Abstract
The outbreak of COVID-19 has created a global public health crisis. Little is known about the protective factors of this infection. Therefore, preventive health measures that can reduce the risk of infection, progression and severity are desperately needed. This review discussed the possible roles of vitamin D in reducing the risk of COVID-19 and other acute respiratory tract infections and severity. Moreover, this study determined the correlation of vitamin D levels with COVID-19 cases and deaths in 20 European countries as of 20 May 2020. A significant negative correlation (p = 0.033) has been observed between mean vitamin D levels and COVID-19 cases per one million population in European countries. However, the correlation of vitamin D with COVID-19 deaths of these countries was not significant. Some retrospective studies demonstrated a correlation between vitamin D status and COVID-19 severity and mortality, while other studies did not find the correlation when confounding variables are adjusted. Several studies demonstrated the role of vitamin D in reducing the risk of acute viral respiratory tract infections and pneumonia. These include direct inhibition with viral replication or with anti-inflammatory or immunomodulatory ways. In the meta-analysis, vitamin D supplementation has been shown as safe and effective against acute respiratory tract infections. Thus, people who are at higher risk of vitamin D deficiency during this global pandemic should consider taking vitamin D supplements to maintain the circulating 25(OH)D in the optimal levels (75–125 nmol/L). In conclusion, there is not enough evidence on the association between vitamin D levels and COVID-19 severity and mortality. Therefore, randomized control trials and cohort studies are necessary to test this hypothesis.
Keywords: COVID-19, SARS-CoV-2, Infections, Vitamin D, Vitamin D supplementation
Introduction
Vitamin D is a steroid hormone, produced endogenously with the effect of ultraviolet radiation on the skin or available from exogenous food sources or dietary supplements. Vitamin D insufficiency is a public health problem affecting over a billion people across all life stages worldwide [1]. In the past decade, several studies demonstrated a potential link between vitamin D deficiency and various diseases, including systemic infection [2], [3], [4]. Vitamin D insufficiency affects the immune functions as vitamin D exerts an immunomodulation role [5], increasing innate immunity by secretion of antiviral peptides [6], [7], which improves mucosal defenses. In clinical studies, low levels of serum vitamin D were associated with acute respiratory tract infections including epidemic influenza [8], [9], [10]. A recent meta-analysis incorporating data from eight observational studies reported that subjects with a serum vitamin D concentration <50 nmol/l (i.e. <20 ng/ml) had a 64% increased risk of community-acquired pneumonia [11]. Some recent reviews hypothesized that vitamin D insufficiency may compromise respiratory immune function, increasing the risk of COVID-19 severity and mortality [12], [13]. There are also some retrospective studies that determined the correlation of vitamin D levels with COVID-19 severity and mortality [14], [15], [16], [17], [18], [19], [20], [21], [22].
The outbreak and fast spreading of SARS-CoV-2 are a global health threat with an unstable outcome worldwide. A recent data reported the antiviral effects of vitamin D, which can hinder viral replication directly, and also be effective in an anti-inflammatory and immunomodulatory way [23]. It seems that SARS-CoV-2 primarily uses the immune evasion process during infection, which is followed by hyper reaction and cytokine storm in some patients [24], as a known pathogenic process of acute respiratory disease syndrome (ARDS) development [25]. SARS-CoV-2 uses angiotensin-converting enzyme 2 as the host receptor to enter into alveolar and intestinal epithelial cells [26]. Subsequent dysregulation of the renin–angiotensin system may lead to excess cytokine production resulting in prospective fatal ARDS [25].
Considering the differences in the severity and fatality of COVID-19 in the globe, it is important to understand the reasons behind it. Improvement of immunity through better nutrition might be a considerable factor. The nutrient such as vitamin D shows significant roles in immune function. However, little is known about the role of vitamin D in preventing COVID-19 infection and fatality. This study evaluated the correlation of vitamin D concentrations with COVID-19 cases and deaths per one million of the population in 20 European countries using data from the COVID-19 pandemic data portal [27] for 20 May 2020 (most countries after peak). This review also discussed the possible preventing role of vitamin D in acute respiratory tract infections. Furthermore, the available studies that determined the role of vitamin D in COVID-19 severity and mortality have been discussed. PubMed, Google Scholar, Web of Science, Scopus, Cochrane Central Register of Controlled Trials, and medRXiv were searched for relevant literature about the role of vitamin D in COVID-19 infections, severity, and mortality.
Vitamin D and mechanisms to decrease viral infections
Some recent reviews demonstrated some pathways by which vitamin D decreases the risk of microbial infections [28], [29], [30], [31]. Vitamin D follows different mechanisms in reducing the risk of viral infection and mortality. To reduce the risk of common cold, vitamin D uses three pathways: physical barrier, cellular natural immunity, and adaptive immunity [32]. A recent review also supported the possible role of vitamin D in decreasing the risk of COVID-19 infections and mortality [12]. These comprise maintaining of cell junctions, and gap junctions, increasing cellular immunity by decreasing the cytokine storm with influence on interferon γ and tumor necrosis factor α [12] and regulating adaptive immunity through inhibiting T helper cell type 1 responses and stimulating of T cells induction [33]. Vitamin D supplementation was also found to enhance CD4+ T cell count in HIV infection [34].
One of the major manifestations of severe SARS-CoV-2 infection is lymphopenia [35]. In both the mouse models and in human cell lines, vitamin D exerted activity in lung tissue and played protective effects on experimental interstitial pneumonitis [36]. Several in vitro studies demonstrated that vitamin D plays a significant role in local “respiratory homeostasis” either by stimulating the exhibition of antimicrobial peptides or by directly interfering with the replication of respiratory viruses [37]. Vitamin D insufficiency can, therefore, be involved in ARDS and heart failure [12] and these are the manifestations of severely ill COVID-19 subjects. Therefore, vitamin D deficiency promotes the renin-angiotensin system (RAS), which may lead to chronic cardiovascular disease (CVD) and reduced lung function [38]. People with such comorbidities account for a higher percentage of severe ill cases in COVID-19 [35]. Although, many studies supported the immunomodulatory characteristics of vitamin D and its significant role in the maintenance of immune homeostasis; well-designed randomized controlled trials are required to elucidate the plausible role of vitamin D in protective immune responses against respiratory microbes and in preventing various types of acute respiratory tract infections.
The relevance of vitamin D to COVID-19
Yet, it is important to fully elucidate the virulence mechanisms of COVID-19, several cellular mechanisms including Papain-like protease (PLpro)-mediated replication, dipeptidyl peptidase-4 receptor (DPP-4/CD26) binding, disruption of M-protein mediated type-1 IFN induction and MDA5 and RIG-I host-recognition evasion have been recognized in the closely-related COVID-MERS virus [39], [40]. Of the above processal, human DPP-4/CD26 has been exhibited to connect with the S1 domain of the COVID-19 spike glycoprotein, suggesting that it could also be a salient virulence factor in Covid-19 infection [41]. The expression of the DPP-4/CD26 receptor is reduced significantly in vivo upon the correctness of vitamin D insufficiency [42]. There is also an indication that maintaining of vitamin D may reduce some of the unfavorable downstream immunological sequelae thought to extract poorer clinical outcome in Covid-19 infection, such as interleukin 6 elevation, delayed interferon-gamma response[37], and, a negative prognostic marker in subjects with acutely-ill pneumonia [43], including those having Covid-19.
Epidemiological and clinical observations regarding COVID-19
Some clinical and epidemiological studies support to outline the hypothesis regarding COVID-19 and its relationship with vitamin D status. Recent studies indicated that COVID-19 is associated with the increased generation of pro-inflammatory cytokines, C-reactive protein (CRP), ARDS, pneumonia, and heart failure [44], [45], [46], [47]. In China, chronic fatality rates were 6-10% for people with chronic respiratory tract disease, cardiovascular disease, hypertension, and diabetes [12], [48]. In other studies, serum concentrations of 25(OH)D were inversely associated with pro-inflammatory cytokines, IL-6, increased CRP, and increased risk of pneumonia, ARDS, diabetes and heart failure [11], [49], [50], [51], [52], [53], [54]. In randomized control trials, vitamin D supplementation has been shown to reduce the risk of respiratory diseases [55], [56]. A placebo-controlled trial with 5660 subjects showed that vitamin D supplementation significantly reduces the risk of respiratory tract infections [57]. A review included five clinical studies reported that respiratory tract infections were significantly lower in the vitamin D supplementation group than the control group [58]. Another study included 25 randomized controlled trials, with 10,933 participants in total from 14 different countries indicated the beneficial effects of vitamin D supplementation in reducing the risk of at least one acute respiratory tract infection [59].
Correlation of vitamin D levels with COVID-19 cases and deaths in the European population
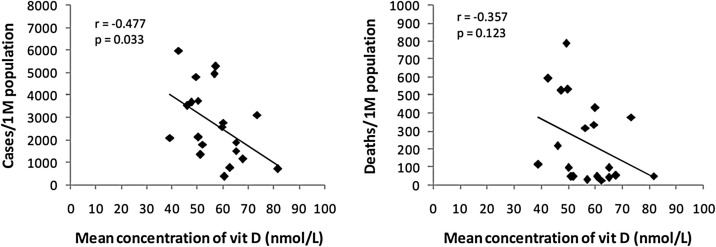
The data about the number of COVID-19 cases and deaths per one million of the population in 20 European countries (Table 1 ) were obtained for 20 May 2020 (all countries after peak) from the global coronavirus pandemic data portal at https://www.worldometers.info/coronavirus/ [27]. The data for mean serum vitamin D concentrations of these 20 countries were obtained from a previous publication [60]. In Pearson's correlation coefficient test, a significant correlation (r = -0.477, p = 0.033) was observed between mean concentrations of vitamin D and number of COVID-19 cases/1 M population (Table 1 and Fig. 1 ). While, the relationship between mean vitamin D concentrations and the number of COVID-19 deaths/1 M population was not significant (r = -0.357, p = 0.123). In the present analysis, the negative correlation between vitamin D levels and COVID-19 cases is stronger than that (r = -0.444, p = 0.050) observed on 8 April 2018 (most countries before the peak) in a previous analysis [19]. However, the negative correlation of vitamin D with COVID-19 deaths in the present analysis seems to be more insignificant than the previous analysis (Table 1). The number of cases and deaths per million populations varied from country to country at these two time periods. There is a probability that there exists a cause–effect relationship. The underlying differences in the age distribution, population composition, pre-existing medical conditions and variation in medication uses might be potential confounding variables that could affect the correlation.
Table 1.
The number of COVID-19 cases and deaths per one million of population at two different time period in 20 European countries.
| Countries | Mean vitamin D (nmol/L) | 8 April 2020 |
20 May 2020 |
||
|---|---|---|---|---|---|
| Cases/1 M population | Death/1 M population | Cases/1 M population | Death/1 M population | ||
| Iceland | 57 | 4736 | 18 | 5287 | 29 |
| Norway | 65 | 1123 | 19 | 1530 | 43 |
| Sweden | 73.3 | 834 | 68 | 3124 | 380 |
| Finland | 67.7 | 449 | 7 | 1163 | 55 |
| Denmark | 65 | 933 | 38 | 1920 | 97 |
| UK | 47.4 | 895 | 105 | 3690 | 526 |
| Ireland | 56.4 | 1230 | 48 | 4932 | 319 |
| Netherlands | 59.5 | 1199 | 131 | 2595 | 336 |
| Belgium | 49.3 | 2019 | 193 | 4818 | 790 |
| Germany | 50.1 | 1309 | 25 | 2131 | 98 |
| France | 60 | 1671 | 167 | 2782 | 431 |
| Switzerland | 46 | 2686 | 103 | 3548 | 219 |
| Italy | 50 | 2306 | 292 | 3750 | 535 |
| Spain | 42.5 | 3137 | 314 | 5980 | 596 |
| Estonia | 51 | 893 | 18 | 1359 | 48 |
| Czech Republic | 62.5 | 488 | 9 | 773 | 28 |
| Slovakia | 81.5 | 125 | 0.4 | 709 | 51 |
| Hungary | 60.6 | 93 | 6 | 372 | 48 |
| Turkey | 51.8 | 453 | 10 | 1810 | 50 |
| Portugal | 39 | 1289 | 37 | 2101 | 115 |
| Average | 56.8 ± 10.6 | 1393.4 ± 1129.9 | 80.4 ± 94.6 | 2718.7 ± 1632.3 | 239.7 ± 233.3 |
| Correlation (r) | −0.444 | −0.438 | −0.477 | −0.357 | |
| p-value | 0.050 | 0.053 | 0.033 | 0.123 | |
Data source: Vitamin D concentration from the reference Lips et al. [60], and COVID-19 cases and deaths from worldwide COVID-19 pandemic data portal at https://www.worldometers.info/coronavirus/[27]. Pearson correlation coefficient test (2-tailed) was applied to determine the correlation of vitamin D concentration with COVID-19 cases and death per one million population.
Fig. 1.
Correlation between vitamin D levels and the number of COVID-19 cases and deaths/1 M population in 20 European countries. Data source: vitamin D concentration from the reference Lips et al. [60], and COVID-19 cases and mortality as of 20 May 2020 from worldwide COVID-19 pandemic data portal at https://www.worldometers.info/coronavirus/[27].
Other available studies on the role of vitamin D in COVID-19 infections and outcomes
Up to now, there is a lack of clinical trials and cohort studies in determining the preventing role of vitamin D in COVID-19 infections and severity. However, there are some retrospective observational studies that determined the correlation between vitamin D levels and COVID-19 cases and severity (Table 2 ); the findings were not consistent for all studies. For example, a retrospective study used a database of 212 patients with infection of SARS-CoV-2 and measured serum levels of vitamin D from three hospitals in South Asian countries [61]. The author observed a significant difference in the mean levels of vitamin D within the mild (78 nmol/L), ordinary (68.5 nmol/L), severe (53 nmol/L) and critical cases of COVID-19 (p < 0.001). The author noted a significant association between vitamin D status and clinical outcomes (p < 0.001). In Singapore, a small cohort observational study [62] included 43 cases from a tertiary academic hospital reported that fewer COVID-19 patients who received combined oral doses of vitamin D (1000 IU), Mg (150 mg), and vitamin B12 (500 μg) required subsequent oxygen therapy compared to controls (3/17 vs. 16/26, p = 0.006). It was observed that patients treated with combined vitamin D, Mg and vitamin B12 showed significant protective effects against clinical deterioration (p = 0.041) even after adjusting for age, gender and comorbidities [62]. Low levels of vitamin D have also been reported in severe COVID-19 patients and patients with pre-existing medical conditions [17], [20]. In Belgium, a retrospective observational study consisted of 186 positive cases and 2717 negative controls, reported a significant (p = 0.0016) low median of vitamin D in COVID-19 patients compared to the control subjects [16]. Another study used datasets from different parts of the world and demonstrated a 15% reduction in the number of severe COVID-19 cases given a normal vitamin D status within a population (Daneskhar et al.). An Indonesian retrospective cohort study included 780 cases reported that older and male cases with pre-existing medical conditions and below-normal vitamin D levels are associated with higher odds of death [22]. In that study, after adjustment of confounders (age, sex and comorbidity), vitamin D status showed a strong relationship with COVID-19 mortality [22]. A retrospective study in the mainland of USA included a large number of cases demonstrated that Sunlight and vitamin D, with latitude as an indicator, possibly associated with reduced risks for both COVID-19 cases and mortality [21].
Table 2.
Correlation of vitamin D concentrations with COVID-19 infections and outcomes in recent studies.
| Reference | Country | n | Population type | Study design | Vit D doses | Outcomes |
|---|---|---|---|---|---|---|
| Tan et al., 2020 [62] | Singapore (a tertiary academic hospital) | 43 | Adults, age ≥50 yrs | Cohort observational (15 Jan–15 April 2020) |
Vitamin D 1000 IU, Mg 150 mg, and vitamin B12 500 μg (oral) | (i) A fewer patients who received vitamin D, Mg and vitamin B12 required subsequent oxygen therapy compared to controls (3/17 vs. 16/26, p = 0.006) (ii) In multivariate analysis, patients treatment with vitamin D, Mg and vitamin B12 showed a significant protective effects against clinical deterioration (p = 0.041) after adjusting for age, gender and comorbidities |
| Present study | 20 European countries | Cases and death/1 M population | Adults | Retrospective (as of 20 May 2020) | NA | A significant negative correlation was observed for levels of mean vitamin D with COVID-19 cases (p = 0.033) but not with death (p = 0.123) per million of population |
| IIie et al., 2020 [19] | 20 European countries | Cases and death/1 M population | Adults | Retrospective (as of 8 April 2020) | NA | A negative correlation was observed between levels of mean vitamin D and COVID-19 cases (p = 0.050) and death (p = 0.053) per million of population |
| Alipio 2020 [61] | Southern Asian countries (three hospitals) |
222 | NA | Retrospective multicentre study | NA | (i) The differences in the mean levels of vitamin D were significant within the mild, ordinary, severe and critical cases of COVID-19 (p < 0.001) (ii) Vitamin D status showed a significant association with clinical outcomes (p < 0.001) |
| Lau et al., 2020 [20] | USA (a single tertiary academic medical center) | 20 | Adults, mean age 65.2 yrs | Retrospective observational study (27 March–21 April 2020) |
NA | A high vitamin D insufficiency was observed in ICU patients (84.6%) than in the floor patients (57.1%) (p = 0.29) |
| Glicio et al., 2020 [17] | South Asia (two tertiary medical centers) | 176 | Adults, age ≥60 yrs | Retrospective (as of 5 May 2020) |
NA | (i) Severe patients had a low level of vitamin D than mild patients (ii) Subjects with pre-existing medical conditions had a low level of vitamin D |
| Hastie et al., 2020 [18] | UK (UK Biobank data 2006–2010 for vitamin D and ethnicity) | 449 | Adults, age 37–73 yrs | Cross-sectional (16 March–14 April 2020) | NA | (i) Vitamin D levels showed a significant association with COVID-19 infection in univariate analysis (p = 0.013) but not after adjustment for confounders (p = 0.208) (ii) Ethnicity showed a significant association with COVID-19 infection univariably |
| Darling et al., 2020 [15] | UK (UK Biobank data 2006–2010 for BMI, vitamin D and ethnicity) | 580 cases and 723 control | Adults, mean age 57.7 yrs | Retrospective | NA | (i) No significant difference was observed for vitamin D levels between COVID-19 cases and control group (ii) Vitamin D status was significantly lower in those of Asian, Black and Mixed ethnicity (p < 0.0010) compared with those of White ethnicity (iii) Vitamin D levels were significantly lower in those with obesity (p < 0.001). Overweight or obese person; living in London; being male and being of Asian, Black or Mixed ethnicity was associated with a higher odd of positive cases (iv) In regression model, the interaction between BMI and vitamin D status did not predict test result in the available data set |
| Li et al., 2020 [21] | Mainland of USA (48 states and Columbia district) |
– | 1,609,488 cases and 91,094 deaths | Retrospective (22 Jan–23 May 2020) | NA | (i) Latitudes were marginally associated with cases (p = 0.0792) and deaths (p = 0.0599) (ii) Sunlight and vitamin D, with latitude as an indicator, possibly associated with reduced risks for both COVID-19 cases and mortality |
| De Smet et al., 2020 [16] | Belgium (Central network hospital) | 186 cases, 2717 controls | Adults, median age 71 yrs (cases), 68 yrs (control) | Retrospective observational (1 March–7 April 2020) | NA | (i) Patients with COVID-19 had significantly a low median value of vitamin D and higher vitamin D deficiency compared to control subjects (p = 0.0016, p = 0.0005, respectively) (ii) This difference were more pronounced in male COVID-19 subjects than male control subjects that increased with advancing radiological stage and were not confounded vit D-impacted comorbidities |
| Daneshkhah et al., 2020 [14] | Hospitals and clinics from different parts of the world | 5000 cases | Age up to 80 yrs | As of March 21, 2020 | NA | About 15% reduction in the number of severe COVID-19 cases given a normal vitamin D status within a population |
| Raharusuna et al., 2020 [22] | Indonesia (Government hospital) | 780 cases | Adults, mean age 54.5 yrs | Retrospective cohort study (2 March 2–24 April 2020) | NA | (i) In univariate analysis, older and male cases with pre-existing medical condition and below normal vitamin D levels were associated with higher odds of death (ii) After adjustment of confounders (age, sex and comorbidity), vitamin D levels showed a strong relationship with COVID-19 mortality |
On the other hand, a study used UK Biobank data (2006–2010) for vitamin D status, and ethnicity and correlated with COVID-19 cases [18]; no significant difference was observed for vitamin D levels with COVID-19 cases after adjustment of potential confounders. However, ethnicity showed a significant association with COVID-19 infection in univariate analysis. Another study used the same UK Biobank data (2006–2010) for BMI, vitamin D status and ethnicity and noted some interesting observations [15]. The authors did not find any significant difference for mean vitamin D levels between COVID-19 cases and the control group. Vitamin D status was significantly lower in those of Asian, Black and Mixed ethnicity (p < 0.001) compared with those of White ethnicity. Moreover, vitamin D levels were significantly lower in those with obesity (p < 0.001); overweight or obese male subjects living in London and being of Asian, Black or Mixed ethnicity were associated with a higher odd of positive test cases. However, in the regression model, the interaction between BMI and vitamin D status did not predict test results in the available dataset.
It is important to note that there are some potential factors that are associated with COVID-19 severity and mortality, such as age, sex, ethnicity, comorbidities and co-infection. A study determined the impact of comorbidities on 1590 COVID-19 patients in China and reported that COVID-19 patients with any comorbidity (hypertension, diabetes) produced poorer clinical outcomes than those without [63]. Another study in South Korea indicated an association of comorbidities with COVID-19 infection and severe clinical courses [64]. A systematic review and meta-analysis demonstrated that age and the underlying disease including hypertension, cardiovascular disease, and respiratory system disease are possible risk factors for severe COVID-19 patients compared to non-severe patients [65]. Besides comorbidities, co-infections might be another important determinant of severity and mortality in COVID-19 patients. Both bacterial and fungal co-infections have been identified in COVID-19 patients [66]. Currently, there is no clear evidence that people living with HIV are at higher risk of COVID-19 infection. However, people living with HIV who have a compromised immune system need extra caution to prevent COVID-19 infections. The importance of co-infections in the severity of respiratory diseases has been widely studied; however, they are understudied in the COVID-19 outbreak. Therefore, more data on co-infections are required to determine their impacts on COVID-19 severity and mortality.
Beneficial effects of vitamin D supplementation
COVID-19 has been declared a global pandemic by the World Health Organization. Yet, information is limited about the potential protective factors of this infection. Presently, there is no clear evidence that vitamin D supplementation prevents the severity and mortality of COVID-19. There are some registered randomized trials in evaluating the role of vitamin D in COVID-19 infections and severities but have not yet reported their findings. Up to now, there is a small cohort study described above [16] that demonstrated the protective effects of combined vitamin D, Mg and vitamin B12 against clinical deterioration of COVID-19. In a previous meta-analysis, vitamin D supplementation has been shown as safe and effective in preventing acute respiratory tract infections [59]. They also added that subjects who had severe vitamin D deficiency experienced the maximum benefits from the supplementation. The authors also noticed that the protective role of vitamin D was high in subjects with a baseline serum 25(OH)D levels <25 nmol/L compared to those with serum 25(OH)D concentrations >25 nmol/L [59]. In the same study, subgroup analysis indicated that daily or weekly intake of vitamin D (without additional bolus doses) showed protective effects against acute respiratory tract infection, especially in persons with vitamin D deficiency. D supplementation also found to increase gene expression related to antioxidation (glutathione reductase modifier subunit) [67]. The increased production of glutathione spares the use of vitamin C, which has potential antimicrobial activities [68], [69], and has been suggested to prevent and treat COVID-19 infection [12]. Up to now, there is limited evidence that supplementation of vitamin D at 20–50 μg/day has any adverse health effects. Indeed, supplementation of vitamin D with doses up to 100 μg/day is safe for adults [70], [71], and many expert groups now suggest supplementation in older people, although at lower levels than it. A study reported that intake of vitamin D supplement at 100–250 μg/day over 6 weeks increases the baseline serum concentration of 25(OH)D from 2 to 3 folds, respectively, without any adverse health effects [72].
Thus from literature, it is worth to suggest take up to 250 μg/day for a month which is effective to increase the serum levels of 25(OH)D into the optimal range between 75 and125 nmol/L. The dose can be reduced to 100 μg/day after one month to maintain the circulating concentrations of 25(OH)D [70], [71]. However, future clinical trials may be worth evaluating the potency of different vitamin D dosing plans on acute respiratory tract infections, for example once per week, which can be easier for implementation. The serum response to the given dose is largely varied between the individuals due to differences in demographic and biological variables, such as ethnicity, age, duration of exposure, seasonal variations, body mass index, intake of certain medications, baseline concentration of vitamin D, genetics and type of vitamin D supplements [73], [74]. Moreover, heterogeneity of the population as well as the dose of vitamin D needs to be considered during determining the preventing role of vitamin D in COVID-19. To evaluate the impacts of vitamin D supplementation in randomized controlled trials, emphasis should be given on study design based on serum concentration of 25(OH)D rather than administered dose concentration [74]. A recent review also suggested magnesium supplementation with vitamin D supplements as magnesium helps in regulating phosphate and calcium homeostasis [12]. The enzymes involved in vitamin D metabolism seem to need magnesium which plays an important role as a cofactor in enzymatic reactions especially in the kidney and liver [75].
Conclusions and recommendations
In the randomized trials and meta-analysis, vitamin D supplementation has been shown to have protective effects against respiratory tract infections; therefore, people who are at higher risk of vitamin D deficiency during this global pandemic should consider taking vitamin D supplements to maintain the circulating 25(OH)D in the optimal levels (75–125 nmol/L). Some retrospective studies demonstrated a correlation between vitamin D and COVID-19 cases and outcomes, while other studies did not find the correlation when confounding variables are adjusted. Yet, there is insufficient evidence on the association between vitamin D levels and COVID-19 severity and mortality. Therefore, randomized controlled trials and large-scale cohort studies are necessary to test this hypothesis.
Funding
None.
Conflict of interest
The author has no conflict of interest to declare.
Ethical approval
Not required.
References
- 1.Holick M.F. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocrine Metab Disord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 2.Dankers W., Colin E.M., van Hamburg J.P., Lubberts E. Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol. 2017;7:697. doi: 10.3389/fimmu.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Infante M., Ricordi C., Sanchez J., Clare-Salzler M.J., Padilla N., Fuenmayor V. Influence of vitamin d on islet autoimmunity and beta-cell function in type 1 diabetes. Nutrients. 2019;11:2185. doi: 10.3390/nu11092185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouillon R., Marcocci C., Carmeliet G., Bikle D., White J.H., Dawson-Hughes B. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocrine Rev. 2019;40:1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greiller C.L., Martineau A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gombart A.F., Borregaard N., Koeffler H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 7.Wang T.-T., Dabbas B., Laperriere D., Bitton A.J., Soualhine H., Tavera-Mendoza L.E. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285:2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannell J.J., Vieth R., Umhau J.C., Holick M.F., Grant W.B., Madronich S. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannell J.J., Vieth R., Willett W., Zasloff M., Hathcock J.N., White J.H. Cod liver oil, vitamin A toxicity, frequent respiratory infections, and the vitamin D deficiency epidemic. Ann Otol Rhinol Laryngol. 2008;117:864–870. doi: 10.1177/000348940811701112. [DOI] [PubMed] [Google Scholar]
- 10.Ginde A.A., Mansbach J.M., Camargo C.A. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y.-F., Luo B.-A., Qin L.-L. The association between vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine. 2019;98 doi: 10.1097/MD.0000000000017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watkins J. British Medical Journal Publishing Group; 2020. Preventing a covid-19 pandemic. [Google Scholar]
- 14.Daneshkhah A., Agrawal V., Eshein A., Subramanian H., Roy H.K., Backman V. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients [preprint] Infect Dis (except HIV/AIDS) 2020 doi: 10.1101/2020.04.08.20058578. [DOI] [Google Scholar]
- 15.Darling A.L., Ahmadi K.R., Ward K.A., Harvey N.C., Couto Alves A., Dunn-Waters D.K. Vitamin D status, body mass index, ethnicity and COVID-19: Initial analysis of the first-reported UK Biobank COVID-19 positive cases (n 580) compared with negative controls (n 723) [preprint] Infect Dis (except HIV/AIDS) 2020 doi: 10.1101/2020.04.29.20084277. [DOI] [Google Scholar]
- 16.De Smet D., De Smet K., Herroelen P., Gryspeerdt S., Martens G.A. Vitamin D deficiency as risk factor for severe COVID-19: a convergence of two pandemics [preprint] Infect Dis (except HIV/AIDS) 2020 doi: 10.1101/2020.05.01.20079376. [DOI] [Google Scholar]
- 17.Glicio EJ. Vitamin D level of mild and severe elderly cases of COVID-19: a preliminary report. Available at SSRN: https://ssrn.com/abstract=3593258; 2020.
- 18.Hastie C.E., Mackay D.F., Ho F., Celis-Morales C.A., Katikireddi S.V., Niedzwiedz C.L. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabet Metab Syndr: Clin Res Rev. 2020;14:561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020 doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau F.H., Majumder R., Torabi R., Saeg F., Hoffman R., Cirillo J.D. Vitamin D insufficiency is prevalent in severe COVID-19 [preprint] Infect Dis (except HIV/AIDS) 2020 doi: 10.1101/2020.04.24.20075838. [DOI] [Google Scholar]
- 21.Li Y, Li Q, Zhang N, Liu Z. Sunlight and vitamin D in the prevention of coronavirus disease (COVID-19) infection and mortality in the United States (preprint, in review); 2020. doi:10.21203/rs.3.rs-32499/v1.
- 22.Raharusun P., Priambada S., Budiarti C., Agung E., Budi C. Patterns of COVID-19 mortality and vitamin D: an Indonesian study. SSRN J. 2020 doi: 10.2139/ssrn.3585561. [DOI] [Google Scholar]
- 23.Teymoori-Rad M., Shokri F., Salimi V., Marashi S.M. The interplay between vitamin D and viral infections. Rev Med Virol. 2019;29:e2032. doi: 10.1002/rmv.2032. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Military Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakovac H. COVID-19 and vitamin D—is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318:E589. doi: 10.1152/ajpendo.00138.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.https://worldometers.info/coronavirus/.
- 28.Coussens A.K. The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease. Photochem Photobiol Sci. 2017;16:314–338. doi: 10.1039/c6pp00355a. [DOI] [PubMed] [Google Scholar]
- 29.Lang P.O., Aspinall R. Vitamin D status and the host resistance to infections: what it is currently (not) understood. Clin Ther. 2017;39:930–945. doi: 10.1016/j.clinthera.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Gruber-Bzura B.M. Vitamin D and influenza—prevention or therapy? Int J Mol Sci. 2018;19:2419. doi: 10.3390/ijms19082419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rondanelli M., Miccono A., Lamburghini S., Avanzato I., Riva A., Allegrini P. Self-care for common colds: the pivotal role of vitamin D, vitamin C, zinc, and Echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds—practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid-Based Complement Altern Med. 2018;2018 doi: 10.1155/2018/5813095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantorna M.T., Snyder L., Lin Y.-D., Yang L. Vitamin D and 1, 25 (OH) 2D regulation of T cells. Nutrients. 2015;7:3011–3021. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez N., Aguilar-Jimenez W., Rugeles M.T. The potential protective role of vitamin D supplementation on HIV-1 infection. Front Immunol. 2019;10:2291. doi: 10.3389/fimmu.2019.02291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian Y., Rong L. Covid-19 and vitamin D-authors’ reply. Aliment Pharmacol Ther. 2020 doi: 10.1111/apt.15764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsujino I., Ushikoshi-Nakayama R., Yamazaki T., Matsumoto N., Saito I. Pulmonary activation of vitamin D3 and preventive effect against interstitial pneumonia. J Clin Biochem Nutr. 2019;65:245–251. doi: 10.3164/jcbn.19-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zdrenghea M.T., Makrinioti H., Bagacean C., Bush A., Johnston S.L., Stanciu L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017;27:e1909. doi: 10.1002/rmv.1909. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y., Liu T., Yao L., Xing Y., Zhao X., Fu J. Chronic vitamin D deficiency induces lung fibrosis through activation of the renin–angiotensin system. Sci Rep. 2017;7:1–10. doi: 10.1038/s41598-017-03474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skariyachan S., Challapilli S.B., Packirisamy S., Kumargowda S.T., Sridhar V.S. Recent aspects on the pathogenesis mechanism, animal models and novel therapeutic interventions for Middle East respiratory syndrome coronavirus infections. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCartney D.M., Byrne D.G. Optimisation of vitamin D status for enhanced immuno-protection against Covid-19. Irish Med J. 2020;113:58. [PubMed] [Google Scholar]
- 41.Vankadari N., Wilce J.A. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microb Infect. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komolmit P., Charoensuk K., Thanapirom K., Suksawatamnuay S., Thaimai P., Chirathaworn C. Correction of vitamin D deficiency facilitated suppression of IP-10 and DPP IV levels in patients with chronic hepatitis C: a randomised double-blinded, placebo-control trial. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0174608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miroliaee A.E., Salamzadeh J., Shokouhi S., Sahraei Z. The study of vitamin D administration effect on CRP and Interleukin-6 as prognostic biomarkers of ventilator associated pneumonia. J Crit Care. 2018;44:300–305. doi: 10.1016/j.jcrc.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 44.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.26097. jmv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surveillances V. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 49.Poudel-Tandukar K., Poudel K.C., Jimba M., Kobayashi J., Johnson C.A., Palmer P.H. Serum 25-hydroxyvitamin d levels and C-reactive protein in persons with human immunodeficiency virus infection. AIDS Res Hum Retroviruses. 2013;29:528–534. doi: 10.1089/aid.2012.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dancer R.C., Parekh D., Lax S., D’Souza V., Zheng S., Bassford C.R. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS) Thorax. 2015;70:617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manion M., Hullsiek K.H., Wilson E.M., Rhame F., Kojic E., Gibson D. Vitamin D deficiency is associated with IL-6 levels and monocyte activation in HIV-infected persons. PLoS ONE. 2017;12:e0175517. doi: 10.1371/journal.pone.0175517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu D., Zhang J., Ma C., Yue Y., Zou Z., Yu C. Link between community-acquired pneumonia and vitamin D levels in older patients. Z Gerontol Geriatr. 2018;51:435–439. doi: 10.1007/s00391-017-1237-z. [DOI] [PubMed] [Google Scholar]
- 53.Hou Y.-M., Zhao J.-Y., Liu H.-Y. Impact of serum 25-hydroxyvitamin D on cardiac prognosis in Chinese patients with heart failure. Br J Nutr. 2019;122:162–171. doi: 10.1017/S0007114519000795. [DOI] [PubMed] [Google Scholar]
- 54.Pittas A.G., Dawson-Hughes B., Sheehan P., Ware J.H., Knowler W.C., Aroda V.R. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520–530. doi: 10.1056/NEJMoa1900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Autier P., Mullie P., Macacu A., Dragomir M., Boniol M., Coppens K. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabet Endocrinol. 2017;5:986–1004. doi: 10.1016/S2213-8587(17)30357-1. [DOI] [PubMed] [Google Scholar]
- 56.Rejnmark L., Bislev L.S., Cashman K.D., Eiríksdottir G., Gaksch M., Grübler M. Non-skeletal health effects of vitamin D supplementation: a systematic review on findings from meta-analyses summarizing trial data. PLoS ONE. 2017;12:e0180512. doi: 10.1371/journal.pone.0180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergman P., Lindh ÅU, Björkhem-Bergman L., Lindh J.D. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2013;8:e65835. doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charan J., Goyal J., Saxena D., Yadav P. Vitamin D for prevention of respiratory tract infections: a systematic review and meta-analysis. J Pharmacol Pharmacother. 2012;3:300. doi: 10.4103/0976-500X.103685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lips P., Cashman K.D., Lamberg-Allardt C., Bischoff-Ferrari H.A., Obermayer-Pietsch B., Bianchi M.L. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019:P23–P54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 61.Alipio M. Vitamin D supplementation could possibly improve clinical outcomes of patients infected with coronavirus-2019 (COVID-2019). Available at SSRN 3571484; 2020.
- 62.Tan C.W., Ho L.P., Kalimuddin S., Cherng B.P.Z., Teh Y.E., Thien S.Y. A cohort study to evaluate the effect of combination Vitamin D. Magnesium and Vitamin B12 (DMB) on progression to severe outcome in older COVID-19 patients [preprint] Infect Dis (except HIV/AIDS) 2020 doi: 10.1101/2020.06.01.20112334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guan W., Liang W., Zhao Y., Liang H., Chen Z., Li Y. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ji W., Huh K., Kang M., Hong J., Bae G.H., Lee R. Effect of underlying comorbidities on the infection and severity of COVID-19 in South Korea [preprint] Infect Dis (except HIV/AIDS) 2020 doi: 10.1101/2020.05.08.20095174. [DOI] [Google Scholar]
- 65.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lei G.-S., Zhang C., Cheng B.-H., Lee C.-H. Mechanisms of action of vitamin D as supplemental therapy for pneumocystis pneumonia. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01226-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mousavi S., Bereswill S., Heimesaat M.M. Immunomodulatory and antimicrobial effects of vitamin C. Eur J Microbiol Immunol (Bp) 2019;9:73–79. doi: 10.1556/1886.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colunga Biancatelli R.M.L., Berrill M., Marik P.E. The antiviral properties of vitamin C. Expert Rev Anti Infect Ther. 2020;18:99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- 70.Vieth R., Kimball S., Hu A., Walfish P.G. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3:8. doi: 10.1186/1475-2891-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bischoff-Ferrari H.A., Shao A., Dawson-Hughes B., Hathcock J., Giovannucci E., Willett W.C. Benefit-risk assessment of vitamin D supplementation. Osteoporos Int. 2010;21:1121–1132. doi: 10.1007/s00198-009-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Charoenngam N., Shirvani A., Kalajian T.A., Song A., Holick M.F. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: a randomized, double-blinded, dose-response study. Anticancer Res. 2020;40:551–556. doi: 10.21873/anticanres.13984. [DOI] [PubMed] [Google Scholar]
- 73.Mazahery H., von Hurst P. Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients. 2015;7:5111–5142. doi: 10.3390/nu7075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fabbri A., Infante M., Ricordi C. Editorial-Vitamin D status: a key modulator of innate immunity and natural defense from acute viral respiratory infections. Eur Rev Med Pharmacol Sci. 2020;24:4038–4042. doi: 10.26355/eurrev_202004_20876. [DOI] [PubMed] [Google Scholar]
- 75.Uwitonze A.M., Razzaque M.S. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181–189. doi: 10.7556/jaoa.2018.037. [DOI] [PubMed] [Google Scholar]