Abstract
Background
Coronavirus disease 2019 (COVID-19) is posing a huge threat to human health worldwide. We aim to investigate the immune status of CD8+ T and NK cells in COVID-19 patients.
Methods
The count and immune status of lymphocytes were detected by flow cytometry in 32 COVID-19 patients and 18 healthy individuals.
Results
As the disease progression in COVID-19 patients, CD8+ T and NK cells were significantly decreased in absolute number but highly activated. After patients' condition improved, the count and immune status of CD8+ T and NK cells restored to some extent. GrA+CD8+ T and perforin+ NK cells had good sensitivity and specificity for assisting diagnosis of COVID-19.
Conclusions
As the disease progression, the declined lymphocytes in COVID-19 patients might lead to compensatory activation of CD8+ T and NK cells. GrA+CD8+ T and perforin+ NK cells might be used as meaningful indicators for assisting diagnosis of COVID-19.
Keywords: COVID-19, CD8+ T cells, NK cells, Perforin, Granzyme
Highlights
-
•
As disease progression, CD8+ T and NK cells were decreased but highly activated.
-
•
After effective treatment, the immune status of CD8+ T and NK cells restored.
-
•
GrA+ CD8+ T and perforin+ NK cells could assist diagnosis of COVID-19.
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by a new coronavirus SARS-CoV-2 has spread widely and rapidly around the world since it was first reported in Wuhan, China, in December 2019. Until May 16, 2020, locations with confirmed COVID-19 cases included 216 countries and 4,347,935 cases were confirmed. A total of 297,241 patients died from this viral infection until May 16 [1]. WHO officially announced COVID-19 as a “pandemic”. A model-based analysis combining about 70,000 COVID-19 cases at home and abroad reported that the fatality rate of COVID-19 increased with age, and that the mortality in patients over 80 years of age even reached 10 times of the overall mortality in China [2]. However, the phenomenon of low incidence and mild symptoms in children with COVID-19 was common in many countries [3]. Guan WJ et al. confirmed the following points by analysis of inpatients in 575 domestic hospitals: COVID-19 patients with comorbidity would be poorer in clinical outcomes than those without, and a large number of comorbidities were closely correlated with worse clinical outcomes [4]. Besides, the diagnosis and treatment protocol for COVID-19 in China has been updated to the trial version 7, and the clinical classification of the disease remained mild, moderate, severe and critical cases. A confusing phenomenon is that why some people suffer from severe or even critical illness while some others only show slight clinical symptoms, and the prognosis is so various. Yufang Shi et al. proposed that traditional immunology viewpoints couldn't explain the broad spectrum of the disease, and suggested that the course of COVID-19 should be divided into immune defense-based protective phase and inflammation-driven damaging phase [5].
The morbidity and mortality of infectious diseases can be caused by direct damage to the host by the pathogen or collateral damage to the host tissue by the excessive immune response to the pathogen [6,7]. After infected with COVID-19, whether for clinical or basic research, the patients' immune status both need to be clarified urgently. The guideline of new edition for COVID-19 as mentioned above suggests that the progressive decline of peripheral lymphocytes is one of the clinical early warning indicators for severe and critical cases in adults, and many studies have also reported lymphopenia, mainly for the reduction of CD8+ T cells, in COVID-19 patients [[8], [9], [10], [11], [12], [13]]. In an autopsy report about one died case with COVID-19, Fu-Sheng Wang and his colleague found that the count of peripheral CD8+ T cells was substantially reduced, while cell immune status was hyperactivated; meanwhile, CD8+ T cells were found to harbour high concentrations of cytotoxic granules including perforin and granzyme [14]. It is well known that as primary cytotoxic lymphocytes, CD8+ T and NK cells play a vital role in the control of pathogen infection by mediating cellular immunity and cytotoxic function [[15], [16], [17]]. However, the immune status of CD8+ T and NK cells in COVID-19 patients with different severity is still largely unclear.
Wenzhou is the city with the largest number of COVID-19 cases except Wuhan in China. The First Affiliated Hospital of Wenzhou Medical University, as a designated hospital for treatment or referral of critical patients in Wenzhou, has accumulated some experience in the diagnosis and treatment of COVID-19 patients. In this study, we focus on the dynamic change in the number of lymphocyte subsets and monitor the immune status of CD8+ T and NK cells in COVID-19 patients with different severity. It may help provide new ideas for understanding the immune response mechanism of COVID-19 patients with different severity.
2. Materials and methods
2.1. Patients
The diagnosis and clinical classification of COVID-19 patients was performed according to the Guideline of the Diagnosis and Treatment of New Coronavirus Pneumonia (trial version 7) published by the National Health Commission of China. Thirty-two confirmed COVID-19 patients were recruited in this study, comprising of 13 mild cases (merging the mild and moderate types defined in the guideline, male/female: 6/7; age: 59.69 ± 13.38), 10 severe cases (male/female: 7/3; age: 62.80 ± 13.03) and 9 critical cases (male/female: 6/3; age: 67.78 ± 17.22). No statistically significant differences in age and sex between groups. As a designated hospital for treatment or referral of critical patients, some of these recruited patients, especially of critical patients, were referred from other hospitals. Consistent with previous studies, many patients had fever and cough upon admission. The clinical features of COVID-19 patients were consistent with those reported by Guan WJ and his colleagues [4]. Eighteen age- and sex-matched healthy individuals without SARS-CoV-2 infection were recruited as healthy controls (HCs). Written informed consent was obtained from all subjects. The study was approved by the ethics committee of The First Affiliated Hospital of Wenzhou Medical University.
2.2. Laboratory examination
Laboratory confirmation of the SARS-CoV-2 was performed by The First Affiliated Hospital of Wenzhou Medical University according to the Chinese CDC protocol. Throat-swab specimens were collected from all patients and all samples were maintained in viral-transport medium for laboratory testing. Infection with other respiratory viruses including influenza A virus, influenza B virus, Coxsackie virus, respiratory syncytial virus, parainfluenza virus, and enterovirus were excluded by RT-PCR. Specimens, including sputum or alveolar lavatory fluid, blood, urine, and feces, were cultured to identify pathogenic bacteria or fungi that might be associated with the SARS-CoV-2 infection. All tests were performed according to the product manual.
2.3. Flow cytometry analysis
To detect the phenotypic and functional characteristics of cytotoxic lymphocytes (CD8+T cells and NK cells), 2 mL of peripheral blood was used for cell surface markers staining, and then fixed and permeabilized for intracellular cytokine staining. FACSCanto II flow cytometer (BD, USA) was used to determine fluorescence intensity, Flowjo software (TreeStar) was used to analyze the data. Cell staining was performed with the following antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-human CD3 mAb (Biolegend, USA), allophycocyanin-Cy7(APC/Cy7)-conjugated anti-human CD8 mAb (Biolegend, USA), Brilliant™ Violet 510(BV-510)-conjugated anti-human CD56 mAb (Biolegend, USA),Brilliant™ Violet 421(BV-421)-conjugated anti-human perforin mAb (Biolegend, USA), APC-conjugated anti-human Granzyme A (GrA) mAb (Biolegend, USA), phycoerythrin(PE)-conjugated anti-human Granzyme B (GrB) mAb (Biolegend, USA), Peridin chlorophyll protein (PerCP)-conjugated anti-human CD3 mAb (BD Biosciences, California, USA), PE-conjugated anti-human CD8 mAb (BD Biosciences, California, USA), APC-conjugated anti-human CD38 mAb (BD Biosciences, California, USA), APC-conjugated anti-human HLA-DR mAb (BD Biosciences, California, USA), APC-conjugated anti-human CD4 mAb (BD Biosciences, California, USA), and APC-conjugated anti-human CD19 mAb (BD Biosciences, California, USA). The gate strategy of CD4+ T, CD8+ T, B, and NK cells was executed as CD3+CD4+, CD3+CD8+, CD3−CD19+ and CD3−CD16+/CD56+, respectively.
2.4. Statistical analyses
Data were expressed as the mean (Standard deviation) and median (Interquartile range) for continuous variables with and without normal distribution, and were analyzed by GraphPad Prism version 8.0 (GraphPad Software, Inc., San Diego, CA, USA). Multiple comparisons were made among the different groups using the Kruskal-Wallis H nonparametric test followed by Dunn's test as the post-test. Comparisons of various individuals were performed with the unpaired t-test and Mann-Whitney U test. The Paired t-test and Wilcoxon matched-pairs test were used for statistical analyses before and after remission. Diagnostic value of each indicator was appraised using the receiver operation characteristic (ROC) curves. The 95% confidence interval was utilized to calculate the sensitivity, specificity and consistency, and the cut-off value was selected when the Jordan index was at its maximum. For all tests, a two-sided P value <.05 was considered to be significant. Statistical significance is indicated as follows: ****p < .0001, ***p < .001, **p < .01, and *p < .05.
3. Results
3.1. Distribution of peripheral lymphocyte subsets in COVID-19 patients
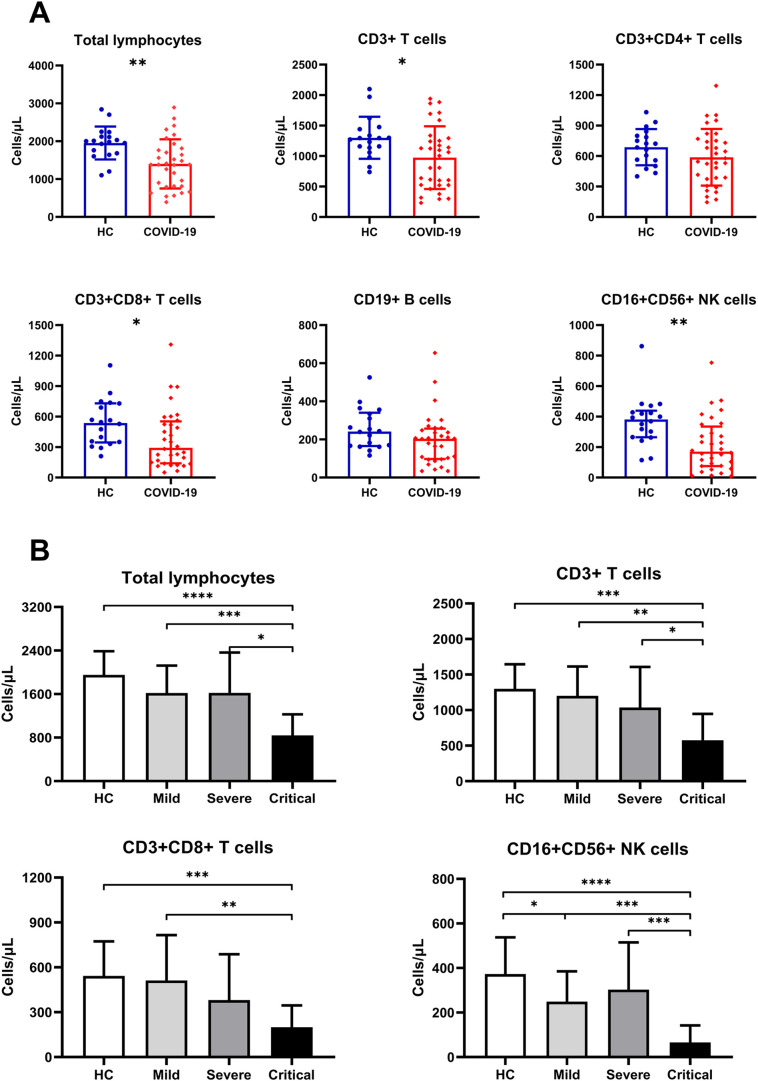
We detected the absolute number of circulating total lymphocytes and found that lymphocytes in COVID-19 patients decreased significantly as compared with HCs (Fig. 1A). In COVID-19 patients, the sustained decrease of lymphocytes in the Critical group was observed as compared with the other three groups (Fig. 1B). To determine the changes of different lymphocyte subsets in COVID-19 patients, we performed flow cytometry to determine total T cells, CD4+ T and CD8+ T cell subsets, B cells, and NK cells. Similar to the findings of total lymphocytes, sustained decrease in total T, CD8+T and NK cell count was observed in COVID-19 patients compared to HCs (Fig. 1A). In COVID-19 patients, the sustained decrease of total T and NK cells in the Critical group was observed compared to the other three groups (Fig. 1B), and CD8+ T cell count in the Critical group was significantly decreased as compared with HCs and the Mild group (Fig. 1B). However, no significant difference in CD4+ T and B cell count was observed between COVID-19 patients and HCs (Fig. 1A).
Fig. 1.
Distribution of peripheral lymphocyte subsets in COVID-19 patients. (A) The absolute number of all lymphocytes was decreased compared to HCs except for CD4+T and B cells in COVID-19 patients. (B) The absolute number of total lymphocytes, total T, CD8+T and NK cells was decreased significantly as the disease progression in COVID-19 patients, especially in the Critical group. *, P < .05; **, P < .01; ***, P < .001; ****, p < .0001.
3.2. Immune status of CD8+ T and NK cells in COVID-19 patients
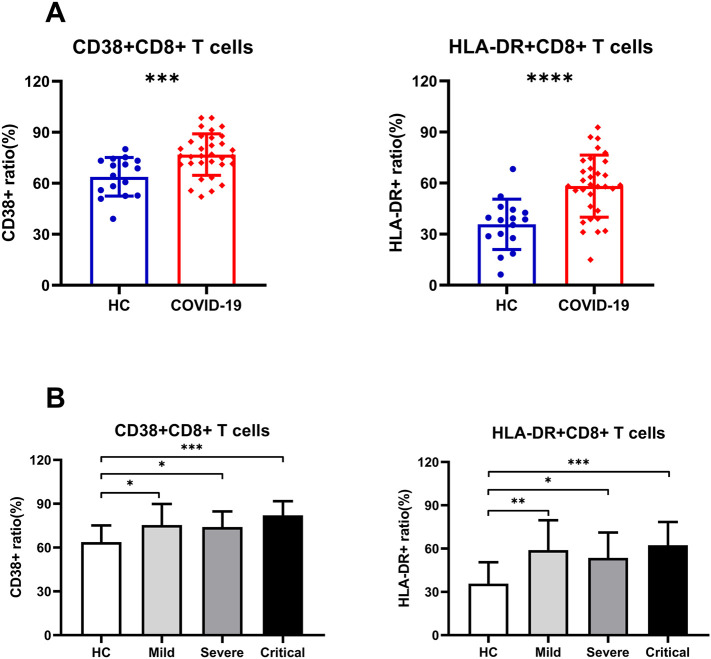
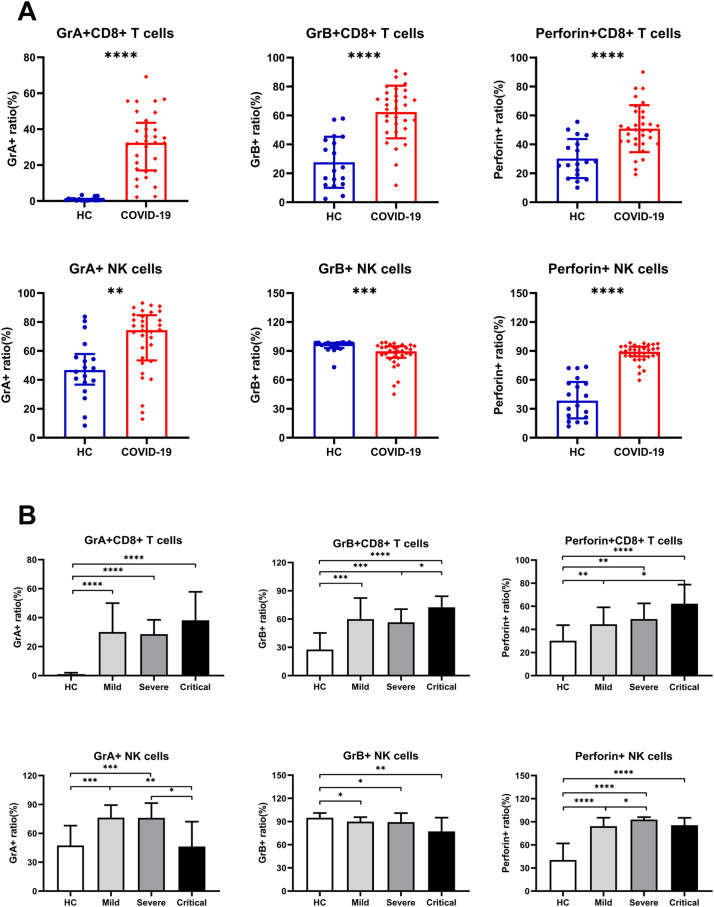
Expression of CD38 and HLA-DR was detected to analyze the activation status of CD8+ T cells and found that circulating CD8+ T cells from COVID-19 patients had higher expression of those two markers compared to HCs (Fig. 2A), while the expression of CD38 and HLA-DR in CD8+ T cells was not significantly different among the severity of the disease (Fig. 2B). We then detected the expression of GrA, GrB and perforin on CD8+ T and NK cells to understand their cytotoxic potential. We found that CD8+ T cells in COVID-19 patients had higher expression of cytotoxic granules (GrA, GrB and perforin) compared to HCs (Fig. 3A). Moreover, in COVID-19 patients, we found that the expression of GrB and perforin on CD8+ T cells in the Critical group was significantly increased as compared with the Severe group and Mild group (Fig. 3B), respectively; while GrA expression on CD8+ T cells was not significantly different among different severity of disease (Fig. 3B). In addition, we found that the expression of GrA and perforin on NK cells in COVID-19 patients was significantly increased compared to HCs (Fig. 3A). In comparing diseases of different severity, we found the expression of GrA on NK cells in the Mild and Severe groups was significantly increased as compared with HCs and the Critical group (Fig. 3B). The expression of perforin on NK cells in the Severe group was significantly increased as compared with the Mild group (Fig. 3B). However, the expression of GrB on NK cells in COVID-19 patients was significantly decreased compared to HCs (Fig. 3A), but it had no significant relationship with the severity of the disease (Fig. 3B).
Fig. 2.
The activation status of CD8+ T cells in COVID-19 patients. (A) The expression of CD38 and HLA-DR on CD8+ T cells of COVID-19 patients was significantly increased as compared with HCs. (B) The expression of CD38 and HLA-DR on CD8+ T cells was not significantly different among the severity of the disease.
Fig. 3.
The cytotoxic potential of CD8+ T cells and NK cells in COVID-19 patients. (A) CD8+ T cells in COVID-19 patients had higher expression of cytotoxic granules (GrA, GrB and perforin) compared to HCs. And the expression of GrA and perforin on NK cells in COVID-19 patients was also significantly increased while GrB expression was decreased compared to HCs. (B) There are slight differences in the expression of perforin and granzyme in COVID-19 patients of different severity. The expression of GrB and perforin on CD8+ T cells in the Critical group was significantly increased as compared with the Severe or Mild group; the expression of GrA on NK cells in the Mild and Severe groups was significantly increased as compared with HCs and the Critical group; the expression of perforin on NK cells in the Severe group was significantly increased as compared with the Mild group. The expression of GrA on CD8+ T cells and GrB on NK cells was not significantly different among different severity of the disease.
3.3. Characteristics of CD8+ T and NK cells in COVID-19 patients after remission
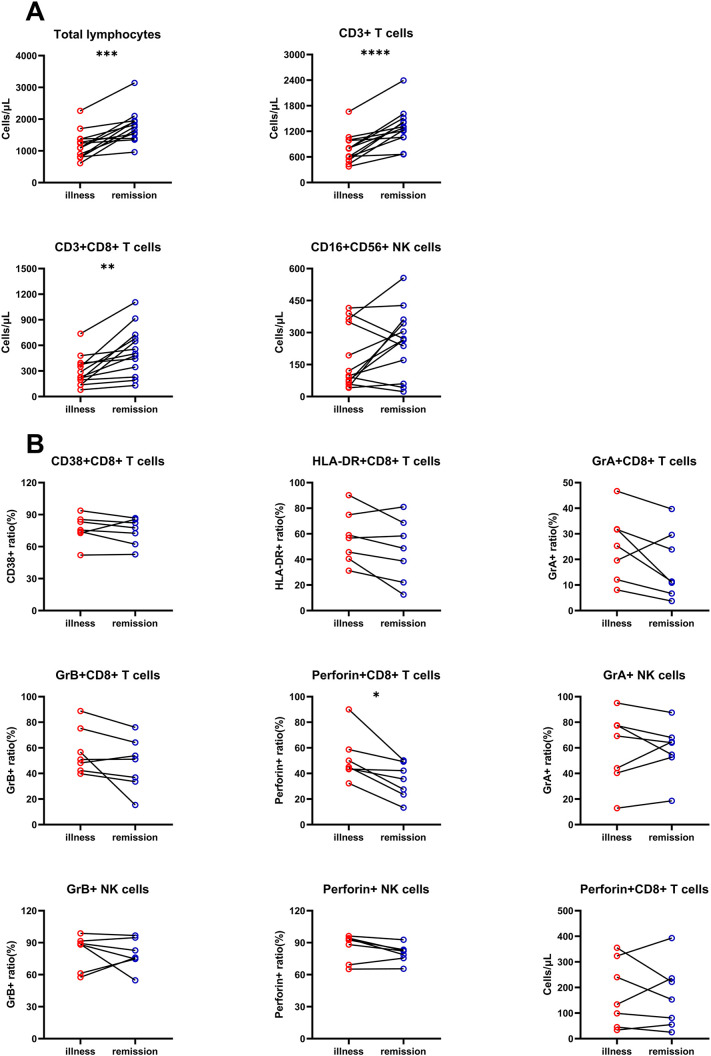
In order to study the relationship between lymphocyte changes and patients' condition, we first detected the absolute number of lymphocyte subsets before and after remission in 9 severe patients and 4 critical patients. We found that the absolute number of total lymphocytes, total T cells, and CD8+ T cells in the remission group was significantly higher than those in the illness group. Although the absolute number of NK cells was not changed significantly after remission, it had a tendency to increase compared to before remission (Fig. 4A). Then, we detected the expression of CD38, HLA-DR, GrA, GrB, and perforin on CD8+ T and NK cells before and after remission in 5 severe patients and 2 critical patients. The expressions of CD38, HLA-DR, GrA, GrB, especially of perforin, on CD8+ T cells all showed a downward trend compared to those before remission (Fig. 4B). However, there was no significant statistical change in perforin+ CD8+ T cell count before and after remission in COVID-19 patients. The tendency of GrA, GrB, and perforin on NK cells did not change significantly before and after remission (Fig. 4B).
Fig. 4.
Characteristics of CD8+ T and NK cells in COVID-19 patients after remission. (A) The absolute number of total lymphocytes, total T, and CD8+ T cells in the remission group was significantly higher than those in the illness group. The absolute number of NK cells had a tendency to increase compared to before remission. (B) The expression of CD38, HLA-DR, GrA, GrB and perforin on CD8+ T cells in the remission group all showed a downward trend compared to the illness group. However, the tendency of GrA, GrB, and perforin expression on NK cells and perforin+ CD8+ T cell counts did not change significantly before and after remission.
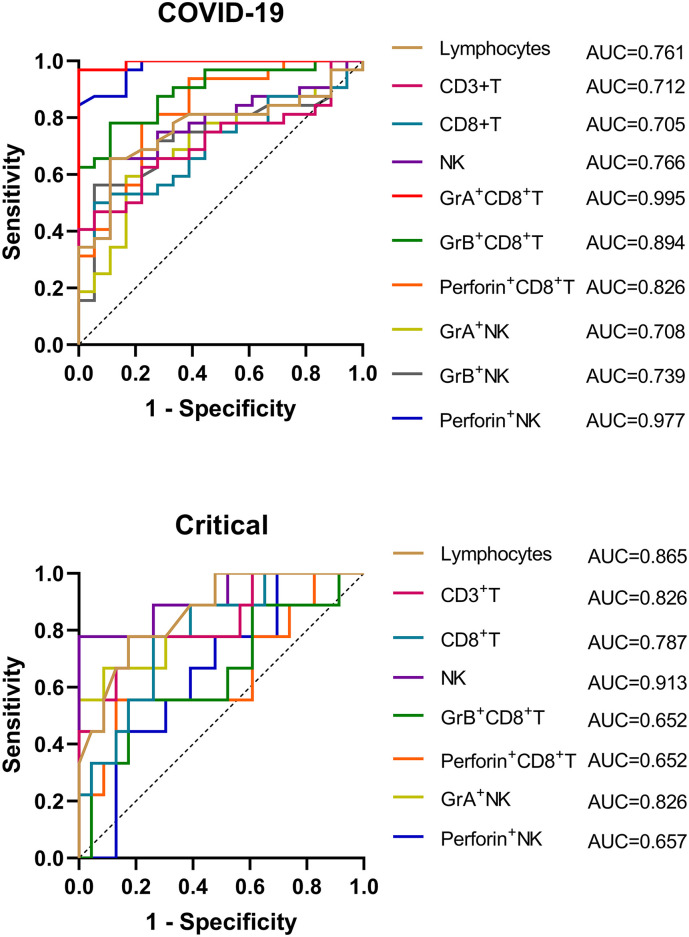
3.4. ROC curve analysis of CD8+ T and NK cells in COVID-19 patients
Through ROC curve analysis, we found that GrA+CD8+ T (AUC, 0.995) and perforin+ NK (AUC, 0.977) cells showed an excellent predictive value for COVID-19 with high sensitivity (96.88%, 84.38%) and specificity (100.00%, 100.00%); followed by GrB+CD8+ T (0.894) and perforin+CD8+ T (0.826) with sensitivity of 78.13% and 78.13%, and specificity of 88.89% and 77.78%, respectively (Fig. 5A). However, other indicators had relatively weak predictive value for COVID-19 (Detailed data are listed in Table 1 ). We further used parameters with different expressions and distributions in the severity of COVID-19 to predict the criticality of the disease. NK cells (AUC, 0.913) and GrA expression (AUC, 0.826) on NK cells showed good value to predict the critical of COVID-19 with high specificity (100.00%, 91.30%), but their sensitivity was not high (77.78%, 66.67%). Besides, total lymphocytes and CD3+ T cells also had good value in distinguishing critical COVID-19 patients with a total AUC of 0.865 and 0.826, respectively. CD8+ T cells had weak predictive value for critical COVID-19 patients with a lower AUC of 0.787 (Fig. 5B). Other parameters were not statistically significant (Detailed data are listed in Table 2). (See Table 2.)
Fig. 5.
ROC curve analysis of lymphocyte subsets in COVID-19 patients. (A) To distinguish COVID-19 patients from healthy controls. GrA+CD8+ T (AUC, 0.995) and perforin+ NK (AUC, 0.977) cells showed the best predictive value for COVID-19. (B) To distinguish critical cases from mild and severe cases in COVID-19 patients. Lymphocytes (AUC, 0.865), CD3+T (AUC, 0.826) cells, NK (AUC, 0.913) and GrA+ NK (AUC, 0.826) cells showed a good value to predict the critical illness. AUC, area under the curve.
Table 1.
Receiver operation characteristic curve analysis of parameters for the diagnosis of COVID-19 patients.
| Parameters | AUC | P | 95% CI | Cut-off value | Jordan index | sensitivity | specificity |
|---|---|---|---|---|---|---|---|
| Lymphocytes | 0.761 | 0.0024 | 0.627–0.896 | <1580.00 | 0.545 | 65.63% | 88.89% |
| CD3+T | 0.712 | 0.0137 | 0.571–0.853 | <814.30 | 0.413 | 46.88% | 94.44% |
| CD8+T | 0.705 | 0.0171 | 0.563–0.847 | <286.80 | 0.444 | 50.00% | 94.44% |
| NK | 0.766 | 0.0020 | 0.633–0.898 | <237.70 | 0.545 | 65.63% | 88.89% |
| GrA + CD8 + T | 0.995 | <0.0001 | 0.982–1.000 | >4.47 | 0.969 | 96.88% | 100.00% |
| GrB + CD8 + T | 0.894 | <0.0001 | 0.808–0.980 | >46.95 | 0.670 | 78.13% | 88.89% |
| Perforin + CD8 + T | 0.826 | 0.0001 | 0.706–0.947 | >40.20 | 0.559 | 78.13% | 77.78% |
| GrA + NK | 0.708 | 0.0153 | 0.561–0.856 | >65.65 | 0.427 | 59.38% | 83.33% |
| GrB + NK | 0.739 | 0.0055 | 0.598–0.879 | <90.35 | 0.507 | 56.25% | 94.44% |
| Perforin + NK | 0.977 | <0.0001 | 0.944–1.000 | >73.70 | 0.844 | 84.38% | 100.00% |
AUC, area under the curve; 95% CI, 95% confidence interval.
Table 2.
Receiver operation characteristic curve analysis of parameters for the diagnosis of critical COVID-19 patients.
Critical.
| Parameters | AUC | P | 95% CI | Cut-off value | Jordan index | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| Lymphocytes | 0.865 | 0.0016 | 0.732–0.998 | <1060.00 | 0.604 | 77.78% | 82.61% |
| CD3+T | 0.826 | 0.0047 | 0.657–0.995 | <609.50 | 0.604 | 77.78% | 82.61% |
| CD8+T | 0.787 | 0.0126 | 0.620–0.955 | <227.50 | 0.517 | 77.78% | 73.91% |
| NK | 0.913 | 0.0003 | 0.790–1.000 | <66.50 | 0.778 | 77.78% | 100.00% |
| GrB + CD8 + T | 0.652 | 0.1867 | 0.427–0.878 | >76.5 | 0.382 | 55.56% | 82.61% |
| Perforin + CD8 + T | 0.652 | 0.1867 | 0.420–0.884 | >62.6 | 0.425 | 55.56% | 86.96 |
| GrA + NK | 0.826 | 0.0047 | 0.654–0.999 | <49.80 | 0.580 | 66.67% | 91.30% |
| Perforin + NK | 0.657 | 0.1731 | 0.457–0.857 | <80.5% | 0.314 | 44.44% | 86.96% |
AUC, area under the curve; 95% CI, 95% confidence interval.
4. Discussion
COVID-19 has emerged as a severe infectious disease and is affecting the whole world, threatening human health and life. Lymphocytes are core components of the immune system to fight against virus intrusion, especially of CD8+ T and NK cells which play a vital role in the clearance of virus [15,16]. However, it's still largely unclear about the characteristic of lymphocyte subsets, especially of CD8+ T and NK cells, in COVID-19 patients.
In this study, we detected the characteristic of lymphocyte subsets in 32 COVID-19 patients with different severity. We found a significant reduction of circulating total lymphocytes in COVID-19 patients compared to healthy controls, which was consistent with previous studies [[8], [9], [10], [11], [12]]. We also found that the decreased lymphocytes in COVID-19 patients might mainly due to the reduction of CD8+ T and NK cells. And with the progression of COVID-19, CD8+ T and NK cells continued to decline, this phenomenon was particularly evident in the Critical group. The loss of CD8+ T and NK cells suggests that specific and non-specific immunity is suppressed to a certain extent during COVID-19. In order to further understand the immune status of CD8+ T and NK cells in COVID-19 patients, we detected the activation status and cytotoxic potential of CD8+ T and NK cells. The results indicated that CD8+ T cells in COVID-19 patients were highly activated and might have a high cytotoxic potential with high expression of perforin and GrA/B, especially in the Critical group. These results suggest that although the number of CD8+ T cells decreases during the progression of COVID-19, their immune status may have a compensatory activation, especially in the critical period. Diao et al. found increasing PD-1 and Tim-3 expression on T cells as COVID-19 patients progressed from prodromal to overtly symptomatic stages [18], which indicated that T cells might be functionally exhausted, such results seem to be inconsistent with our research, probably because the functional status of cells is determined by the co-regulation of many activation, inhibition and apoptosis pathways. Liao et al. [19] found that compared with patients in the moderate group, the number of CD8+ T cells in the severe group was reduced, and these CD8+ T cells expressed high levels of GrA, GrK, which was very similar with our results; As for the different results of GrA and GrK expression between patients with moderate and severe in Liao et al. study [19] and our study, we speculate which may be caused by the difference in the number of statistics. And, of course, there is also a great possibility that in those with severe cases, the number of granzyme A+ / perforin+ CD8+ T cells was still reduced due to the dramatic reduction in total cell counts.
We then detected the cytotoxic potential of NK cells, and found an increase of GrA and perforin expression on NK cells but a decrease of GrB expression in COVID-19 patients. Besides, we found that GrA expression on NK cells was significantly increased in the Mild and Severe groups, however, as the disease progressed, GrA expression on NK cells was significantly decreased in the Critical group, which might due to a compensatory enhancement in the mild and severe stages, but when the disease reached a critical stage, the cell immune status was significantly suppressed and couldn't be compensated. To some extent, this could be confirmed by the fact that the absolute number of NK cells in the Critical group decreased significantly compared to the other three groups.
Perforin is a key effector molecule for T and NK cells to mediate cytolysis. It could lyse cells by coordinating the delivery of GrA and GrB to target cells. The perforin/ granzyme cytolytic pathway is a two-edged sword in viral defense, which could result in viral clearance and host cell damage [[20], [21], [22]]. Therefore, we speculate that as the COVID-19 progression, the over-activated immune status of cytotoxic CD8+ T and NK cells might mediate pathological damage and lead to continuous deterioration of patients.
Besides, we analyzed the changes of absolute number and immune status of lymphocytes in COVID-19 patients before and after remission. We found that compared to the illness group, the absolute number of total lymphocytes, T, CD8+ T, and NK cells in the remission group restoratively elevated. Additionally, the expression of CD38, HLA-DR, GrA, GrB, especially of perforin, on CD8+ T cells in the remission group showed a downward trend compared to those before remission, indicating a decrease in CD8+ T cell activation and cytotoxic potential in COVID-19 patients after remission. However, there was no significant statistical change in perforin+ CD8+ T cell count before and after remission in COVID-19 patients, which suggests an expansion of perforin− CD8+ T cells when in remission, and could be the effect of regulatory T cells plus expansion of memory cells due to resolution of viral infection. However, the tendency of GrA, GrB, and perforin expression on NK cells did not change significantly, which might be that although the number of NK cells recovered during the remission period, the immune status was still suppressed and did not timely recovered. Currently, we still know little about how SARS-CoV-2 precipitates such severe lymphopenia in such a short period of time, which needs further study.
In addition, timely and accurate diagnosis of COVID-19 can give valuable time for patients' treatment. In this study, we found that GrA+CD8+ T (AUC, 0.995; Se, 96.88%; Sp, 100.00%) and perforin+ NK cells (AUC, 0.977; Se, 84.38%; Sp, 100.00%) were valuable indicators for assisting diagnosis of COVID-19. These two indicators might be used as valuable indicators to assist COVID-19 diagnosis. Effectively predict the severity of the disease could help clinicians treat patients timely and transfer patients to the intensive care unit as soon as necessary. In this study, we found that NK cells (Se, 77.78%; Sp, 100.00%) had a good value for assisting diagnosis of critical COVID-19 patients with an AUC of 0.913 followed by total lymphocytes (AUC, 0.865; Se, 77.78%; Sp, 82.61%). However, the expression of perforin and granzymes had no better diagnostic value than total lymphocyte and NK cell counts in the identification of critical COVID-19 patients, although GrA expression on NK cells had a good specificity for critical patients.
In summary, as the course of disease progression, the declined peripheral lymphocyte subsets in COVID-19 patients might lead to compensatory activation of CD8+ T and NK cells. GrA+CD8+ T and perforin+ NK cells might be used as meaningful indicators for assisting diagnosis of COVID-19. These findings might provide a new perspective for the diagnosis and treatment of COVID-19 patients.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province of China (LY16H200004).
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Contributor Information
Yanfan Chen, Email: chenyf2605@163.com.
Xiangyang Lin, Email: linxy1968@126.com.
References
- 1.World Health Organization (WHO). Coronavirus disease (COVID-19) outbreak situation reports. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 16 May 2020.
- 2.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodin P. 2020. Why Is COVID-19 So Mild in Children? J Acta Paediatrica. [DOI] [PubMed] [Google Scholar]
- 4.Guan W.J., Liang W.H., Zhao Y. Comorbidity and its impact on 1590 patients with Covid-19 in China: a Nationwide analysis. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y., Wang Y., Shao C. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palm N.W., Medzhitov R. Not so fast: adaptive suppression of innate immunity. J. Nat. Med. 2007;13(10):1142–1144. doi: 10.1038/nm1007-1142b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K.D., Zhao J., Auh S. Adaptive immune cells temper initial innate responses. Nat. Med. 2007;13(10):1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D., Hu B., Hu C. China; JAMA: 2020. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J.J., Dong X., Cao Y.Y. China; Allergy: 2020. Clinical Characteristics of 140 Patients Infected with SARS-CoV-2 in Wuhan. [DOI] [PubMed] [Google Scholar]
- 12.Borges do Nascimento I.J., Cacic N., Abdulazeem H.M. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J. Clin. Med. 2020:9(4). doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F., Nie J., Wang H. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt M.E., Varga S.M. The CD8 T cell response to respiratory virus infections. J Front. in Immunol. 2018;9:678. doi: 10.3389/fimmu.2018.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Channappanavar R., Zhao J., Perlman S. T cell-mediated immune response to respiratory coronaviruses. J Immunol Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vivier Eric, Tomasello Elena, Baratin Myriam, Walzer Thierry, Ugolini Sophie. Functions of natural killer cells. Nat. Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 18.Diao B., Wang C.H., Tan Y.J. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao M.F., Liu Y., Yuan J. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 20.Kägi D., Ledermann B., Bürki K. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 21.Berke G. The CTL's kiss of death. J Cell. 1995;81(1):9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 22.Topham D.J., Tripp R.A., Doherty P.C. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J J. Immunol. 1997;159(11):5197–5200. [PubMed] [Google Scholar]