Since it emerged in December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread to all parts of the world, infecting more than 5 million people and directly causing more than 340 000 deaths.1 Coronavirus disease 2019 (COVID-19), the disease caused by SARS-CoV-2, was initially viewed as primarily a respiratory disease, in some cases leading to a viral pneumonia. It is now recognised as a complex disorder affecting many body systems, which, especially in older males, can require treatment in ICUs. Amongst those admitted to intensive care, a substantial proportion will die. This new disease has generated a surge of research, with more than 4000 papers published in 1 week in May 2020.2 Here, we attempt to summarise emerging evidence for anaesthetists and intensive care specialists caring for patients with COVID-19.
Infection and host defence
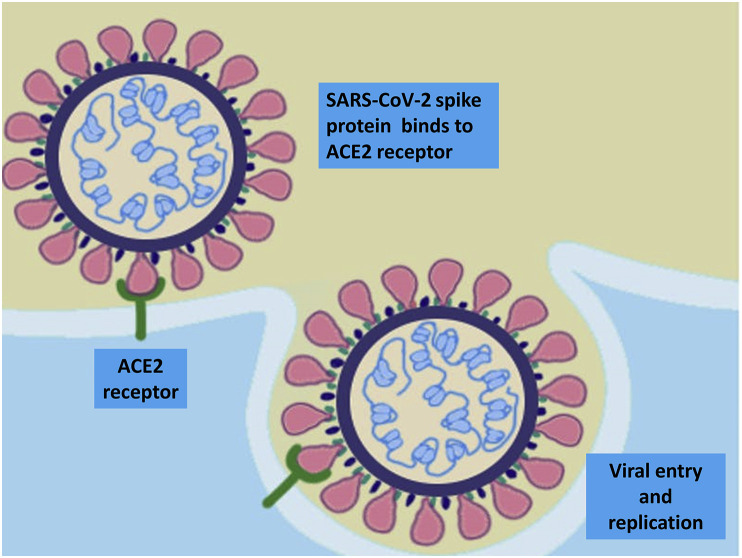
COVID-19 starts when SARS-CoV-2 is transmitted from one human to another via inhalation or oral ingestion of virus-containing droplets. The virus likely enters epithelial cells in the nasal or oral cavity through SARS-CoV-2 spike protein binding with the angiotensin-converting enzyme 2 (ACE2) receptor (Fig 1 ). Virus–receptor binding results in fusion of the viral envelope and cell membranes with entry of the viral nucleocapsid into the cell enabling viral mRNA to hijack host cell ribosomes to translate viral code, thereby leading to generation of viral proteins and ultimately virus replication.3 Initially, there is local propagation of the virus and a limited innate immune response, but at this stage, infected individuals can already infect others. Viral load is highest around the time of symptom onset and decreases over the following 5–7 days, with viable virus no longer cultivable beyond 10 days from symptom onset.4 These features likely account for the high transmissibility of the virus. SARS-CoV-2 propagates and travels down the respiratory tract, and a more robust innate immune response is triggered, characterised by the occurrence of systemic pro-inflammatory cytokines and activated immune cells. By then, COVID-19 may clinically manifest with, in most cases, self-limiting mild-to-moderate symptoms of an upper respiratory tract infection and general symptoms, such as myalgia and fatigue. In about 20% of patients, the virus will infect alveolar cells, once again via the ACE2 receptor.
Fig 1.
SARS-CoV-2 spike protein binding with angiotensin-converting enzyme 2 (ACE2) receptor enables viral envelope fusion with the host cell membrane and subsequent entry of the viral nucleocapsid into the cell.
In the most severely affected patients, an exaggerated immune response occurs as a cytokine ‘storm’, characterised by extreme concentrations of pro-inflammatory cytokines (such as tumour necrosis factor-α and interleukins), granulocyte colony-stimulating factor, and several chemokines.5 This pattern mimics secondary haemophagocytic lymphohistiocytosis, an under-recognised hyperinflammatory syndrome characterised by fulminant hypercytokinaemia and multiorgan failure. It also resembles the cytokine release syndrome that is seen as a complication of chimaeric antigen receptor T-cell therapy for lymphoproliferative malignancies and other forms of cancer. Because of the prominent role of the cytokine interleukin-6 (IL-6) in these processes, treatment of severe COVID-19 with monoclonal anti-soluble IL-6 receptor antibody (tocilizumab) has been proposed.6
COVID-19 coagulopathy and thromboembolic complications
Severe COVID-19 causes a specific coagulopathy that is reminiscent of, but also distinct from, other systemic coagulopathies associated with severe infections, such as disseminated intravascular coagulation or thrombotic microangiopathy.7 Pro-inflammatory cytokines, in particular IL-6, stimulate mononuclear cells to express tissue factor, leading to thrombin generation, thereby initiating a systemic coagulopathy. Superimposed on this low-grade coagulation activation, direct infection of endothelial cells causes release of plasminogen activator (explaining the very high D-dimer concentrations in severe COVID-19) and large von Willebrand factor multimers.8 The massive release of these multimers overwhelms the cleaving capacity of its physiological regulator ADAMTS13 (a disintegrin and metalloprotease) resulting in high concentrations of uncleaved von Willebrand factor mediating the consequent deposit of microvascular platelet thrombi, especially in affected pulmonary vessels.9
Direct viral infection of endothelial cells, which express ACE2 enabling entry of the virus, can result in widespread endothelial dysfunction associated with recruitment of a vascular inflammatory response, which is more exaggerated in patients with pre-existent vascular disease.8 The simultaneous presence of vascular inflammation and coagulopathy might explain the high incidence of thromboembolic complications in patients with COVID-19. Indeed, markers of coagulopathy, such as D-dimer, have been closely associated with thrombotic complications and increased mortality.10
These systemic inflammatory responses manifest as a varied and wide-ranging clinical syndrome. Lung, heart, brain, and kidneys are the organs with major clinicopathological manifestations requiring ICU management to support life.
COVID-19 and the lungs
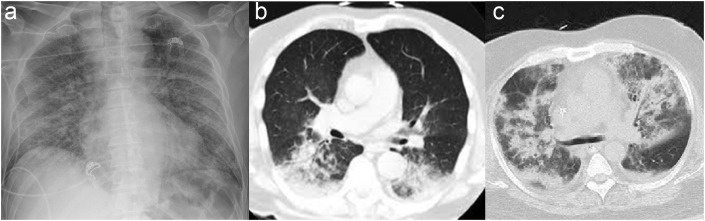
SARS-CoV-2 infection is associated with three main types of lung damage. The typical clinical manifestation is viral pneumonia, seen in the early stages on CT imaging as small focal areas of ground-glass opacity (GGO). These changes are typically prominent in the peripheral lung bases and are bilateral in 75% of patients. Lymphadenopathy, pleural effusions, and lung nodules are uncommon (<5%).11 Patients can exhibit significant hypoxaemia but with preserved lung compliance, a low ventilation-to-perfusion ratio, and low lung recruitability.12 Dual-energy CT findings of preferentially increased perfusion surrounding areas of lung opacification support the notion that loss of regional pulmonary vasoconstriction contributes to these pathophysiological changes, but the mechanism is still unclear. Clinically, ‘tachypnoea without dyspnoea’ is observed, a feature also described in other severe viral pneumonias, including pandemic influenza (H1N1) in 1918. Figure 2 illustrates a plain chest radiograph of COVID-19 pneumonia and CT of COVID-19 pneumonitis and acute respiratory distress syndrome (ARDS).
Fig 2.
(a) Plain chest radiograph of COVID-19 pneumonia. (b) Chest CT of COVID-19 pneumonitis. (c) Chest CT of COVID-19 acute respiratory distress syndrome.
A second manifestation that occurs in a minority of patients usually in the second week from symptom onset is a dysfunctional immune response, in which massive activation of cytokines is triggered. Markedly elevated concentrations of pro-inflammatory cytokines and chemokines attract immune cells to the lungs. The resulting lung damage, with desquamation of alveolar cells, hyaline membrane formation, and pulmonary oedema, is manifest as ARDS, with widespread bilateral consolidation or GGO on imaging accompanied by progression to hypoxaemic respiratory failure.11 This stage corresponds with findings of decreased lung compliance, high right-to-left shunting, and high lung recruitability.12 Postmortem findings include diffuse alveolar damage with patchy inflammation dominated by lymphocytes (CD3-, CD8-, and CD4-positive T cells, and few CD20-positive B cells) and thrombi in small pulmonary arteries.13
These different lung injuries warrant different management strategies. Patients with viral pneumonia may tolerate higher tidal volumes (7–9 ml kg−1) than recommended for ARDS (6 ml kg−1) along with lower than recommended levels of PEEP.14 In contrast, the ARDS-like clinical picture should be managed with classical ARDS Network ventilation strategies, including tidal volumes of 6 ml kg−1, higher levels of PEEP, and trials of prone positioning.14 Considerable controversy exists as to whether noninvasive ventilatory support, such as CPAP, can enable some patients with viral pneumonia and relatively normal lung compliance to avoid invasive mechanical ventilation with some centres claiming considerable success with this approach. Prone positioning is widely practised and has been shown to improve oxygenation, but impact on clinical outcomes is uncertain.15 An unconventional approach that has achieved widespread adoption during the COVID-19 pandemic is ‘self-proning’: the voluntary adoption of the prone position, according to medical advice, in conscious patients receiving noninvasive respiratory support.15
The third type of lung injury arises from venous thromboembolism (VTE), including pulmonary emboli. Despite routine thromboprophylaxis, studies from France, Italy, and the Netherlands report VTE in 35–47% of ICU-treated patients.16 The British Thoracic Society suggests higher doses of thromboprophylaxis for these patients, whilst noting the likely increased risk of bleeding with such an approach and the absence of clinical trial evidence. In the event of unexpected clinical deterioration, a high index of suspicion for VTE is warranted.
COVID-19 and the heart
The basic mechanisms for viral infection and replication described previously have specific relevance with regard to the cardiovascular system. The two groups of patients particularly at risk of severe disease are those with a history of either hypertension or coronary artery disease.17 One explanation might be that ACE2 is a functional receptor for the virus. Whether angiotensin-converting enzyme or angiotensin receptor blocker drugs have an impact on COVID-19 disease remains unclear, and at present, it is recommended that patients continue these drugs.18 Cardiovascular manifestations occur in two forms: direct cardiac injury and indirect damage secondary to haemodynamic compromise. Even during the early phase of mild symptomatic disease, cardiac enzyme release can be observed, indicating myocardial inflammation and damage.19 This is usually subclinical, but may present in its most extreme form as a myocarditis.20 , 21 Even in the subclinical form, cardiac enzyme release is a strong predictor of mortality.19
COVID-19 myocarditis can mimic myocardial infarction, and distinction of myocardial infarction and myocarditis can be challenging with some patients presenting with chest pain as the primary symptoms in the absence of pyrexia and other disease manifestations.22 During the early experience of the disease, patients underwent invasive coronary angiography, but now that this phenomenon is recognised, the widespread ST elevation allows clinical distinction from the more regional ECG changes characteristic of a coronary artery occlusion.
Fatal ventricular arrhythmias in COVID-19 appear to be directly linked to the degree of cardiac enzyme release, and therefore related to myocardial damage rather than arrhythmogenicity of the disease itself.23 Therapies that produce QT prolongation have been associated with torsade de pointes.24 Around 19% of new intensive care admissions would be expected to develop new onset atrial fibrillation,25 but whilst arrhythmias do occur in 16% of patients, atrial fibrillation only makes up a proportion of this and is not a prominent feature in COVID-19.26 This is remarkable given the haemodynamic and inflammatory stress that COVID-19 presents.
Pulmonary embolus in COVID-19 is relatively frequent and can cause catastrophic cardiovascular collapse. Patients with ARDS are vulnerable to pulmonary oedema, but in COVID-19, maintaining a negative fluid balance increases thrombogenicity and exacerbates the cardiovascular collapse associated with pulmonary hypertension. As the right heart begins to fail in the face of high pulmonary vascular resistance, high venous filling pressures are necessary to maintain cardiac output. Adequate hydration/fluid therapy may also mitigate risk of renal failure, which results in 20–30% of these patients needing renal replacement therapy.
COVID-19 and the kidneys
Renal damage may result from direct infection of kidney cells by SARS-CoV-2 or indirect harm secondary to intensive care interventions required to manage failure of other organs. Like endothelial cells, proximal renal tubular cells have ACE2 receptors, and in the kidney, both may be infected by SARS-CoV-2, creating direct and indirect mechanisms of nephron injury.27 As elsewhere in the body, endothelial injury is implicated in formation of microvascular thromboses. Importantly, renal injury may also be a consequence of several critical care interventions,28 for example, where reduced oral or i.v. fluid administration intended to minimise pulmonary vascular congestion results in inadequate renal perfusion. Elevated PEEP and high driving pressures (peak–mean pressure) can impair renal function as a result of reduced renal blood flow caused by increased pressure in the renal veins. Similarly, reduced cardiac output and blood pressure reduce renal blood flow, an effect compounded by the cardiovascular depressant effects of sedation/analgesia.
COVID-19 and the brain
Neurological complications of COVID-19 infection result from a number of factors: direct invasion of neurological tissue by the virus, virus-induced inflammatory changes (including thrombosis), metabolic disturbance (such as hypoxaemia and acidosis), and unintended consequences of medical interventions ranging from sedative medications to the pressure effects of prolonged prone positioning. Direct invasion of neurones could be facilitated via ACE2 receptors, but the presence of these entry points in brain tissue is unconfirmed29 but has been inferred.30 Animal studies have documented other coronavirus infections involving the CNS,31 and in humans, SARS-CoV-1 was found in the brain in post-mortem cases,32 , 33 whilst coronavirus antigen and RNA in the CSF and brain tissue outside of epidemics has been confirmed in patients with primary neurological conditions, such as multiple sclerosis.34 Several case histories from the current COVID-19 pandemic suggest direct viral invasion.
There is increasing recognition of manifestations of neurological disease, reported in 36% of ICU patients in a series from Wuhan with severe respiratory disease.35 The most common presentation in the ICU setting is delirium, which, although well reported in intensive care syndrome itself,36 appears to be even more common in COVID-19 ICU patients.37 Delirium may be associated with the primary disease itself, or may result from the combinations of drug therapy used for sedation in prolonged ventilation, particularly when neuromuscular blocking agents are required to facilitate mechanical ventilation.
Early reports from China indicated a high incidence of other neurological complications in COVID-19. Encephalopathy, presenting as headache with delirium and coma, associated with cerebral oedema and non-inflammatory CSF, was reported in about 20% of ICU patients in one series.38 The aetiology is likely a combination of hypoxaemia and metabolic derangement. An inflammatory COVID encephalitis has also been reported presenting as headache, fever, vomiting, and reduced level of consciousness. Ischaemic stroke was reported in around 5% of severely ill patients in a Wuhan series35 and in 2.5% in a report from Italy.39 Stroke is thought to be related to cytokine release and is associated with thrombocytosis. Finally, there are an increasing number of case reports of other associated neurological presentations, including Guillain–Barré syndrome,40 , 41 Miller Fisher syndrome, meningitis, and subarachnoid haemorrhage.
Conclusions
Early reports described COVID-19 as a respiratory illness, but we now know that it is a much more complex multisystem disorder. There is still a great deal to learn about why it affects people in different ways, although by combining epidemiology and basic science, some clues are emerging. Growing understanding points to potential therapeutic approaches. Interventions are likely to include some combination of antiviral treatment, drugs to protect tissues targeted by the virus (such as those that stabilise endothelium), drugs to manage abnormal physiological states (such as hypercoagulability), and drugs that treat the dysfunctional immune response that is often the terminal event. Survival rates for those needing ICU care are reported at about 50%, and UK Intensive Care National Audit & Research Centre data for those needing combined advanced respiratory, cardiovascular, and renal support are a shocking 19%. There is still a great deal to learn.
Authors' contributions
Conception/drafting/critical revision of this article: all authors.
Declarations of interest
The authors declare that they have no conflicts of interest.
References
- 1.Johns Hopkins University & Medicine . 2020. COVID-19 map.https://coronavirus.jhu.edu/map.html Available from: [Google Scholar]
- 2.Brainard J. 2020. Scientists are drowning in COVID-19 papers. Can new tools keep them afloat? Science.https://www.sciencemag.org/news/2020/05/scientists-are-drowning-covid-19-papers-can-new-tools-keep-them-afloat Available from: [Google Scholar]
- 3.McKee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. 2020;157:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 5.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz-Martinez Y. Tocilizumab: a new opportunity in the possible therapeutic arsenal against COVID-19. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levi M., Thachil J., Iba T., Levy J. Coagulation abnormalities and thrombosis in patients with COVID-19 infection. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levi M., Scully M., Singer M. The role of ADAMTS-13 in the coagulopathy of sepsis. J Thromb Haemost. 2018;16:646–651. doi: 10.1111/jth.13953. [DOI] [PubMed] [Google Scholar]
- 10.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L., Chiumello D., Caironi P. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 15.Caputo N.D., Strayer R.J., Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27:375–378. doi: 10.1111/acem.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middeldorp S., Coppens M., van Haaps T.F. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14888. Advance Access published on May 5, 2020, PMID 32369666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchis-Gomar F., Lavie C.J., Perez-Quilis C., Henry B.M., Lippi G. Angiotensin-converting enzyme 2 and antihypertensives (angiotensin receptor blockers and angiotensin-converting enzyme inhibitors) in coronavirus disease 2019. Mayo Clin Proc. 2020;95:1222–1230. doi: 10.1016/j.mayocp.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inciardi R.M., Adamo M., Lupi L. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried J.A., Ramasubbu K., Bhatt R. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sala S., Peretto G., Gramegna M. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang Y., Chen T., Mui D. Cardiovascular manifestations and treatment considerations in covid-19. Heart. 2020 doi: 10.1136/heartjnl-2020-317056. Advance Access published on April 30, 2020. PMID 32354800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. Advance Access published on March 27, 2020. PMID 32219356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chorin E., Dai M., Shulman E. The QT interval in patients with SARS-CoV-2 infection treated with hydroxychloroquine/azithromycin. Nature Med. 2020 doi: 10.1101/2020.04.02.20047050v1. Preprint posted on April 3, 2020. PMID 32488217. [DOI] [PubMed] [Google Scholar]
- 25.Moss T.J., Calland J.F., Enfield K.B. New-onset atrial fibrillation in the critically ill. Crit Care Med. 2017;45:790–797. doi: 10.1097/CCM.0000000000002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Akker J.P., Egal M., Groeneveld A.B. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care. 2013;17:R98. doi: 10.1186/cc12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 30.De Felice F.G., Tovar-Moll F., Moll J., Munoz D.P., Ferreira S.T. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the central nervous system. Trends Neurosci. 2020;43:355–357. doi: 10.1016/j.tins.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Natoli S., Oliveira V., Calabresi P., Maia L.F., Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur J Neurol. 2020 doi: 10.1111/ene.14277. Advance Access published on April 25, 2020. PMID 32333487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu J., Gong E., Zhang B. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q.L., Ding Y.Q., Hou J.L. Detection of severe acute respiratory syndrome (SARS)-associated coronavirus RNA in autopsy tissues with in situ hybridization. Di Yi Jun Yi Da Xue Bao. 2003;23:1125–1127. [PubMed] [Google Scholar]
- 34.Murray R.S., Brown B., Brian D., Cabirac G.F. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol. 1992;31:525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao L., Wang M., Chen S. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. JAMA Neurol. 2020 doi: 10.1101/2020.02.22.20026500. Preprint posted on February 25, 2020. PMID 32275288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Needham D.M., Davidson J., Cohen H. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 37.Helms J., Kremer S., Merdji H. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheidl E., Canseco D.D., Hadji-Naumov A., Bereznai B. Guillain–Barré syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. 2020;25:204–207. doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain–Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]