Teaser
We review the evolution of our clinical understanding of COVID-19, possible therapeutic targets, and prospects for COVID-19 management.
Introduction
During the early weeks of December 2019, the first case of pneumonia caused by the novel coronavirus defined as COVID-19 by the WHO was reported in Wuhan, the capital city of Hubei province, China [1]. Local daily-wage employees of the Huanan Seafood Market in Wuhan were believed to be the initial COVID-19 carriers, infecting a large proportion of the population of Wuhan 2, 3. Since its emergence and as of, June 24, 2020, COVID-19 had infected ∼85098 individuals across China. At the time of completion of the review-draft (by 24th June 2020), the Iran, Italy, Chile, Peru, Spain, UK, India, Russia, Brazil, and USA had recorded the highest population count of COVID-19 cases, ranging from 209,970 to 2,424,492,819 to 1,573, (www.worldometers.info/coronavirus/). Thus far, ∼ 9,373,424 cases of COVID-19 have been recorded worldwide, with the total number of deaths reaching 480,140, and the number of recovered patients 5,062,840, (www.worldometers.info/coronavirus/).
Although SARS-CoV-2-encoded proteins share similar homologous structures with SARS-CoV, the spike (S) ectodomain of the COVID-19 virus shows a higher binding affinity (∼15 nM) for the angiotensin-converting enzyme 2 (ACE2) receptor protein of the upper bronchial system, which is ∼10–20-fold higher compared with SARS-CoV. Thus, this facilitated the unprecedented transmission of COVID-19 among humans [4]. The SARS-CoV-2 strain can spread through all modes of physical contact, including sneezing and coughing [5].
A higher level of COVID-19 cases, ∼87%, has been recorded among the adult and older population groups (30–79 years of age) [6]. Contradictorily, a moderate (3%) and a small (1%) number of cases have been recorded in the older population group (≥80 years old) and the younger population group (10–19 years) [6]. Concomitantly, people with prior respiratory ailments and metabolic complications, such as type 2 diabetes mellitus, hypertension, cardiovascular complications, and cancer, are highly susceptible to COVID-19 infection with increased mortality 6, 7. Consistent with earlier clinical evidence that SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) affected males more than females [8], the prevalence of COVID-19 has been recorded in the male population at a higher rate compared with that of the female population [3]. It might be that estrogen receptor activation and its associated signaling cascades in females confer increased protection against infection with COVID-19, similar to the results of clinical-based research studies with SARS-CoV and MERS-CoV [9].
Moreover, some patients with SARS-CoV-2 have become infected with virus from asymptomatic carriers who do not show any obvious clinical symptoms, such as flu, tiredness, fever, and dry cough, but are able to pass-on COVID-19 to healthy individuals either by direct or indirect physical contact through nasal droplets and sneezing [10]. In addition to this major barrier to the control of COVID-19 spread, it is difficult to control the spread of disease from recovered patients to healthy individuals 11, 12. In this review, we focus on clinical trials involving drugs against COVID-19, potential clinical therapeutic targets, and the future directions of COVID-19 management.
Clinical lessons learned, current therapeutic molecules, and prospects for COVID-19 management
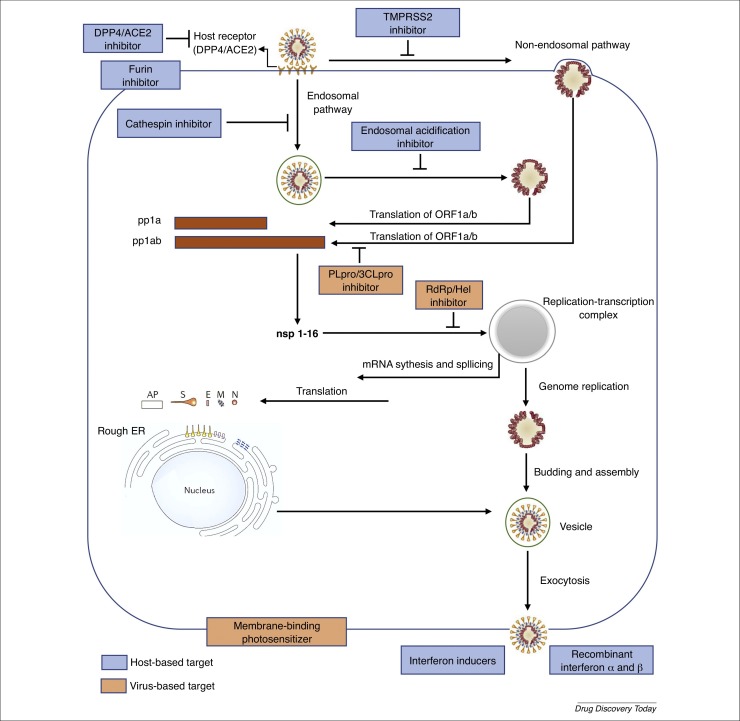
As depicted in Fig. 1 , all nonstructural proteins (NSPs), which represent virus-based targets, are crucial for the design of therapeutic efforts to ameliorate colonization of the virus in the host, especially in the upper bronchial system. The catalytic sites of the functional NSPs of the Coronaviridae can be targeted to attenuate SARS-CoV, MERS-CoV, and SARS-CoV-2 virulence. Moreover, the functional NSPs interact with the host ACE2 to enable coronaviral entry to the cells [13].
Figure 1.
Virus-based and host-based targets against the coronavirus replication cycle. Coronaviruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) gain the entry into the host cell via the endosomal pathway and/or the cell surface non-endosomal pathway. Viral translation, replication, assembling and exocytosis then occur using key proteins, which could be potential therapeutic targets against SARS-CoV-2. Abbreviations: 3CLpro, 3-chymotrypsin-like cysteine protease; ACE2, angiotensin-converting enzyme 2; AP, accessory protein; DPP4, dipeptidyl peptidase 4; E, envelope; ER, endoplasmic reticulum; Hel, helicase; M, membrane; N, nucleocapsid; ORF, open reading frame; PLpro, protease papain-like protease; RdRp, RNA-dependent RNA polymerase; S, spike; TMPRSS2, transmembrane serine proteases 2.
Given the current lack of vaccines to either attenuate or prevent COVID-19 transmission, targeting any of the crucial invasion steps of SARS-CoV-2, such as the virus entry, transcription and translation, genome synthesis and assembly, and virus release would be effective in reversing its pathogenesis and transmission in humans.
To target the initial colonization of SARS-CoV-2, research has focused on the use of potential antiviral agents used against other viruses, such as SARS-CoV, MERS-CoV, hepatitis B, hepatitis C, HIV, and common influenza viruses [14].
Past lessons
During the initial stages of the COVID-19 outbreak, from late December 2019 to early February 2020, during 40,000 cases were confirmed and 850 people died across China, patients in clinical trials were treated with drugs that were found to be generally ineffective at against the virus. One such drug was oseltamivir, selected because COVID-19 symptoms include fever and other symptoms common to influenza infections [15]. Oseltamivir is a potent neuraminidase inhibitor that effectively attenuates the virulence of influenza viruses A and B, but did show any noticeable effects against COVID-19 because the virus does not secrete neuraminidase [16]. Moreover, the treatment of patients with COVID-19 with antibacterial drugs, including moxifloxacin, ceftriaxone, and azithromycin, either as a single drug or in combination, showed little health benefit [17]. In addition, the long-term use of a higher dose of antibiotics during the COVID-19 outbreak was found to be linked with the adverse clinical symptoms of severe respiratory ailments, including hyperinflammation, shock, circulatory impairment, and other organ damage.
Corticosteroids mimicking the natural corticosteroids of the human body have been commonly used to treat patients with defective adrenal glands who fail to synthesize sufficient levels of corticosteroids [18]. However, corticosteroids have also been widely prescribed at a higher dose in patients with immunological disorders, inflammation, and/or an impaired salt/water balance [18]. Nevertheless, there is no clear clinical evidence of the core therapeutic benefits of corticosteroids in treating respiratory symptoms of respiratory syncytial virus (RSV), common influenza viruses, SARS-CoV, and MERS-CoV 19, 20. Research was conducted to evaluate the efficacy of corticosteroids in low to mild doses for treating the adverse clinical symptoms induced by coronaviruses [21]. Concomitantly, patients with COVID-19 were treated with corticosteroids as a supplementary drug for a minimal duration of 3–15 days, with no substantial improvement in symptoms 6, 15. This shortened treatment time was because corticosteroid are well known for their long-term adverse effects and other secondary level complications 16, 22.
Thus, this lack of success with the aforementioned drugs also contributed to the increased mortality among patients with COVID-19 across China, alongside impairments in the early diagnosis and treatment of COVID-19; delayed action from healthcare professionals to break the virus transmission chain; a poor understanding of COVID-19 virulence and its transmission efficacy among the common population; and inadequate clinical diagnostic kits and other essential medical facilities, such as respiratory ventilators, protective medical gowns and gloves, to handle patients critically ill with COVID-19 [23].
Present therapeutics with clinical significance for COVID-19 management
Chemical agents
To manage the emerging COVID-19 outbreak and its associated mortality, ∼300 clinical trials have been performed on patients with COVID-19 in China [24]. Some of the clinical drugs used in these trials have shown promising results in reversing COVID-19 clinical symptoms [25] (Table 1 ).
Table 1.
Summary of anti-SARS-CoV-2 compounds against COVID-19 currently in clinic trials
| Names | Structure | targets | Original indication | Activity against SARS-CoV-2 reported | No. of clinic trials | Refs |
|---|---|---|---|---|---|---|
| Lopinavir/ritonavir | 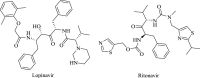 |
Protease; 3CLpro; CYP3A4 | HIV | Yes | 14 | [26] |
| Ribavirin | 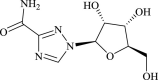 |
RdRp | HCV and RSV | Yes (EC50 = 109.5 μM) | 2 | 27, 28 |
| Chloroquine | 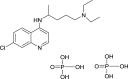 |
Endosomal acidification | Malaria | Yes (EC50 = 1.13 μM) | 20 | [28] |
| Hydroxychloroquine | 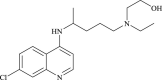 |
Endosomal acidification | Malaria | No | 10 | |
| Arbidol | 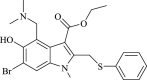 |
Clathrin-dependent trafficking | Influenza | Yes (EC50 = 10–30 μM) | 8 | [29] |
| Dipyridamole | 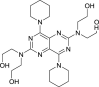 |
Phosphodiesterase | Ischemic heart disease | Yes (EC50 = 100 nM) | 1 | [30] |
| Darunavir/cobicistat | 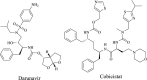 |
3CLpro | HIV | Yes | 2 | [31] |
| Remdesivir | 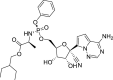 |
RdRp | Ebola virus | Yes (EC50 = 0.77 μM) | 2 | [28] |
| Favipiravir | 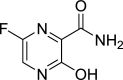 |
RdRp | Influenza | Yes (EC50 = 62 μM) | 4 | [28] |
| Emtricitabine/tenofovir alafenamide | 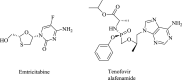 |
Reverse transcriptase | HIV | No | 1 | |
| Azvudine | 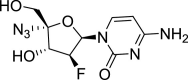 |
Reverse transcriptase | HIV | No | 3 | |
| ASC09/ritonavir (ASC09F) | 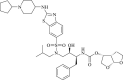 |
3CLpro | HIV | No | 6 | |
| Baloxavir marboxil | 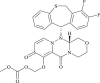 |
Endonuclease | Influenza | No | 2 | |
| Oseltamivir | 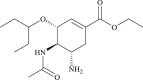 |
Neuraminidase | Influenza | No | 2 |
Abbreviations: 3CLpro: 3-chymotrypsin-like cysteine protease; CYP3A4, cytochrome P450 3A4; HCV, hepatitis C virus; RdRp, RNA-dependent RNA polymerase; RSV, respiratory syncytial virus
To tackle the SARS-CoV epidemic, lopinavir and ritonavir drug combinations [US Food and Drug Administration (FDA) approved] that inhibit viral 3-chymotrypsin-like cysteine protease [33], supplemented with ribavirin, effectively controlled SARS-CoV virulence and the associated mortality rate [34]. Such outcomes resulted in ∼14 clinical trials using lopinavir and ritonavir drug combinations to treat patients with COVID-19. That lopinavir and ritonavir drug combinations effectively attenuated the adverse clinical symptoms, such as fever, of five patients with COVID-19 demands further clinical validation [35]. By contrast, a recent clinical trial using these drug combinations failed to show any noticeable therapeutic effects on the adult patients with COVID-19 over other patients who received standard medication [36]. Thus, there is a need for a more in-depth clinical trial on these drug combinations.
Ribavirin, another FDA-approved effective antiviral drug commonly used to treat hepatitis C virus and RSV, has been widely referred to in combinations with effective antibiotics and/or with or without hormonal treatment [16]. As a potent inhibitor of the viral RNA-dependent RNA polymerase (RdRp), ribavirin effectively controlled COVID-19 virulence when co-administered with ritonavir/lopinavir drug combinations [27].
Given that chloroquine derivatives exhibited a strong inhibitory action against SARS-CoV colonization 16, 37, clinicians showed that chloroquine derivatives together with remdesivir effectively controlled the proliferation of a clinically isolated SARS-CoV-2 strain [28]. A clinical trial using chloroquine phosphate to treat COVID-19 virulence has since been approved by the National Health Commission of the People's Republic of China [38].
Arbidol, a potent antiviral drug targeting virus-associated inflammatory cytokines, has been used to treat patients with COVID-19 and severe pneumonia; it has not shown any adverse effects and, thus, is under study in both China and Russia [39]. In addition, both in vitro and in vivo studies have explored the additional therapeutic advantages of arbidol as a drug that exhibits a strong immune response by disrupting the viral capsid binding the host cell membrane 16, 40. Arbidol has been prioritized with the other potential clinical drugs discussed earlier for clinical trials to tackle the COVID-19 outbreak, supported by the sixth edition of Guidelines for the Prevention, Diagnosis, and Treatment of Novel Coronavirus-induced Pneumonia [XX].
Dipyridamole, an antiplatelet and phosphodiesterase inhibitor drug that targets intracellular cAMP/cGMP levels, including positive-stranded RNA viruses, has been suggested to be an effective antiviral drug 41, 42. Dipyridamole effectively inhibited the replication of SARS-CoV at a half-maximal effective concentration (EC50) concentration of 100 nM in vitro [30]. This clinical proficiency of dipyridamole emphasized its possible usage as an adjuvant to strengthen the immune system as well as to inhibit viral proliferation and hypercoagulation [30].
Darunavir, a potent retrovirus inhibitor, together with cobicistat, which controls cytochrome P4503A (CYP3A) activity resulting in the breakdown of antiviral agents, has been used to treat patients with HIV [43]. Although Darunavir is intended to inhibit viral proteinases [31], research is required to prove its clinical significance in reversing COVID-19 virulence.
Animal studies showed that remdesivir inhibited the virulence of SARS-CoV and MERS-CoV [32]. In vitro research also confirmed that remdesivir treatment profoundly inhibited SARS-CoV-2 proliferation at an EC50 concentration: 0.77–1.76 μM [28].
Favipiravir, which markedly inhibits influenza-dependent RNA polymerase, exhibits profound antiviral activity against many viruses, including arenavirus, bunyavirus, and filovirus, which result in fatal hemorrhagic fever 16, 44. In addition to these viruses, favipiravir treatment effectively inhibited the proliferation of SARS-CoV-2 in Vero E6 culture cells, with an EC50 value of 62 μM [32]. Based on this positive outcome, the Ministry of Science and Technology, China recently recommended favipiravir for COVID-19 management in a larger group of patients [45].
Other potential drugs of high therapeutic value include baloxavir and marboxil, which target the viral cap-dependent endonuclease (although this is absent in SARS-CoV-2) [32]; TMC310911 targets viral protease activity [46]; emtricitabine/tenofovir alafenamide and azvudine target the viral reverse transcriptase and have been tested on patients with COVID-19 47, 48. Although these clinical drugs showed promising therapeutic effects against COVID-19 virulence, there is still a need for an in-depth clinical trial to confirm their efficacy in a larger group of patients with COVID-19.
The pharmacokinetics (PK) of all emerging COVID-19 drugs, such as absorption, distribution, metabolism and excretion, including the druggable effect on the host body (pharmacodynamics; PD), can be studied using PK/PD modeling [49]. A PK/PD drug model would reveal the correlation between drug exposure and its associated PD effect in silico [49]. Of the empirical and mechanistic models of PK/PD available, empirical models that comprise direct-link, spline-function, logistic-regression, and circadian models could be used to study disease progression in patients with COVID-19 with or without drug exposure in silico [49]. Mechanistic model studies, which primarily rely on data from clinical biomarkers in the context of COVID-19 virulence in the presence or absence of a drug, would confer a brief clinical understanding of the druggable effect against COVID-19 in humans and other species as well as the determined drug dosage for COVID-19 management [49]. PK/PD analyses are conducted at three various levels: Level 1 reveals the direct correlation between the drug exposure and its relevant response using a plot graph with the measured unbound plasma drug concentration plotted against the relevant PD response (in vivo); this generates the effective drug dosage concentration based on the ratio of mean unbound plasma drug concentration/half-maximal inhibitory concentration (IC50); Level 2 generates the PD response turnover rate, K out, in response to the noticeable changes in the drug and biological system; Level 3 (with the supportive pre-established models) unravels the pharmacological response to the drug at various dosages among the patients involved in the experimental study. The correlation of biomarkers with the generated PK/PD models would also provide a mechanistic understanding of the mechanism of action of the drugs to enable translational research and intersubject drug evaluation on a wider scale [49].
Biological agents
Convalescent plasma therapy using clinically procured blood plasma samples of patients with a particular virus has been adopted to treat patients with SARS-CoV-2 [50]. It has been proposed that convalescent blood plasma (CBP) infusion into such patients would effectively attenuate the pathogenicity of the virus, with its eventual removal from the patient's blood. Although the methodology of CBP transfusion has certain constraints for clinical trials, clinical treatment using the CBP of patients with COVID-19 has been considered to be both promising and effective for treating patients critically ill with COVID-19. To test CBP transfusion on a large scale, certain clinical factors must be taken into consideration, such as the transmission of intermittent pathogens between the donor and the recipient and the precise recruitment of donors with sufficient immunoglobulin titers to produce noticeable effects against the pathogenicity of that particular pathogen in the patients [51].
Bone marrow-derived mesenchymal stem cells (MSCs) have been used to treat patients with acute respiratory distress syndrome (ARDS), with no adverse effects recorded during the relevant trial [52]. To date, there are 13 clinical trials in progress to manage SARS-CoV-2 using MSCs, with promising initial clinical results in seven patients with COVID-19, who showed a remarkable improvement in their clinical condition within 14 days of treatment without any noticeable adverse effects.
Interferon-alpha (IFN-α), a potent immune cytokine released during pathogen infection, improved pulmonary function when coupled with other antiviral agents, such as lopinavir, ritonavir, and remdesivir, in patients with MERS-CoV [53]. The therapeutic combination of ribavirin and IFN-α was acknowledged in the sixth edition of Guidelines for the Prevention, Diagnosis, and Treatment of Novel Coronavirus-induced Pneumonia [XX].
Patients with COVID-19 and other severe health conditions have shown increased circulatory levels of proinflammatory cytokines, especially interleukin 6 (IL-6), which might be responsible for adverse clinical symptoms, such as septic shock, organ tissue damage associated with heart, liver, and kidney, and respiratory dysfunction [54]. In one ongoing clinical trial (ChiCTR2000029765), clinicians are targeting the increased levels of IL-6 using the IL-6-specific monoclonal antibody tocilizumab to treat patients critically ill with COVID-19. Clinical results from 21 Chinese patients with COVID-19 showed a reduced body fever after treatment improved their respiratory function [55]. These results suggested targeting additional circulatory proinflammatory cytokines, such as IL-1 and IL-17, using cytokine-specific neutralizing antibodies [55]. Following successful results from these clinical trials, it would be possible to adopt a proficient biological strategy to treat COVID-19 virulence in immunocompromised patients. A monoclonal antibody labeled ‘CR3022’, raised against the receptor-binding domain of the S membrane glycoprotein of SARS-CoV-2, could benefit patients by disrupting its colonization in the upper respiratory system [56]. Also, monoclonal antibodies generated specifically against the functional proteins of SARS-CoV-2 and/or its potential agonist ACE2, which controls viral entry, would significantly benefit patients in terms of a quick recovery and a restricted transmission rate.
Future clinical strategies for COVID-19 management
To tackle the COVID-19 outbreak within a short timeframe, treatment using drug repurposing against the virus- and host-based targets could resolve similar clinical issues in the future. In the long term, the development of novel-multifaceted-pan-CoV antiviral drugs against coronaviruses might result in an efficacious treatment for SARS-CoV-2. A profound activation of the bitter taste receptors [taste 2 receptor member 4 (T2R4); taste 2 receptor member 38 (T2R38); taste 2 receptor member 43 (T2R43) and taste 2 receptor member 46 (T2R46)] using bitter taste compounds, including nicotine (as agonists), could attenuate COVID-19 virulence with increased intracellular calcium-dependent nitric oxide (NO) production accompanied by reduced secretion of the proinflammatory cytokines in the upper respiratory system 57, 58, 59. This increased NO production further strengthens the ciliary beat frequency with the resulting mucociliary clearance of the invading pathogens [57]. The idea of triggering the innate immune response by the activation of bitter taste receptors, and reducing the production of proinflammatory cytokines by the stimulation of ACE2 and neuronal acetylcholine receptors using nicotine, will be validated in patients with COVID-19 in the near future.
Concluding remarks
All of proposed therapeutic strategies discussed herein, including the on-going clinical trials of COVID-19 management, have to overcome substantial obstacles, such as the possibility of spontaneous mutation of SARS-CoV-2, restricted animal models for preclinical studies, a lack of patients for clinical study, the high maintenance costs of experimental set-ups, and retention of the sustainability of clinical-based therapeutic study outcomes. In addition, all the basic criteria of each clinical trial must be well studied and abide by the proper clinical guidelines, regardless of study type. Only with such concerted research efforts is the research community likely to be successful in its search for a therapeutic with proven effects against COVID-19.
Conflict of interest
None declared.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Project No.81903623 and U1804283), Henan Province Novel Coronavirus Control and Prevention Emergency Science and Technology Tackling Key Project (201100311500) and Henan Medical Science and Technology Program (2018020601).
References
- 1.WHO . WHO; 2020. Novel Coronavirus – China. [Google Scholar]
- 2.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12:24. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai C.C. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Ruan Q. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlberg J. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am. J. Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaillon S. Sexual dimorphism in innate immunity. Clin. Rev. Allergy Immunol. 2019;56:308–321. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 10.Hu Z. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan L. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie X. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zumla A. Coronaviruses—drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan Y. Advance of promising targets and agents against COVID-19 in China. Drug Discov. Today. 2020;25:810–812. doi: 10.1016/j.drudis.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen N. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yousefi B. A global treatments for coronaviruses including COVID-19. J. Cell Physiol. 2020 doi: 10.1002/jcp.29785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKay L.I., J.A C. Physiologic and pharmacologic effects of corticosteroids, Ch. 62. In: Kufe D.W., editor. Holland–Frei Cancer Medicine. 6th edn. BC Decker; 2003. [Google Scholar]
- 19.Siemieniuk R.A. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann. Intern. Med. 2015;163:519–528. doi: 10.7326/M15-0715. [DOI] [PubMed] [Google Scholar]
- 20.Russell C.D. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stockman L.J. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang L. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H. Early lessons from the frontline of the 2019-nCoV outbreak. Lancet. 2020;395:687. doi: 10.1016/S0140-6736(20)30356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chinese Clinical Trials Register . Chinese Clinical Trials Register; 2020. Clinical Trials about COVID-19 in China. [Google Scholar]
- 25.Liu W. Effective chemicals against novel coronavirus (COVID-19) in China. Curr. Top. Med. Chem. 2020;20:603–605. doi: 10.2174/1568026620999200305145032. [DOI] [PubMed] [Google Scholar]
- 26.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 27.Lennon A. Four potential treatments for SARS-CoV-2 currently under testing. Journal. 2020 https://www.labroots.com/trending/drug-discovery-and-development/17097/4-potential-treatments-sars-cov-2-currently-testing#. [Google Scholar]
- 28.Wang M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X.W. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X. Therapeutic effects of dipyridamole on COVID-19 patients with coagulation dysfunction. medRxiv. 2020;29:2020. doi: 10.1101/2020.02.27.20027557. [DOI] [Google Scholar]
- 31.Beck B.R. Predicting commercially available antiviral drugs that may act on the novel coronavirus (2019-nCoV), Wuhan, China through a drug–target interaction deep learning model. bioRxiv. 2020 doi: 10.1101/2020.01.31.929547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 33.Nukoolkarn V. Molecular dynamic simulations analysis of ritonavir and lopinavir as SARS-CoV 3CL(pro) inhibitors. J. Theor. Biol. 2008;254:861–867. doi: 10.1016/j.jtbi.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu C.M. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young B.E. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao B. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincent M.J. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao J. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 39.Boriskin Y.S. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr. Med. Chem. 2008;15:997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 40.Blaising J. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fata-Hartley C.L., Palmenberg A.C. Dipyridamole reversibly inhibits mengovirus RNA replication. J. Virol. 2005;79:11062–11070. doi: 10.1128/JVI.79.17.11062-11070.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gresele P. Anti-platelet therapy: phosphodiesterase inhibitors. Br. J. Clin. Pharmacol. 2011;72:634–646. doi: 10.1111/j.1365-2125.2011.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deeks E.D. Cobicistat: a review of its use as a pharmacokinetic enhancer of atazanavir and darunavir in patients with HIV-1 infection. Drugs. 2014;74:195–206. doi: 10.1007/s40265-013-0160-x. [DOI] [PubMed] [Google Scholar]
- 44.Furuta Y. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wehner M. China says it found a flu drug that can treat coronavirus. BGR. 2020 March 18. [Google Scholar]
- 46.Gupta S., Senapati S. Mechanism of inhibition of drug-resistant HIV-1 protease clinical isolates by TMC310911: a molecular dynamics study. J. Mol. Struct. 2019;1198:126893. [Google Scholar]
- 47.Nirogi R. Simultaneous quantification of a non-nucleoside reverse transcriptase inhibitor efavirenz, a nucleoside reverse transcriptase inhibitor emtricitabine and a nucleotide reverse transcriptase inhibitor tenofovir in plasma by liquid chromatography positive ion electrospray tandem mass spectrometry. Biomed. Chromatogr. 2009;23:371–381. doi: 10.1002/bmc.1125. [DOI] [PubMed] [Google Scholar]
- 48.Wang R.R. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug–resistant strains than lamivudine in vitro. PLoS ONE. 2014;9:e105617. doi: 10.1371/journal.pone.0105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu X.Q., Wilson A.G. The role of pharmacokinetic and pharmacokinetic/pharmacodynamic modeling in drug discovery and development. Future Med. Chem. 2010;2:923–928. doi: 10.4155/fmc.10.181. [DOI] [PubMed] [Google Scholar]
- 50.Cheng Y. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong H.K. Practical limitations of convalescent plasma collection: a case scenario in pandemic preparation for influenza A (H1N1) infection. Transfusion. 2010;50:1967–1971. doi: 10.1111/j.1537-2995.2010.02651.x. [DOI] [PubMed] [Google Scholar]
- 52.Wilson J.G. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir. Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheahan T.P. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lythgoe M.P., Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol. Sci. 2020;41:363–382. doi: 10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian X. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah A.S. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Devillier P. The pharmacology of bitter taste receptors and their role in human airways. Pharmacol. Ther. 2015;155:11–21. doi: 10.1016/j.pharmthera.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Farsalinos K. Editorial: Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol. Rep. 2020;7:658–663. doi: 10.1016/j.toxrep.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]