a consequence of these attacks, between the 4th October and 20th November 2001, a total of 22 anthrax cases (11 by inhalation and 11 by cutaneous contact) were reported, five being fatal [85]. More than 30 people tested positive for B. anthracis spores' exposure and nearly 32,000 individuals underwent antibiotic prophylaxis [86].
Following the receipt of letters by the US Senators, the Federal Bureau of Investigation (FBI) started a massive investigation in collaboration with the CDC, Department of Defense (DOD), Intelligence Community, US Department of Justice, Armed Forces Institute of Pathology (AFIP), US Army Medical Research Institute of Infectious Diseases (USAMRIID), US Postal Inspection Service (USPIS), and 29 external laboratories (universities, government and private) [86].
From the 22nd September to 14th November 2001, researchers collected biological evidence from several sources and locations: 4 powder-containing envelopes, 17 clinical samples from infected patients and 106 samples taken at locations along the path traveled by the envelopes (Florida, Washington, D.C., New Jersey, New York, and Connecticut).
On the 19th February 2010, more than eight years after the anthrax letters, the “Amerithrax Investigative Summary” was published and officially closed the FBI’s investigation. Bruce Ivins, a microbiologist and bio-defense researcher from the US Army Medical Research Institute of Infectious Diseases (USAMRIID), in Fort Detrick, working on anthrax vaccines and treatment was incriminated as the sole perpetrator of these BT acts. However, on the 29th July 2008, Dr. Ivins committed suicide through an overdose of Tylenol with codeine. Therefore, it has impossible to obtain a legal resolution concerning his guilt or innocence [87]. The government’s case was considered circumstantial, based on the genetic similarity between the genetic features of the anthrax spores found in the letters and the cultures grown at Dr. Ivin’s laboratory (strain RMR-1029) [88]. Curiously, during the early stages of the investigation, the FBI counted on the collaboration with Dr. Ivins [89]
1.3. Biocrime
Biocrime can be defined as the use of a disease-causing agent or toxin to kill, debilitate or cause panic for a specific individual or a limited group of individuals, motivated by personal reasons such as revenge, jealousy, or the desire for monetary gain by extortion [37,39]. Therefore, the main differences between BC and BT are the number of people affected and the motivation behind the attack. Usually, the perpetrators of these crimes have both the required scientific knowledge and ready access to the biological agent to be used. The use of MF in BC investigations can be illustrated by the paradigmatic cases of the intended dissemination of S. dysenteriae Type 2 [90], HIV [[91], [92], [93]], and hepatitis B (HBV) and C (HCV) viruses [[94], [95], [96]]. In such cases, it is of primordial importance to establish the source of the bioagent through the comparison of the isolate found in/on the victim and that associated with the perpetrator [39].
1.3.1. Diphtheria toxin injection
The first documented case of the use of toxins with criminal intentions is associated with the murder of Captain Vassilli Buturlin, in 1910 (St. Petersburg, Russia). Patrick O’Brien de Lacy (his brother-in-law), with the assistance of Vladimir Pantchenko (a medical doctor), injected Captain Buturlin with diphtheria toxin as a consequence of an inheritance dispute. The initial plan was to inject the victim with cholera (at that time, endemic in the city) but instead, they administered the diphtheria toxin [97].
1.3.2. Typhoid fever
During nine years (1909–1918) the French insurance broker Henri Girard convinced his victims to buy life insurance policies and to make him their primary beneficiary. To obtain the death benefits, Mr. Girard and his collaborators spiked the victims’ food with S. enterica serotype Typhi and natural toxins from the mushrooms belonging to the Amanita genus. Girard was studying bacteriology at the time of the first murder. Mr. Girard was responsible for the murder of Louis Pernotte and Madame Monin and for infecting or poisoning six others. Arrested in August 1918, Mr. Girard committed suicide while awaiting trial by ingesting one of his microorganism cultures (most likely typhoid) secretly hidden among his personal belongings. Before his death, Mr. Girard confessed to these crimes and implicated four accomplices that were then convicted and given sentences ranging from two years imprisonment to life imprisonment with ward work [20].
From 1935–1936, the Japanese physician Dr. Tei-Sabro Takahashi was involved in five incidents of infecting several competing colleagues, their families, and his wife using pastries spiked with S. enterica serovar Typhi. As a result of these incidents, 17 colleagues were infected and three subsequently died. Dr. Tei-Sabro Takahashi was arrested by the Saitama Prefecture Police Department in 1937 and a year later he was convicted and sentenced to the death penalty [20,98].
Three years later, in 1939, the Japanese physician Dr. Kikuko Hirose, offered Ritsuo Kato (her former husband) pastries spiked with S. enterica serovar Typhi and S. enterica serovar Paratyphi. The pastries were then shared with his family and colleagues from Kawaike Elementary School, resulting in twelve individuals becoming infected and one death. Dr. Hirose was found guilty by the Supreme Court and sentenced to eight years imprisonment [20,51].
1.3.3. Cholera and typhus
In the ten years comprehended between 1902 and 1912, the German variety artist Karl Hopf (also known as Athos) attempted to poison his father, mother, two children, and three former wives. Mr. Hopf studied chemistry and worked as a pharmacist in London, having experience in drugs' handling. When his third wife, Wally Siewec, became severely sick due to gastrointestinal illness, suspicions were raised against Mr. Hopf. A police search in his dwellings discovered morphine, opium, cyanide, digitalis, arsenic, and live cultures of typhus, cholera, and other pathogenic agents. Mr. Hopf was detained in April 1913 and judged in January 1914. He was found guilty of poisoning his two children and his first wife, and of attempting to poison his second and third wives. Mr. Hopf was sentenced to death and guillotined in March 1914 at the Royal Prison Preungesheim in Frankfurt [20].
1.3.4. Several pathogens
In 1916, the New Yorker Magazine asserted that Arthur Warren Waite, a dentist who later become known as “The Playboy Poisoner”, made several attempts to acquire pathogens, such as those causing pneumonia, tuberculosis, influenza, and diphtheria. Trying to obtain a financial advantage, Dr. Waite first killed Mrs. Hannah Peck (his mother-in-law) by spiking her food with a mixture of diphtheria and influenza. The same procedure was unsuccessfully adopted in the attempt to kill John Peck (his father-in-law), using a nasal spray spiked with tuberculosis and then with influenza and typhoid. When John Peck survived, Dr. Waite used chlorine gas and arsenic. Dr. Waite was found guilty and was sentenced to death in the electric chair at Sing Sing Correctional Facility, in New York, on 24 May 1917 [99].
1.3.5. Bubonic plague
In 1933, in Calcutta (India), Dr. Taranath Bhatacharyna (a physician trained in bacteriology) assisted by Benoyendra Chandra Pandey injected the arm of Amarendra Pandey (Benoyendra’s half-brother) with a lethal dose of Y. pestis. The dispute was initiated by the division of their father’s estate and because of Amarendra’s flamboyant lifestyle. Three years after, both perpetrators were convicted and sentenced to death [20].
1.3.6. Dysentery
In 1964, Mitsuru Suzuki, a Japanese medical doctor with training in bacteriology, was detained for infecting four of his colleagues using dysentery-contaminated sponge cake. Subsequently, Dr. Suzuki was associated with a series of dysentery and typhoid fever outbreaks involving nearly 200 patients and four deaths. The team of prosecutors argued that Dr. Suzuki’s dissertation included studies of S. enterica serovar Typhi recovered from several patients and that a culture of these bacteria was reported as stolen from Japan’s National Institutes of Health [102,103].
In 1996, an outbreak of S. dysenteriae type 2 took place at a large medical center in Texas. Twelve members of the laboratory staff became indisposed after eating baked goods (muffins and doughnuts) anonymously left in the lunchroom, between the night and morning shift. Identification, serotyping, and pulsed-field gel electrophoresis revealed that the stool isolates from nine of the victims were identical to the S. dysenteriae strain retrieved from an uneaten muffin in the lunchroom and to a laboratory's stock culture of the same pathogen that was partially-missing from the laboratory stockpile [90]. On 28 August 1997, Diane Thompson (a member of the lab staff) was accused of infecting 12 of her colleagues and was later sentenced to 20 years imprisonment [104].
1.3.7. HIV
In 1990, Graham Farlow, an asymptomatic HIV-positive inmate from a New South Wales (Australia) prison, stabbed officer Geoffrey Pearce with a needle containing HIV-positive blood. A few months later, the officer tested HIV-positive and died seven years after the attack [20].
In 1992, Jennifer Jackson informed Brian Stewart (her former husband) that their 11-month old son Brryan Jackson was hospitalized after an asthma attack. After several arguments concerning child support and paternity between the couple, Mr. Stewart injected Brryan with HIV-positive blood. Mr. Stewart, a phlebotomist working at a laboratory in Barnes Hospital (St. Louis, Missouri), has drawn extra tubes of blood from patients and took home all the equipment necessary to inject the child. Four years after, Brryan was diagnosed with acquired immunodeficiency syndrome (AIDS) and three opportunistic infections. Although Mr. Stewart pleaded innocent, in 1998, he was convicted and sentenced to life imprisonment for attempted murder [100].
In 1993, Iwan E. visited Gina O. (both perpetrator and victim were not identified in court using their surnames), his former girlfriend, to pay her some money she had lent him. In the middle of an argument in the apartment kitchen, E. was allegedly threatened with a knife and responded by injecting blood tainted with HIV (previously collected from E’s friend) in the arm of O. Three months after this episode, O. tested HIV-positive. Although self-defense was claimed, the incriminating testimony of E.’s friend supported the conviction of attempted murder, and E was sentenced to 12 years imprisonment [101].
In 1994, with the pretext of giving a vitamin B-12 injection, Richard J. Schmidt, a gastroenterologist, injected Janice Trahan with a mixture of blood or blood-products infected with HIV-1 and HCV, obtained from two patients under his care. Before the crime, Trahan tried to finish the 10 year-long extramarital affair with Dr. Schmidt. In 1995, Trahan was confirmed as HIV-positive and accused Dr. Schmidt of infecting her. During the following investigation, all men previously involved with Trahan were tested and found to be HIV-negative. The donor of the infected blood was identified, and the comparative phylogenetic analyses performed (env and reverse transcriptase genes) revealed this patient as the source for the virus. The case of The State of Louisiana vs. Richard J. Schmidt remains as the first example were phylogenetic analyses of HIV-1 sequences were admitted and used as evidence in a criminal proceeding in the United States [92].
1.4. The role of microbial forensics
Microbial forensics is a relatively new scientific discipline, established in 2003 as a direct consequence of the necessity to identify the strain used in the US. MF relies on knowledge obtained from other basic and applied sciences along with non-traditional forensic science disciplines; microbiology, microbial ecology, public health epidemiology, microbial genomics, and toxicology, along with bioinformatic analysis and process engineering [81].
In 2007, the National Research Council Committee on Metagenomics (US) highlighted metagenomics’ effectiveness in the fields of bio-defense and MF ‘to precisely identify and characterize microbes that have played a role in war, terrorism, and crime events, thus contributing to discovering the source of the microbes and the party responsible for their use’ [105] (please see below for further information on metagenomics).
Therefore, MF efforts focus on the elucidation of the following questions:
-
•
What was the pathogenic microbial agent used?
-
•
Where did it came from?
-
•
Where are the natural reservoirs located?
-
•
What are the possible transmission routes involved?
-
•
Are there any vectors involved?
-
•
What probable targets are affected?
-
•
How did the microorganism evolve?
-
•
Are there toxins involved?
-
•
To which antibiotics are the microorganisms sensitive or resistant to?
-
•
Was the microorganism genetically modified or chemically treated to enhance its features such as virulence or dispersion capacity?
These questions can be divided into two main domains: scientific and legal (or criminal) attribution. Scientific attribution aims to determine the origin of a given pathogenic agent to a known origin to the highest degree of scientific confidence (and exclude other possible source origins) [84,106]. In the case of a BW, BT, and BC is crucial to detect the pathogen as soon as possible to minimize the health hazards associated with its dispersion. This can be achieved with the creation of biosecurity programs via international collaboration, with the collective aim to periodically monitor public places not only to define the baseline concentration of microorganisms, to include variations such as seasonal fluctuation but also to detect the sudden increase of non-expected microorganism [82,83]. Furthermore, the information gathered can be applied to develop a better preparedness response plan and to discourage BT attacks. Legal attribution aims to determine who was responsible for the acts of interest (BW, BT, or BC) or who was otherwise implicated, according to the principles recognized and applied within a legal system [84,106].
Legal attribution intends to determine with respect who was responsible for the acts of interest (biocrime, bioterrorism or biowarfare) or who was otherwise implicated, according to the principles recognized and applied within a legal system [84,106].
Another fundamental factor to consider is sample collection and preservation as the entire process is reliant on appropriate and efficient sampling procedures; to maximize recovery of microorganisms and to maintain the integrity of the sample. The validation of the results is also an important factor to enable the use of such evidence to clarify the facts to determine, possibly in a court of law, whether a crime is likely to have been committed or not.
In most cases, MF is applied to entirely new situations (sampling area, location, type of agent). Hence, instead of applying the stringent standard operating protocols (SOP) to process the crime scene, it is desirable to adapt the existing rules to a combination of previous knowledge from specialists, their research expertise, and common sense [3,4].
Samples collection and preservation is a fundamental step of the entire process. It limits the validation of the results and may prevent samples from constituting the evidence necessary for the clarification of a given crime.
The value of the evidence, even when carefully collected and preserved, can be lost if the chain of custody is not properly constituted. The chain of custody is frequently recognized as the weakest link in a criminal investigation. It refers to the procedure of careful and chronological documentation of evidence, establishing its link to a criminal offense. From the beginning to the end of a judicial process, it is essential to demonstrate and document each step, ensuring evidence "tracking" and "integrity" from the crime scene to the courtroom. The collection of samples must be carefully performed by specialized technicians, using the appropriate equipment (e.g., suits, gloves, masks) to minimize contamination of the sample and to avoid the risk of infection. Samples must be accompanied by a record documenting; who collected it, under what conditions and the methods used for its collection, where and how the sample was preserved (e.g., temperature, relative humidity), and who had access and conducted any scientific work on the sample [3,4].
The first techniques for microorganism identification and detection were limited to phenotypic methods, associated with antigenic and/or antimicrobial resistance profiles. These methods only allow resolution at the genus and/or species level [3]. Then, in the late 1980s, these methods were complemented with nucleic acid-based methods (e.g., DNA fingerprinting, whole-genome sequencing, and microarray analysis). Such methods improved taxonomic resolution to the isolate/strain level, they are independent of the changes observed in the phenotypic characteristics due to the influence of environmental factors. Moreover, nucleic acid-based methods also decreased the turnaround time between sample collection and result availability [2,12].
In the early 2000s, high-throughput sequencing (HTS) [also referred to as massive parallel sequencing (MPS) technology or next-generation sequencing (NGS)] was introduced. Due to its increased multiplexing capacity, this method complies with different approaches, either allowing for whole-genome sequencing (WGS) of a single microorganism (e.g., viruses, bacteria, or fungi) or sequencing all the microbial species present in complex samples or matrices collected from a given environment or individual (metagenomics) [2]. In 2014, HTS approaches were introduced in routine diagnostics, for the investigation of outbreaks and transmissions, and used for genotyping of highly-resistant microorganisms. Therefore, clinical microbiologists or infectious disease specialists frequently resort to HTS, in collaboration with molecular microbiologists and infection control professionals. HTS has frequently been proven as suitable for studies of forensic epidemiology, concerning source identification, outbreak detection, transmission routes, pathogen evolution, and to identify the dynamics of multi-drug resistant pathogens, as reviewed in Oliveira et al., 2018 [12].
The main advantages of HTS over classical Sanger sequencing are:
-
(i)
high-throughput capacity, hundreds of millions of sequencing reactions can be accomplished in parallel, enabling full sequencing of an entire bacterial genome in just one or two instrument runs;
-
(ii)
a single protocol can be applied for all microorganisms for identification and genotyping;
-
(iii)
DNA cloning is eliminated, exclusively depending on libraries preparation, in a cell-free system;
-
(iv)
no prior knowledge about the sequence of a particular gene/genome is needed since HTS can read the DNA templates randomly distributed throughout the entire genome, and then de novo genome assembly can be applied;
-
(v)
no need for isolation and culture of the microorganism of interest, a particularly critical aspect as many strains are unable to grow in culture media, allowing the identification of microorganisms present at trace levels and also those previously undetected by conventional methods;
-
(vi)
cost (usually less than US$1000 (US) per genome, depending on the genome length) and the reduction in turnaround time (only a few hours) [2].
The main disadvantage of HTS is associated with data storage and bioinformatics analysis. Depending on the platform used, in a single run, 30,000–500,000 sequence reads of 50–700 nucleotides can be generated. Due to the extremely sizeable quantity of data collected, the process deeply depends on storage ability and bioinformatics capacity to produce valuable data and to evaluate the quality of the HTS platform [2].
During the last two decades, the advances in HTS technology, coupled with developments in machine learning, resulted in the production of extremely large amounts of high-quality sequence data that can be used for the prompt analysis of microbial communities from environmental samples [107,108]. Metagenomics bypasses two of the major limitations associated with the “classical” studies of microbial communities: it is culture-independent, provides information on the true diversity of a given ecosystem (99% of microorganisms in nature that have not yet been cultivated), and offers insights into the complex metabolic pathways associated with these microorganisms (e.g., antimicrobial resistance, or virulence factors) [[109], [110], [111]].
However, certain challenges must be addressed before the widespread application of metagenomics in the MF context. On one hand, the whole process – sample collection, maker selection, computational and bioinformatics, and metagenome analysis benchmarking and standards - presents several experimental biases [[112], [113], [114]], limiting its application to MF. The development of validated experimental protocols and SOPs would ensure the required sensitivity, specificity, precision, accuracy, reproducibility, repeatability, the limit of detection, reportable range, false positive and negative ranges, and robustness of this method [107,115]. Although there has been an increased interest in HTS [MiSeq™ (Illumina, CA) or the Ion S5™ (ThermoFisher Scientific, UK)] by some members of the forensic community, the vast majority of forensic laboratories are more familiar with and still using Sanger sequencing technologies [116].
For the widespread application of MF, the forensic community needs to be further educated. Experts must address all the requirements of transparency (e.g., application of legal standards for genetic privacy and reliable standards) and accuracy of the data produced (e.g., correct use of databases and informatics tools). End-users (e.g., crime scene investigators, lawyers, judges, and juries) must be responsive to these new developments, recognizing both its possibilities and constraints.
Stable isotope [i.e., hydrogen (2H/1H), carbon (13C/12C), nitrogen (15N/14N), and oxygen (18O/16O)] ratios, can render unparalleled information in forensic investigations concerning the assignment of a source to a given microorganism. Microorganisms use these elements as water and nutrients supplies incorporating the isotopic variations in its nucleic acids. It has been suggested these variations can be used to trace specific batches of media or with specific sources of water, allowing to determine from which laboratory the microorganisms were produced. However, the high replication rate of microorganisms results in a rapid turnover of these elements [117,118].
2. Conclusions
Criminals and terrorists often consider biological agents (i.e., virus, bacteria, or bacterial toxin) as an attractive alternative to conventional weapons. Bioweapons production is associated with relatively low cost, microorganisms are relatively accessible, they can be simple to produce and to deliver while avoiding detection and even the threat of their use can induce fear among individuals and potential widespread social disruption.
The release of a biological weapon is intended to induce diseases or even death. Usually, these are naturally occurring microorganisms but, sometimes, they can be engineered to become harmful by increasing their ability to cause or spread disease or to resist known therapeutic approaches.
Throughout this review, it has been demonstrated that despite its form (either BW, BT, or BC) bioterror is a historical fact, almost present since the dawn of times. Therefore, facing this reality, experts must be aware that the correct question is not if we will have another attack using biological agents as weapons but rather when will be the next attack using biological agents as weapons.
With this in mind, it is essential to plan for a timely and effective response to the release and dissemination of a biological agent, including the ability to obtain a reliable and informative classification of the agent(s) being used. Controlled access to data held about global collections of representative strains, along with research to characterize biological agents that are less well studied and more difficult to culture, would aid in this effort. Preventative and early detection measures such as comprehensive environmental monitoring should also be implemented.
It is also essential to manage the expectations of law enforcement agencies, the general public, policymakers, and the scientific community along with highlighting the range of capabilities of microbial forensics.
CRediT authorship contribution statement
Manuela Oliveira: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Gabriella Mason-Buck: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. David Ballard: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Wojciech Branicki: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. António Amorim: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing.
Acknowledgments
This work was financed by FEDER - Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 - Operational Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT- Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Inovação in the framework of the project "Institute for Research and Innovation in Health Sciences" (POCI-01-0145-FEDER-007274).
References
- 1.Amorim A. Introduction to the special issue on forensic genetics: non-human DNA (Guest editor: Antonio Amorim) Open Forensic Sci. J. 2010:3. [Google Scholar]
- 2.Oliveira M., Amorim A. Microbial forensics: new breakthroughs and future prospects. Appl. Microbiol. Biotechnol. 2018;102:10377–10391. doi: 10.1007/s00253-018-9414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira M., Arenas M., Pinto N., António A. Genética forense no humana. In: Caballero Barrio., editor. Genética Forense: Del laboratorio a los Tribunales, M.C. Crespillo Márquez and P. 2019. pp. 291–318. (Madrid, Ediciones Díaz de Santos) [Google Scholar]
- 4.Arenas M., Pereira F., Oliveira M., Pinto N., Lopes A.M., Gomes V., Carracedo A., Amorim A. Forensic genetics and genomics: much more than just a human affair. PLoS Genet. 2017;13:e1006960. doi: 10.1371/journal.pgen.1006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budowle B., Schutzer S.E., Einseln A., Kelley L.C., Walsh A.C., Smith J.A., Marrone B.L., Robertson J., Campos J. American Association for the Advancement of Science; 2003. Building Microbial Forensics as a Response to Bioterrorism. [DOI] [PubMed] [Google Scholar]
- 6.Murch R.S. Microbial forensics: building a national capacity to investigate bioterrorism. Biosecur. Bioterror. 2003;1:117–122. doi: 10.1089/153871303766275781. [DOI] [PubMed] [Google Scholar]
- 7.Danley L. Duties and difficulties of investigating and prosecuting biocrimes. J. Biosecur. Biosaf. Biodefense Law. 2012:3. [Google Scholar]
- 8.Schutzer S.E., Budowle B., Atlas R.M. Biocrimes, microbial forensics, and the physician. PLoS Med. 2005;2:e337. doi: 10.1371/journal.pmed.0020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allard M.W., Bell R., Ferreira C.M., Gonzalez-Escalona N., Hoffmann M., Muruvanda T., Ottesen A., Ramachandran P., Reed E., Sharma S. Genomics of foodborne pathogens for microbial food safety. Curr. Opin. Biotechnol. 2018;49:224–229. doi: 10.1016/j.copbio.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Araújo R., Pereira F., Asch Bv. Handbook of Forensic Genetics: Biodiversity and Heredity in Civil and Criminal Investigation. 2017. Applications of DNA-based methods in food forensics; pp. 493–517. [Google Scholar]
- 11.González-Candelas F. Handbook of Forensic Genetics: Biodiversity and Heredity in Civil and Criminal Investigation. World Scientific; 2017. Molecular epidemiology and evolution concepts in microbial forensics; pp. 561–582. [Google Scholar]
- 12.Oliveira M., Arenas M., António A. New trends in microbial epidemiology: can an old dog learn new tricks? Ann. Microbiol. Immunol. 2018;1:1–7. [Google Scholar]
- 13.Di Pasquale S., Paniconi M., Auricchio B., Orefice L., Schultz A.C., De Medici D. Comparison of different concentration methods for the detection of hepatitis A virus and calicivirus from bottled natural mineral waters. J. Virol. Methods. 2010;165:57–63. doi: 10.1016/j.jviromet.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Knutsson R., Van Rotterdam B., Fach P., De Medici D., Fricker M., Löfström C., Ågren J., Segerman B., Andersson G., Wielinga P. Accidental and deliberate microbiological contamination in the feed and food chains—how biotraceability may improve the response to bioterrorism. Int. J. Food Microbiol. 2011;145:S123–S128. doi: 10.1016/j.ijfoodmicro.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Khardori N., Kanchanapoom T. Overview of biological terrorism: potential agents and preparedness. Clin. Microbiol. Newsl. 2005;27:1–8. [Google Scholar]
- 16.Atlas R.M. Combating the threat of biowarfare and bioterrorism: defending against biological weapons is critical to global security. BioScience. 1999;49:465–477. [Google Scholar]
- 17.Eitzen E.M. Medical Aspects of Chemical and Biological Warfare. 1997. Use of biological weapons; pp. 437–450. [Google Scholar]
- 18.Szinicz L. History of chemical and biological warfare agents. Toxicology. 2005;214:167–181. doi: 10.1016/j.tox.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Christopher L.G.W., Cieslak L.T.J., Pavlin J.A., Eitzen E.M. Biological warfare: a historical perspective. JAMA. 1997;278:412–417. [PubMed] [Google Scholar]
- 20.Carus W.S. Government Printing Office; 2017. A Short History of Biological Warfare: From Pre-history to the 21st Century. [Google Scholar]
- 21.Barras V., Greub G. History of biological warfare and bioterrorism. Clin. Microbiol. Infect. 2014;20:497–502. doi: 10.1111/1469-0691.12706. [DOI] [PubMed] [Google Scholar]
- 22.Beeching N.J., Dance D.A., Miller A.R., Spencer R.C. Biological warfare and bioterrorism. BMJ. 2002;324:336–339. doi: 10.1136/bmj.324.7333.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rath J. Biological weapons, war crimes, and WWI. Science. 2002;296:1235–1237. doi: 10.1126/science.296.5571.1235c. [DOI] [PubMed] [Google Scholar]
- 24.Davison N. 2005. The Role of Scientific Discovery in the Establishment of the First Biological Weapons Programmes. [Google Scholar]
- 25.Reinhardt K. The Entomological Institute of the Waffen-SS: evidence for offensive biological warfare research in the third Reich. Endeavour. 2013;37:220–227. doi: 10.1016/j.endeavour.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 26.King W., Guillemin J. The price of alliance: Anglo-American intelligence cooperation and Imperial Japan’s criminal biological warfare programme, 1944–1947. Intell. Natl. Secur. 2019;34:263–277. [Google Scholar]
- 27.Eitzen E.M., Takafuji E.T. Medical Aspects of Chemical and Biological Warfare. 1997. Historical overview of biological warfare; pp. 415–423. [Google Scholar]
- 28.Frischknecht F. The history of biological warfare. EMBO Rep. 2003;4:S47. doi: 10.1038/sj.embor.embor849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franz D.R., Parrott C.D., Takafuji E.T. Vol. 425. 1997. The US biological warfare and biological defense programs. (Medical Aspects of Chemical And Biological Warfare). 36. [Google Scholar]
- 30.Roffey R., Tegnell A., Elgh F. Biological warfare in a historical perspective. Clin. Microbiol. Infect. 2002;8:450–454. doi: 10.1046/j.1469-0691.2002.00501.x. [DOI] [PubMed] [Google Scholar]
- 31.Hilleman M.R. Overview: cause and prevention in biowarfare and bioterrorism. Vaccine. 2002;20:3055–3067. doi: 10.1016/s0264-410x(02)00300-6. [DOI] [PubMed] [Google Scholar]
- 32.Tucker J.B. 1999. Biological Weapons in the Former Soviet Union: an Interview with Dr. Kenneth Alibek. [Google Scholar]
- 33.Zilinskas R.A. National Defense University Press; 2014. The Soviet Biological Weapons Program and Its Legacy in Today’s Russia. [Google Scholar]
- 34.Davis C.J. Nuclear blindness: an overview of the biological weapons programs of the former Soviet Union and Iraq. Emerging Infect. Dis. 1999;5:509. doi: 10.3201/eid0504.990408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zilinskas R.A. Iraq’s biological weapons: the past as future? JAMA. 1997;278:418–424. [PubMed] [Google Scholar]
- 36.Flibbert A. After Saddam: regional insecurity, weapons of mass destruction, and proliferation pressures in postwar Iraq. Polit. Sci. Q. 2003;118:547–567. [Google Scholar]
- 37.Jansen H.-J., Breeveld F.J., Stijnis C., Grobusch M.P. Biological warfare, bioterrorism, and biocrime. Clin. Microbiol. Infect. 2014;20:488–496. doi: 10.1111/1469-0691.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavlin J.A. Epidemiology of bioterrorism. Emerg. Infect. Dis. 1999;5:528. doi: 10.3201/eid0504.990412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehman D.C. Forensic microbiology. Clin. Microbiol. Newsl. 2014;36:49–54. [Google Scholar]
- 40.Rasko D.A., Worsham P.L., Abshire T.G., Stanley S.T., Bannan J.D., Wilson M.R., Langham R.J., Decker R.S., Jiang L., Read T.D. Bacillus anthracis comparative genome analysis in support of the Amerithrax investigation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5027–5032. doi: 10.1073/pnas.1016657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ecker D.J., Sampath R., Willett P., Wyatt J.R., Samant V., Massire C., Hall T.A., Hari K., McNeil J.A., Büchen-Osmond C. The Microbial Rosetta Stone Database: a compilation of global and emerging infectious microorganisms and bioterrorist threat agents. BMC Microbiol. 2005;5:19. doi: 10.1186/1471-2180-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CDC, C.f.D.C.P Possession, use, and transfer of select agents and toxins--Addition of Bacillus Cereus biovar Anthracis to the HHS list of select agents and toxins. Interim final rule and request for comments. Fed. Regist. 2016;81:63138. [PubMed] [Google Scholar]
- 43.Gilad J., Harary I., Dushnitsky T., Schwartz D., Amsalem Y. Burkholderia mallei and Burkholderia pseudomallei as bloterrorism agents: national aspects of emergency preparedness. IMAJ. 2007;9:499. [PubMed] [Google Scholar]
- 44.Yagupsky P., Baron E.J. Laboratory exposures to brucellae and implications for bioterrorism. Emerging Infect. Dis. 2005;11:1180. doi: 10.3201/eid1108.041197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes R.K. Diphtheria, other corynebacterial infections, and anthrax. Harrisons Principles Internal Med. 2001;1:909–914. [Google Scholar]
- 46.Glazunova O., Roux V., Freylikman O., Sekeyova Z., Fournous G., Tyczka J., Tokarevich N., Kovacova E., Marrie T.J., Raoult D. Coxiella burnetii genotyping. Emerg. Infect. Dis. 2005;11:1211. doi: 10.3201/eid1108.041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedewald V.E. Clinical Guide to Bioweapons and Chemical Agents. 2008. E coli 0157: h7; pp. 60–62. [Google Scholar]
- 48.Oyston P.C., Sjöstedt A., Titball R.W. Tularaemia: bioterrorism defense renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2004;2:967. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 49.Lee K.-M., Runyon M., Herrman T.J., Phillips R., Hsieh J. Review of Salmonella detection and identification methods: aspects of rapid emergency response and food safety. Food Control. 2015;47:264–276. [Google Scholar]
- 50.Azad A.F., Radulovic S. Pathogenic rickettsiae as bioterrorism agents. Ann. N. Y. Acad. Sci. 2003;990:734–738. doi: 10.1111/j.1749-6632.2003.tb07452.x. [DOI] [PubMed] [Google Scholar]
- 51.Anderson A.M., Varkey J.B., Petti C.A., Liddle R.A., Frothingham R., Woods C.W. Non-O1 Vibrio cholerae septicemia: case report, discussion of literature, and relevance to bioterrorism. Diagn. Microbiol. Infect. Dis. 2004;49:295–297. doi: 10.1016/j.diagmicrobio.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 52.Riedel S. Baylor University Medical Center Proceedings. (Taylor & Francis); 2005. Plague: From Natural Disease to Bioterrorism; pp. 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villar R.G., Elliott S.P., Davenport K.M. Botulism: the many faces of botulinum toxin and its potential for bioterrorism. Infect. Dis. Clin. North Am. 2006;20:313–327. doi: 10.1016/j.idc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Omernik A., Płusa T. Toxins of Clostridium perfringens as a natural and bioterroristic threats. Pol. Merkur. Lekarski. 2015;39:149–152. [PubMed] [Google Scholar]
- 55.Clarke S. Bacteria as potential tools in bioterrorism, with an emphasis on bacterial toxins. Br. J. Biomed. Sci. 2005;62:40–46. doi: 10.1080/09674845.2005.11732685. [DOI] [PubMed] [Google Scholar]
- 56.Ler S.G., Lee F.K., Gopalakrishnakone P. Trends in detection of warfare agents: detection methods for ricin, staphylococcal enterotoxin B and T-2 toxin. J. Chromatogr. A. 2006;1133:1–12. doi: 10.1016/j.chroma.2006.08.078. [DOI] [PubMed] [Google Scholar]
- 57.Bronze M.S., Huycke M.M., Machado L.J., Voskuhl G.W., Greenfield R.A. Viral agents as biological weapons and agents of bioterrorism. Am. J. Med. Sci. 2002;323:316–325. doi: 10.1097/00000441-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Mushtaq A., El-Azizi M., Khardori N. Category C potential bioterrorism agents and emerging pathogens. Infect. Dis. Clin. North Am. 2006;20:423–441. doi: 10.1016/j.idc.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sidwell R.W., Smee D.F. Viruses of the Bunya-and Togaviridae families: potential as bioterrorism agents and means of control. Antiviral Res. 2003;57:101–111. doi: 10.1016/s0166-3542(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 60.Mandell R., Flick R. Rift Valley fever virus: a real bioterror threat. J. Bioterror. Biodef. 2011:2. [Google Scholar]
- 61.Pigott D.C. Hemorrhagic fever viruses. Crit. Care Clin. 2005;21:765–783. doi: 10.1016/j.ccc.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krug R.M. The potential use of influenza virus as an agent for bioterrorism. Antiviral Res. 2003;57:147–150. doi: 10.1016/s0166-3542(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 63.Madjid M., Lillibridge S., Mirhaji P., Casscells W. Influenza as a bioweapon. J. R. Soc. Med. 2003;96:345–346. doi: 10.1258/jrsm.96.7.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenfield R.A., Lutz B.D., Huycke M.M., Gilmore M.S. Unconventional biological threats and the molecular biological response to biological threats. Am. J. Med. Sci. 2002;323:350–357. doi: 10.1097/00000441-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Shurtleff A. Bioterrorism and emerging infectious disease-antimicrobials, therapeutics and immune-modulators. SARS coronavirus. IDrugs. 2004;7:91–95. [PubMed] [Google Scholar]
- 66.Lam S.-K. Nipah virus—a potential agent of bioterrorism? Antiviral Res. 2003;57:113–119. doi: 10.1016/s0166-3542(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 67.Strassberg B.A. A pandemic of terror and terror of a pandemic: american cultural responses to HIV/AIDS and bioterrorism. Zygon. 2004;39:435–463. [Google Scholar]
- 68.Gibbs P. The foot-and-mouth disease epidemic of 2001 in the UK: implications for the USA and the “war on terror”. J. Vet. Med. Educ. 2003;30:121–132. doi: 10.3138/jvme.30.2.121. [DOI] [PubMed] [Google Scholar]
- 69.Breman J.G., Henderson D. Mass Medical Soc.; 1998. Poxvirus Dilemmas—Monkeypox, Smallpox, and Biologic Terrorism. [DOI] [PubMed] [Google Scholar]
- 70.Pennington H. Smallpox and bioterrorism. Bull. World Health Organ. 2003;81:762–767. [PMC free article] [PubMed] [Google Scholar]
- 71.Whitley R.J. Smallpox: a potential agent of bioterrorism. Antiviral Res. 2003;57:7–12. doi: 10.1016/s0166-3542(02)00195-x. [DOI] [PubMed] [Google Scholar]
- 72.Bourzac K. Smallpox: historical review of a potential bioterrorist tool. J. Young Investig. 2002:3. [Google Scholar]
- 73.Darling R.G., Catlett C.L., Huebner K.D., Jarrett D.G. Threats in bioterrorism I: CDC category a agents. Emerg. Med. Clin. North Am. 2002;20:273–309. doi: 10.1016/s0733-8627(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 74.Moran G.J. Threats in bioterrorism. II: CDC category B and C agents. Emerg. Med. Clin. North Am. 2002;20:311–330. doi: 10.1016/s0733-8627(01)00003-7. [DOI] [PubMed] [Google Scholar]
- 75.Carus W.S. Encyclopedia of Bioterrorism Defense. 2005. RISE: a case study. 1-1. [Google Scholar]
- 76.Carus W.S. Biological Threats in the 21st Century: the Politics, People, Science and Historical Roots. 2016. RISE, the Rajneeshees, Aum Shinrikyo and Bruce Ivins. 171. [Google Scholar]
- 77.Yabroff A. Encyclopedia of Bioterrorism Defense. 2005. Dark harvest. [Google Scholar]
- 78.Hammond P., Carter G. Springer; 2016. From Biological Warfare to Healthcare: Porton Down; pp. 1940–2000. [Google Scholar]
- 79.Bernett B.C. Naval Postgraduate School Monterey CA; 2006. US Biodefense & Homeland Security: Toward Detection & Attribution. [Google Scholar]
- 80.Koehler S.A. Defense Against Biological Attacks. Springer; 2019. The science of forensic epidemiology in detecting a biological attack (bioterrorism) pp. 69–104. [Google Scholar]
- 81.Council N.R. National Academies Press; 2014. Science Needs for Microbial Forensics: Developing Initial International Research Priorities. [PubMed] [Google Scholar]
- 82.Cáliz J., Triadó-Margarit X., Camarero L., Casamayor E.O. A long-term survey unveils strong seasonal patterns in the airborne microbiome coupled to general and regional atmospheric circulations. Proc. Natl. Acad. Sci. U. S. A. 2018;115:12229–12234. doi: 10.1073/pnas.1812826115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murch R.S. Bioattribution needs a coherent international approach to improve global biosecurity. Front. Bioeng. Biotechnol. 2015;3:80. doi: 10.3389/fbioe.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murch R.S. Designing an effective microbial forensics program for law enforcement and national security purposes. Arch. Immunol. Ther. Exp. 2014;62:179–185. doi: 10.1007/s00005-014-0289-7. [DOI] [PubMed] [Google Scholar]
- 85.Keim P.S., Budowle B., Ravel J. Microbial Forensics. Elsevier; 2011. Microbial forensic investigation of the anthrax-letter attacks; pp. 15–25. [Google Scholar]
- 86.Council N.R. National Academies Press; 2011. Review of the Scientific Approaches Used During the FBI’s Investigation of the 2001 Anthrax Letters. [PubMed] [Google Scholar]
- 87.Bush L.M., Perez M.T. The anthrax attacks 10 years later. Ann. Intern. Med. 2012;156:41–44. doi: 10.7326/0003-4819-155-12-201112200-00373. [DOI] [PubMed] [Google Scholar]
- 88.Cole L.A. The Anthrax letters: challenges and lessons. Med. Resp. Terror Threats. 2010;65:25. [Google Scholar]
- 89.Willman D. Bantam; 2011. The Mirage Man: Bruce Ivins, the Anthrax Attacks, and America’s Rush to War. [Google Scholar]
- 90.Kolavic S.A., Kimura A., Simons S.L., Slutsker L., Barth S., Haley C.E. An outbreak of Shigella dysenteriae type 2 among laboratory workers due to intentional food contamination. JAMA. 1997;278:396–398. [PubMed] [Google Scholar]
- 91.Scaduto D.I., Brown J.M., Haaland W.C., Zwickl D.J., Hillis D.M., Metzker M.L. Source identification in two criminal cases using phylogenetic analysis of HIV-1 DNA sequences. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21242–21247. doi: 10.1073/pnas.1015673107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Metzker M.L., Mindell D.P., Liu X.-M., Ptak R.G., Gibbs R.A., Hillis D.M. Molecular evidence of HIV-1 transmission in a criminal case. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14292–14297. doi: 10.1073/pnas.222522599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lemey P., Van Dooren S., Van Laethem K., Schrooten Y., Derdelinckx I., Goubau P., Brun-Vezinet F., Vaira D., Vandamme A.-M. Molecular testing of multiple HIV-1 transmissions in a criminal case. Aids. 2005;19:1649–1658. doi: 10.1097/01.aids.0000187904.02261.1a. [DOI] [PubMed] [Google Scholar]
- 94.Muchmore S. Former dentist faces another lawsuit. Tulsa World. 2013;18:A12. [Google Scholar]
- 95.González-Candelas F., Bracho M.A., Wróbel B., Moya A. Molecular evolution in court: analysis of a large hepatitis C virus outbreak from an evolving source. BMC Biol. 2013;11:76. doi: 10.1186/1741-7007-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vandamme A.-M., Pybus O.G. Viral phylogeny in court: the unusual case of the Valencian anesthetist. BMC Biol. 2013;11:83. doi: 10.1186/1741-7007-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lax A.J. Oxford University Press; 2005. Toxin: the Cunning of Bacterial Poisons. [Google Scholar]
- 98.Budowle B., Murch R., Chakraborty R. Microbial forensics: the next forensic challenge. Int. J. Legal Med. 2005;119:317–330. doi: 10.1007/s00414-005-0535-y. [DOI] [PubMed] [Google Scholar]
- 99.Buhk T.T. Arcadia Publishing; 2014. Poisoning the Pecks of Grand Rapids: The Scandalous 1916 Murder Plot. [Google Scholar]
- 100.Colgrass N. USA Today; 2016. Son, 24, Forgives Dad Who Injected Him With HIV: ‘That’s All I Can Do’. [Google Scholar]
- 101.In Digiborn; 1994. Moord Op Termijn Met Aidsbloed. [Google Scholar]
- 102.Katona P., Carus S. Global Biosecurity. Routledge; 2010. The history of bioterrorism, biowarfare, and biocrimes; pp. 63–82. [Google Scholar]
- 103.Morse S.A. Microorganisms and Bioterrorism. Springer; 2006. Historical perspectives of microbial bioterrorism; pp. 15–29. [Google Scholar]
- 104.Zilinskas R.A. Encyclopedia of Bioterrorism Defense. 2005. Diane Thompson: a case study. 1-1. [Google Scholar]
- 105.National Research Council . National Academies Press; 2007. The New Science of Metagenomics: Revealing the Secrets of Our Microbial Planet. [PubMed] [Google Scholar]
- 106.Khan A.S., Amara P.S., Morse S.A. Microbial Forensics. Elsevier; 2020. Forensic public health: epidemiological and microbiological investigations for biosecurity; pp. 105–122. [Google Scholar]
- 107.Budowle B., Connell N.D., Bielecka-Oder A., Colwell R.R., Corbett C.R., Fletcher J., Forsman M., Kadavy D.R., Markotic A., Morse S.A. Validation of high throughput sequencing and microbial forensics applications. Investig. Genet. 2014;5:9. doi: 10.1186/2041-2223-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ditzler G., Rosen G., Polikar R. Forensic identification with environmental samples. 2012 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP) 2012:1861–1864. [Google Scholar]
- 109.Forbes J.D., Knox N.C., Ronholm J., Pagotto F., Reimer A. Metagenomics: the next culture-independent game changer. Front. Microbiol. 2017;8:1069. doi: 10.3389/fmicb.2017.01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aßhauer K.P., Wemheuer B., Daniel R., Meinicke P. Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics. 2015;31:2882–2884. doi: 10.1093/bioinformatics/btv287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alves Ld.F., Westmann C.A., Lovate G.L., de Siqueira G.M.V., Borelli T.C., Guazzaroni M.-E. Metagenomic approaches for understanding new concepts in microbial science. Int. J. Genomics. 2018:2018. doi: 10.1155/2018/2312987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Woerner A.E., Novroski N.M., Wendt F.R., Ambers A., Wiley R., Schmedes S.E., Budowle B. Forensic human identification with targeted microbiome markers using nearest neighbor classification. Forensic Sci. Int. Genet. 2019;38:130–139. doi: 10.1016/j.fsigen.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 113.You H.S., Lee S.H., Ok Y.J., Kang H.-G., Sung H.J., Lee J.Y., Kang S.S., Hyun S.H. Influence of swabbing solution and swab type on DNA recovery from rigid environmental surfaces. J. Microbiol. Methods. 2019;161:12–17. doi: 10.1016/j.mimet.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 114.McLaren M.R., Willis A.D., Callahan B.J. Consistent and correctable bias in metagenomic sequencing experiments. Elife. 2019:8. doi: 10.7554/eLife.46923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schmedes S.E., Sajantila A., Budowle B. Expansion of microbial forensics. J. Clin. Microbiol. 2016;54:1964–1974. doi: 10.1128/JCM.00046-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mason-Buck G., Graf A., Elhaik E., Robinson J., Pospiech E., Oliveira M., Moser J., Lee P.K.H., Githae D., Ballard D., Bromberg Y., Casimiro-Soriguer C.S., Dhungel E., Ahn T.-H., Kawulok J., Loucera C., Ryan F., Walker A.R., Zhu C., Mason C.E., Amorim A., Court D.S., Branicki W., Labaj P. DNA based methods in intelligence-moving towards metagenomics. Preprints. 2020;2020 [Google Scholar]
- 117.Kreuzer-Martin H.W., Lott M.J., Dorigan J., Ehleringer J.R. Microbe forensics: oxygen and hydrogen stable isotope ratios in Bacillus subtilis cells and spores. Proc. Natl. Acad. Sci. U. S. A. 2003;100(3):815–819. doi: 10.1073/pnas.252747799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kreuzer-Martin H.W., Jarman K.H. Stable isotope ratios and forensic analysis of microorganisms. Appl. Environ. Microbiol. 2007;73(12):3896–3908. doi: 10.1128/AEM.02906-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trevisanato S.I. The ‘Hittite plague’, an epidemic of tularemia and the first record of biological warfare. Med. Hypotheses. 2007;69:1371–1374. doi: 10.1016/j.mehy.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 120.Grmek M.D. Ruses de guerre biologiques dans l'Antiquité. Rev. Études Grecques. 1979;92:141–163. [PubMed] [Google Scholar]
- 121.Frischknecht F. The history of biological warfare: Human experimentation, modern nightmares and lone madmen in the twentieth century. EMBO Rep. 2003;4:S47–S52. doi: 10.1038/sj.embor.embor849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Geissler E., van Courtland Moon J.E. Oxford University Press; 1999. Biological and Toxin Weapons: Research, Development and Use from the Middle Ages to 1945. [Google Scholar]
- 123.Wheelis M. Biological warfare at the 1346 siege of Caffa. Emerging Infect. Dis. 2002;8:971. doi: 10.3201/eid0809.010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Noah D.L., Huebner K.D., Darling R.G., Waeckerle J.F. The history and threat of biological warfare and terrorism. Emerg. Med. Clin. North Am. 2002;20:255–271. doi: 10.1016/s0733-8627(01)00002-5. [DOI] [PubMed] [Google Scholar]
- 125.Riedel S. Baylor University Medical Center Proceedings. Taylor & Francis; 2004. Biological warfare and bioterrorism: a historical review; pp. 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hugh‐Jones M. Wickham Steed and German biological warfare research. Intell. Natl. Secur. 1992;7:379–402. [Google Scholar]
- 127.Harris S.H. Routledge; 1932. Factories of Death: Japanese Biological Warfare, 1995-45, and the American Cover-up. [Google Scholar]
- 128.Harris S.H. 2002. Scientists and the Cover-Up. [Google Scholar]