Highlights
-
•
The absolute values of CD3+, CD4+, and CD8+ T cells are lower in COVID-19 patients.
-
•
The levels of IL-6 and IL-10 are increased in COVID-19 patients.
-
•
The absolute values of CD4+ T cells are decreased in COVID-19 patients.
-
•
The severity of lung lesions predicts poor clinical outcomes.
-
•
CT signal may be a predictor of the transition from mild to severe.
Keywords: 2019 novel coronavirus, Lymphocyte subsets, Cytokines, Radiology
Abstract
Objectives
To study the correlations of lymphocytes and cytokines between changes of lung lesion volumes in patients with COVID-19, and to predict these correlations.
Methods
93 patients with COVID-19 were divided into mild and severe groups. The data of lymphocyte subgroups and cytokines were collected, the imaging characteristics were measured, and correlation analysis was performed to analyze the differences.
Results
60 mild and 33 severe patients were included. Lymphocyte subsets decreased in both groups. The reduction percentages of the absolute lymphocytes value in mild and severe groups were 32% and 64%, respectively. The lung CT lesion volume of all patients was 241.45 ± 282.92 cm3, among which the mild group was 151.29 ± 226.04 cm3, and the severe group was 405.38 ± 304.90 cm3, respectively. In critically ill patients, the decrease of the absolute value of CD4+ T cells and the increase of IL-6 levels are significantly correlated with the volume of lung lesions.
Conclusions
The absolute values of CD3+, CD4+, and CD8+ T cells are lower in patients with COVID-19, while the levels of IL-6 and IL-10 are increased. The severity of lung lesions predicts poor clinical outcomes and may be a predictor of the transition from mild to severe.
Introduction
A group of unexplained pneumonia patients was been found in Wuhan, China, in December 2019. Some of them developed severe symptoms of acute respiratory infections, and some rapidly developed into acute respiratory distress syndrome and other serious complications. The Chinese Center for Disease Control and Prevention (CDC) identified a novel β-coronavirus in airway epithelial cells of patients; it was named 2019-nCoV by the WHO (Munster et al., 2020). So far, infections have also been found in other cities in China and more than a dozen countries globally, and there is increasing evidence that human-to-human transmission exists (Phan et al., 2020, Guan et al., 2020, Huang et al., 2020).
Previous studies have shown that increased pro-inflammatory cytokines in the serum of patients with SARS are associated with lung inflammation and extensive lung injury (Metlay et al., 2019). There have been many reports that most 2019-nCoV patients have chest CT manifestations of pneumonia, typically showing bilateral ground-glass shadows and patchy shadows; a few CT images may appear as consolidation shadows and interstitial lesions. Laboratory results show that the lymphocytes count in most patients decreased (Wang et al., 2020, Wong et al., 2004, Metlay et al., 2019, Mahallawi et al., 2018); with a gradually worsening disease, the lymphocytes absolute count continued to decline (Mahallawi et al., 2018). There are reports in the literature that the pro-inflammatory cytokines IL-2, IL-6, IL-8, IL-10, and TNF-α are elevated in some 2019-nCoV patients (Wong et al., 2004, Metlay et al., 2019). The purpose of this study is to investigate changes in lymphocyte counts and cytokine levels induced by 2019-nCoV and their effects on lung lesions, to determine the severity of the disease, and to select markers that could prompt early clinical intervention.
Methods
Patient informed consent
This study was a single-center retrospective study that recruited 93 2019-nCoV patients admitted to Taizhou Public Health Medical Center in Zhejiang Province from January 17 to February 12, 2020. Taizhou Public Health Center is the designated hospital for 2019-nCoV patients in Taizhou City, Zhejiang Province. According to the arrangements of the Chinese government, all patients diagnosed with 2019-nCoV pneumonia in Taizhou City, according to the WHO Interim Guidelines (Guan et al., 2020), were obligated to be transferred to this center, where they were given standard diagnosis and treatment. RT-Realtime PCR confirmed that all patients were positive for the novel coronavirus nucleic acid. All patient data have been reported to the WHO. According to the WHO NCP Interim Guidelines, patients were divided into mild and severe (including severe and critical) groups. This study was approved by the Ethics Committee of Enze Hospital, Zhejiang Enze Medical Group (Center), and written informed consent was obtained from the patients before the collection of retrospective data.
Data acquisition
The patient’s clinical symptoms, signs, laboratory test results, and treatment measures are all from electronic medical records. Data related to other medical institutions are obtained directly by communicating with their attending physicians. The clinical results were followed up until February 27, 2020. The laboratory tests involved in this study include the absolute value of lymphocytes, CD3+ T, CD4+ T, CD8+ T, B cell, NK cell, IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ.
Measurement of CT lesions
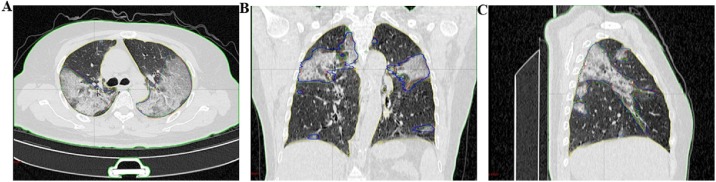
We defined the patients’ lung lesions, including ground glass shadows, patch shadows, consolidation areas, interstitial lesions, and nodular shadows as our regions of interest (ROI) (Figure 1). To ensure the accuracy of the measurement, we had three physicians (all with more than five years of experience) measure the ROI area. The number of measurements for each patient depends on the extent and scope of the lung lesions. The average value is the final measurement value.
Figure 1.
A 65-year-old female patient was admitted to the hospital due to “fever and cough for 8 days”; B coronal position; C, sagittal position; (the red, yellow and green lines are the regions of interest drawn by three senior doctors, with volumes of 596.61 cm3, 585.71 cm3, and 622.23 cm3, respectively).
Statistical analysis
Baseline characteristics, laboratory findings, and radiology were compared using Chi-square analysis. The variables of CT lesion area, lymphocytes, and cytokines were compared using independent group t-tests. The relationship of CT lesion area and peripheral blood lymphocytes was examined using the χ2 test. All statistical analyses were performed using SAS version 7.0 software. For unadjusted comparisons, a 2-sided α of less than 0.05 was considered statistically significant.
Results
2019. -nCoV patients
93 patients with 2019-nCoV infection were included in our study. Patients were divided into mild and severe groups according to the WHO NCP Interim Guidelines. Table 1 summarizes the characteristics of 2019-nCoV patients, among which 60 were in the mild group (65%). and 33 were in the severe group (35%). The median age of the patients in the mild group was 44 (44.5 ± 12.5) years-old, and the median age of the patients in the severe group was 54(53.9 ± 12.5) years. There was no statistical difference. This study shows that the most common symptoms of patients with this disease are fever (75%), followed by cough (65%), fatigue (24%), dyspnea (22%), myalgia (6%), sore throat (12%), and diarrhea (9%) are rare. Twenty-four patients had at least one underlying disease, including 14 cases of hypertension, seven cases of diabetes, and two cases of COPD. The severe group (42%) was statistically different from the mild group (17%); the number of patients with diabetes in the severe group was more than that in the mild group, P < 0.05
Table 1.
Baseline characteristics of patients infected with 2019-nCoV.
| All patients (n = 93) | Mild group (n = 60) | Severe group (n = 33) | P-value | |
|---|---|---|---|---|
| Characteristics | ||||
| Age, years | 49 (47.9 ± 13.2) | 44 (44.5 ± 12.5) | 54 (53.9 ± 12.5) | 0.0009 |
| Sex | ||||
| Men | 49 (53%) | 31(52%) | 18 (55%) | 0.7902 |
| Women | 44 (47%) | 29(48%) | 15 (45%) | 0.7902 |
| Current smoking | 5 (5%) | 4 (7%) | 1 (3%) | 0.4569 |
| Comorbidity | 24 (26%) | 10 (17%) | 14 (42%) | 0.0066 |
| Hypertension | 14 (15%) | 6 (10%) | 8 (24%) | 0.0661 |
| Diabetes | 7 (8%) | 1 (2%) | 6 (18%) | 0.0039 |
| Chronic obstructive pulmonary disease | 2 (2%) | 1 (2%) | 1 (3%) | 0.6645 |
| Signs and symptoms | ||||
| Fever | 70 (75%) | 45 (75%) | 25 (76%) | 0.9354 |
| Cough | 60 (65%) | 38 (63%) | 22 (67%) | 0.7479 |
| Sputum production | 21 (23%) | 14(23%) | 7 (21%) | 0.8149 |
| Dyspnoea | 20 (22%) | 8 (13%) | 12 (36%) | 0.0097 |
| Myalgia | 6 (6%) | 5 (8%) | 1 (3%) | 0.3192 |
| Fatigue | 22 (24%) | 18 (30%) | 4 (12%) | 0.0522 |
| Diarrhea | 8 (9%) | 6 (10%) | 2 (6%) | 0.5168 |
| Headache | 5 (5%) | 3 (5%) | 2 (6%) | 0.8282 |
| Dizzyness | 7 (8%) | 6 (10%) | 1 (3%) | 0.2229 |
| Pharyngalgia | 11 (12%) | 8 (13%) | 3 (9%) | 0.5444 |
Data are n (%) unless specified otherwise.
Laboratory and radiology
Table 2 shows the results of laboratory tests after admission. 40/93 patients (43%) had an absolute decrease in lymphocytes, among which 19 (32%) patients were mild, and 21 (64%) patients were severe; there were statistical differences. T-cell subsets were tested in 73 patients, including 48 mild patients and 25 severe patients. Among them, the absolute value of CD8+ T cell decreased in 34 (47%) patients, among which 17 patients were mild (35% of total mild patients), 17 cases of severe (68% of severe cases); there was a statistical difference P < 0.05. It was observed that some patients had a decrease in CD3+ T cell (55%), CD4+ T cell (53%), B cell (21%), and NK cell (32%), but there was no statistical difference between mild or severe groups. All 93 patients were tested for IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ. There were 72 (77%) patients with elevated IL-6, of which 42 were in mild patients (70% of the mild group), and 30 were in severe patients (91% of the severe group); there was a statistical difference between the two groups. There were 29 (31%) patients with elevated IL-10, of which 13 were mild (22% of the mild group), and 16 were severe (48% of the severe group); there was a statistical difference between the two groups. IL-2, IL-4, TNF-α, and IFN-γ were normal in all patients.
Table 2.
Laboratory findings of patients infected with 2019-nCoV.
| All patients |
Mild group |
Severe group |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| Level | N | n, % | N | n, % | N | n, % | ||
| lymphocytes | Decreased | 93 | 40 (43%) | 60 | 19(32%) | 33 | 21(64%) | 0.0029 |
| CD3+T | Decreased | 73 | 40(55%) | 48 | 22(46%) | 25 | 18(72%) | 0.0957 |
| CD4+T | Decreased | 73 | 39(53%) | 48 | 21(44%) | 25 | 18(72%) | 0.0676 |
| CD8+T | Decreased | 73 | 34(47%) | 48 | 17(35%) | 25 | 17(68%) | 0.0263 |
| B Cell | Decreased | 73 | 15(21%) | 48 | 12(25%) | 25 | 3(12%) | 0.1711 |
| NK Cell | Decreased | 73 | 23(32%) | 48 | 13(27%) | 25 | 10(40%) | 0.3557 |
| IL-2 | Normal | 93 | 93 (100%) | 60 | 60(100%) | 33 | 33 (100%) | NA |
| IL-4 | Normal | 93 | 93 (100%) | 60 | 60(100%) | 33 | 33 (100%) | NA |
| IL-6 | Increased | 93 | 72 (77%) | 60 | 42 (70%) | 33 | 30 (91%) | 0.0210 |
| IL-10 | Increased | 93 | 29(31%) | 60 | 13(22%) | 33 | 16(48%) | 0.0076 |
| TNF-α | Normal | 93 | 93 (100%) | 60 | 60 (100%) | 33 | 33 (100%) | NA |
| IFN-γ | Normal | 93 | 93 (100%) | 60 | 60 (100%) | 33 | 33 (100%) | NA |
All patients underwent chest CT scans; 71 (76%) patients had lesions involving three or more lung lobes, including all 33 critically ill patients. Lung CT showed ground-glass opacity or patchy shadowing in 88 (95%) patients, 50 (54%) patients with a thickened lobular septum, 40 (43%) with consolidation, and 25 (27%) combined with nodular shadows, there were four (4%) mild patients with no abnormal chest CT. Among them, severe patients had much more chances of consolidation than mild patients, which was statistically different (Table 3 ).
Table 3.
Radiographic findings of patients infected with 2019-nCoV.
| All patients (n = 93) | Mild group (n = 60) | Severe group (n = 33) | P value | |
|---|---|---|---|---|
| Ground-glass opacity or patchy shadowing | 88 (95%) | 55 (92%) | 33 (100%) | 0.0882 |
| Septal thickening | 50 (54%) | 29 (48%) | 21 (64%) | 0.1567 |
| Consolidation | 40 (43%) | 19 (32%) | 21 (64%) | 0.0029 |
| Nodule | 25 (27%) | 15 (25%) | 10 (30%) | 0.5810 |
| Number of lobes involved ˂3 | 18 (19%) | 18 (30%) | 0 (0%) | 0.0004 |
| Number of lobes involved ≥3 | 71 (76%) | 38 (63%) | 33 (100%) | 0.0004 |
| Normal | 4 (4%) | 4 (7%) | 0 (0%) | 0.0004 |
As shown in Table 4 , the absolute values of lymphocytes, CD3+ T cell, CD4+ T cell, and CD8+ T cell in the severe group had a more significant decrease than those in the mild group (P < 0.05). The levels of IL-6 and IL-10 in the severe group were higher than those in the mild group, which was statistically significant (P < 0.05). The lesion volumes in lung CT in all patients were 241.45 ± 282.92 cm3. The volume of lung lesions in the mild group, 151.29 ± 226.04 cm3, was significantly smaller than that in the severe group (405.38 ± 304.90 cm3, P < 0.001).
Table 4.
Comparison of lymphocyte subsets, cytokines and CT lesion volume in peripheral blood between mild and severe patients.
| All patients |
Mild group |
Severe group |
P-value | ||||
|---|---|---|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | ||
| Lymphocytes, ×109/l | 93 | 1.1 (0.7) | 60 | 1.25 (0.63) | 33 | 0.9 (0.5) | 0.0001 |
| CD3+T cell, /μl | 73 | 715.5 (577.13) | 48 | 821.86 (526.19) | 25 | 465.28 (505.67) | 0.0011 |
| CD4+T cell, /μl | 73 | 403.5 (258.8) | 48 | 475.68 (263.57) | 25 | 277.07 (306.09) | 0.001 |
| CD8+T cell, /μl | 73 | 260.11 (252.78) | 48 | 302.35 (256.25) | 25 | 185.75 (166.3) | 0.0062 |
| B cell, /μl | 73 | 151.36 (121.25) | 48 | 146.37 (136.01) | 25 | 154.06 (89.84) | 0.6811 |
| NK cell, l/μl | 73 | 198.28 (137.65) | 48 | 212.47 (160.21) | 25 | 176.25 (123.55) | 0.1907 |
| CD4+T/CD8+T | 73 | 1.58 (0.97) | 48 | 1.68 (0.9) | 25 | 1.46 (0.96) | 0.4376 |
| IL-2, pg/ml | 93 | 1.3 (1.14) | 60 | 1.35 (1.16) | 33 | 1.07 (0.55) | 0.1202 |
| IL-4, pg/ml | 93 | 1.63 (1.08) | 60 | 1.67 (1.06) | 33 | 1.52 (0.91) | 0.5513 |
| IL-6, pg/ml | 93 | 5.97 (12.81) | 60 | 4.63 (11.58) | 33 | 12.66 (22.01) | 0.0427 |
| IL-10, pg/ml | 93 | 3.55 (3.38) | 60 | 3.49 (2.13) | 33 | 4.93 (6.13) | 0.011 |
| TNF-α, pg/ml | 93 | 1.26 (1.11) | 60 | 1.36 (1.3) | 33 | 1.13 (0.77) | 0.1164 |
| IFN-γ, pg/ml | 93 | 2.02 (1.53) | 60 | 1.99 (1.64) | 33 | 2.1 (1.51) | 0.7734 |
| CT lesion volume, cm3 | 93 | 119.65 (302.16) | 60 | 80.49 (145.51) | 33 | 334.25 (396.13) | 0.0001 |
Correlation of lung lesion volume with lymphocyte subsets and cytokines
In the mild group, the volume of the lung lesions was related to the absolute value of CD3+ T cells and in severe patients to the absolute value of CD8+ T cells. However, the volume of the lung lesions was significantly correlated to the absolute value of CD4+ T cells, P < 0.05. Although all patients’ NK cells were within the normal range, the absolute value of the NK cells in the severe group was negatively correlated with the volume of lung lesions, P < 0.05 (Table 5). Whether in the light group or the severe group, the size of the lung lesion volume was positively correlated with the increase of IL-6, P < 0.05 (Table 6 ).
Table 5.
Correlation analysis between CT lesion volume and peripheral blood lymphocytes, cytokines.
| Mild group (n = 60) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lymphocytes | CD3+T | CD4+T | CD8+T | B Cell | NK Cell | CD4+T/CD8+T | |||
| r | −0.2418 | −0.343 | −0.258 | −0.3285 | −0.141 | −0.123 | 0.2218 | ||
| P value | 0.0628 | 0.0170 | 0.0756 | 0.0226 | 0.3385 | 0.4023 | 0.1298 | ||
| Severe group (n = 33) | |||||||||
| Lymphocytes | CD3+T | CD4+T | CD8+T | B Cell | NK Cell | CD4+T/CD8+T | |||
| r | −0.4707 | −0.362 | −0.404 | −0.255 | −0.0851 | −0.496 | −0.1414 | ||
| P value | 0.0057 | 0.0754 | 0.0451 | 0.2180 | 0.6860 | 0.0117 | 0.5001 | ||
Table 6.
Correlation analysis between CT lesion volume and cytokines.
| Mild group (n = 60) | ||||||
|---|---|---|---|---|---|---|
| IL-2 | IL-4 | IL-6 | IL-10 | TNF-α | IFN-γ | |
| r | −0.127 | 0.0184 | 0.3479 | 0.2143 | 0.0907 | 0.0642 |
| P value | 0.3322 | 0.8892 | 0.0065 | 0.1001 | 0.4905 | 0.6260 |
| Severe group (n = 33) | ||||||
| IL-2 | IL-4 | IL-6 | IL-10 | TNF-α | IFN-γ | |
| r | −0.039 | 0.0382 | 0.5001 | 0.2413 | 0.0766 | −0.1812 |
| P value | 0.8270 | 0.8327 | 0.0030 | 0.1761 | 0.6719 | 0.3130 |
Results
Similar to the study by Mahallawi et al. (2018), severe patients are generally older and have more comorbidities. In in this study we suggests that age and comorbidities are risk factors for adverse outcomes.
The clinical characteristics of 2019-nCoV infection are similar to those of previous beta coronaviruses such as SARS-CoV and MERS-CoV infection. In this study, the majority of patients presented with fever and dry cough, and a few presented dyspnea, sore throat, nasal congestion, and diarrhea. Most patients’ lung CT showed a bilateral distribution of ground glass shadow, patchy shadow, and lobular septum thickening. Patients may show consolidation shadows and nodular shadows; a few patients have no obvious abnormalities in lung CT, similar to previous studies (Wang et al., 2020, Wong et al., 2004, Metlay et al., 2019, Mahallawi et al., 2018). The average age in this study is 49 years old. In this study there was no significant difference between men and women, suggesting that 2019-nCoV is generally susceptible to the population, which is different from previous reports (Wang et al., 2020).
Lymphocyte subsets play an essential role in human cellular immune regulation. Studies have shown that the drastic reduction in the total number of lymphocytes indicates that the coronavirus has consumed many immune cells and inhibited the body’s cellular immune function. The damage to T lymphocytes may be a critical factor leading to the deterioration of patients’ conditions (Wan et al., 2020). Recently, the literature also pointed out that the decline of the absolute value of lymphocytes and the severity of chest CT manifestations predict poor clinical outcomes (Wang et al., 2020), consistent with the current literature (Venet et al., 2009). Our study also showed that the absolute number of lymphocytes decreased in most patients, including CD3, CD4, CD8, B cells, and NK cells. In comparison with mild and severe patients, we found that CD4+ T, CD8+ T decreased, IL-6, IL-10 elevation is statistically significant; it was found that patients with more profound lung lesions have more severely reduced lymphocytes counts, and the reduction is negatively correlated to the area of lung lesions. It can be seen that, like SARS-CoV, 2019nCoV may mainly affect Lymphocytes, especially T (B, NK) lymphocytes, causing cellular immune deficiency.
SARS-CoV infection can induce a series of pro-inflammatory cytokines (IL-1β, IL-6, and IL-12) and Th1 cytokine IFN-γ significantly increased (Leemans et al., 2002), MERS-CoV infection can also induce the release of inflammatory factors (IFN-γ, TNF-α, IL-15, and IL-17) (Liu et al., 2017), and recently published literature also shows that the serum levels of IL1β, IL-4.IL-6, IL-10, IFN-γ, IP10, MCP1, and TNF-α are increased in 2019-nCoV infected patients (Wong et al., 2004). This study found that the serum levels of IL-2, TNF-α, and IFN-γ were normal in patients, which is different from MERS-CoV infection and recent studies. 2019-nCoV infection also led to the suppression of inflammation-associated Th2-related increase of IL-4 and IL10, which is different from SARS-CoV infection, which may be one of the reasons for the milder symptoms of 2019-nCoV infection patients compared with SARS-nCoV infected patients (Leemans et al., 2002).
This study found that the serum levels of IL-2, TNF-α, and IFN-γ were normal in patients, which is different from MERS-CoV infection and recent studies (Wong et al., 2004, Liu et al., 2017;16), suggesting that 2019-nCoV infection may not cause an inflammatory response.
T-helper-1 (Th1) cells.
SImilar to previous studies (Wong et al., 2004), 2019-nCoV infection led to an increase in IL-6 and IL10 associated with T-helper-2 (Th2), which inhibits inflammation; the levels of IL-6 and IL-10 increased significantly in severe patients compared with mild patients, which is different from SARS-CoV infection (Leemans et al., 2002). This study’s results indicate that Th2-cell-associated IL-6 and IL-10 are involved in humoral immunity in patients with severe disease, which is higher than those in patients with mild disease, indicating that patients with severe 2019-nCoV infection have stronger immune suppression. The above results suggest that we need to further study the response characteristics of Th1 and Th2 in 2019-nCoV infection to clarify its pathogenesis.
In this paper Th2 related cytokines IL-6, IL-6, and IL-10 are low or normal, which is similar to previous SARS and other viral pneumonia findings (Venet et al., 2009) (6, 10 are not similar). Still, there are also studies showing that 2019-nCoV Infection causes increased secretion of T-helper-2 (Th2) cytokines (such as IL-4 and IL-10) that suppress inflammation (Wong et al., 2004), which is different from SARS-CoV infection (Leemans et al., 2002). This study’s results indicate that Th2 cell-associated IL-6 and IL-10 in the severe group are higher than those in the mild group, indicating that the immunosuppressive effect of patients with severe infection is stronger. The above results suggest that we need to further study the response characteristics of Th1 and Th2 in 2019-nCoV infection to clarify its pathogenesis.
Infection with SARS-CoV (Metlay et al., 2019), MERS-CoV (Leemans et al., 2002), and 2019-nCoV can induce an increase in cytokines, and early studies have shown that increased inflammatory cytokines are related to lung inflammation and acute lung damage (Metlay et al., 2019), IL-6 may play a pro-inflammatory role in pulmonary inflammation (Mo et al., 1995) and is closely related to mortality in ARDS patients (Parsons et al., 2005). Our study observed that IL-6 increased significantly in patients with severe inflammation in the lungs, and its level changes accordingly as inflammation increases and absorbs. It is suggested that the IL-6 monoclonal antibody may reduce lung inflammation and be used to treat 2019-nCoV infections. It also suggests that for severe patients, early detection, early application of immunoglobulins to boost patients’ anti-infective ability, early application of corticosteroids, reduction of alveolar damage, and pulmonary exudation may improve patient prognosis.
Our study is not flawless. First, as a retrospective study, some clinical data is not complete. Not all patients were tested for lymphocyte subsets and cytokines. A lot of cytokines are not included, such as IL-1β, IL-8, IL-12, IP-10, CSF, and transforming growth factor (TGF). It is, therefore, difficult to clearly explain the changes of various cytokines caused by 2019-nCoV infection and their impact on lung inflammation. In the future, more cytokine and chemokine studies are needed in prospective studies to clarify their potential as a prognostic indicator of the severity of the 2019-nCoV disease. We will continue to follow-up cured and discharged patients, and regularly monitor the peripheral blood lymphocyte subsets, cytokines, chest CT, and lung function to reveal the impact of 2019-nCoV infection on long-term human survival.
Funding
This work was supported by the Science and Technology Foundation of Taizhou (number 1801KY18).
Acknowledgments
This study was funded by the Science and Technology Foundation of Taizhou (number 1801KY18). We thank all patients involved in the study, and we acknowledge all health-care workers involved in the diagnosis and treatment of patients in Taizhou, China.
References
- Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of 2019 novel coronavirus infection in China. The New England Journal of Medicine. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X. Clinical features of patients with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans J.C., Vervoordeldonk M.J., Florquin S. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am J Respir Crit Care Med. 2002;165:1445–1450. doi: 10.1164/rccm.2106045. [DOI] [PubMed] [Google Scholar]
- Liu W.J., Zhao M., Liu K. Tcell immunity of SARSCoV: implications for vaccine development against MERSCoV. Antiviral Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahallawi W.H., Khabour O.F., Zhang Q. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J.P., Waterer G.W., Long A.C. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Disease Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo X.Y., Sarawar S.R., Doherty P.C. Induction of cytokines in mice with parainfluenza pneumonia. J Virol. 1995;69:1288–1291. doi: 10.1128/jvi.69.2.1288-1291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster V.J., Koopmans M., van Doremalen N. A novel coronavirus emerging in China — key questions for impact assessment. N Engl J Med. 2020 doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- Parsons P.E., Eisner M.D., Thompson B.T. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33(January (1)):1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230–232. [DOI] [PubMed] [Google Scholar]
- Phan L.T., Nguyen T.V., Luong Q.C., Nguyen Thinh V., Nguyen Hieu T., Le Hung Q. Importation and human-to-human transmission of a novel coronavirus in Vietnam. New England Journal of Medicine. 2020;382(9) doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venet F., Chung C.S., Huang X. Lymphocytes in the development of lung inflammation: a role for regulatory CD4+ T cells in indirect pulmonary lung injury. J Immunol. 2009;183(September (5)):3472–3480. doi: 10.4049/jimmunol.0804119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S.X., Yi Q.J., Fan S.B. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) MedRxiv preprint. 2020 doi: 10.1101/2020.02.10.20021832. [DOI] [Google Scholar]
- Wang D.W., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.K., Lam C.W.K., Wu A.K.L. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]