Highlights
-
•
We evaluated ECG features of patients hospitalized for COVID-19 pneumonia.
-
•
New ECG abnormalities reflected a wide spectrum of cardiovascular complications.
-
•
The most common manifestations were signs of pericarditis and atrial fibrillation.
-
•
ECG abnormalities showed a late onset from hospitalization.
-
•
ECG abnormalities seem to follow a separate course from the respiratory infection.
Keywords: COVID-19, SARS-CoV-2, Electrocardiography, Prognosis, Heart
Abstract
Background
. The electrocardiographic (ECG) changes which may occur during hospitalization for COVID-19 have not yet been comprehensively assessed.
Patients and methods
. We examined 50 patients admitted to hospital with proven COVID-19 pneumonia. At entry, all patients underwent a detailed clinical examination, 12-lead ECG, laboratory tests and arterial blood gas test. ECG was also recorded at discharge and in case of worsening clinical conditions.
Results
. Mean age of patients was 64 years and 72% were men. At baseline, 30% of patients had ST-T abnormalities, and 33% had left ventricular hypertrophy. During hospitalization, 26% of patients developed new ECG abnormalities which included atrial fibrillation, ST-T changes, tachy-brady syndrome, and changes consistent with acute pericarditis. One patient was transferred to intensive care unit for massive pulmonary embolism with right bundle branch block, and another for non-ST segment elevation myocardial infarction. Patients free of ECG changes during hospitalization were more likely to be treated with antiretrovirals (68% vs 15%, p = 0.001) and hydroxychloroquine (89% vs 62%, p = 0.026) versus those who developed ECG abnormalities after admission. Most measurable ECG features at discharge did not show significant changes from baseline (all p>0.05) except for a slightly decrease in Cornell voltages (13±6 vs 11±5 mm; p = 0.0001) and a modest increase in the PR interval. The majority (54%) of patients with ECG abnormalities had 2 prior consecutive negative nasopharyngeal swabs. ECG abnormalities were first detected after an average of about 30 days from symptoms’ onset (range 12–51 days).
Conclusions
. ECG abnormalities during hospitalization for COVID-19 pneumonia reflect a wide spectrum of cardiovascular complications, exhibit a late onset, do not progress in parallel with pulmonary abnormalities and may occur after negative nasopharyngeal swabs.
1. Introduction
Coronavirus Disease 2019 (COVID-19) is the clinical manifestation of infection with Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) [1], [2]. The clinical course of the infection is characterized by respiratory symptoms (including fever, cough and fatigue) and may progress to pneumonia, acute respiratory distress syndrome (ARDS) and shock [1], [2], [3].
COVID-19 may exert an adverse impact on the heart and cardiovascular system. Some reports described acute cardiovascular syndromes of COVID-19, including myocarditis, acute coronary syndromes, and decompensated heart failure [4], [5], [6], [7].
In this regard, standard electrocardiography (ECG) may represent a crucial test in the diagnosis of myocardial injury or heart rhythm disturbances in patients with SARS-CoV-2. Nonetheless, ECG features of COVID-19 are still undefined, particularly during the acute phase of the disease.
Thus, our main aim was to elucidate ECG abnormalities related to cardiac involvement during hospitalization for COVID-19 pneumonia. Furthermore, we explored the relationship between respiratory function and ECG signs of heart damage.
2. Methods
In the setting of an ongoing registry of patients affected by COVID-19, we analyzed data from patients consecutively hospitalized from March 15 to April 15, 2020 in the Department of Internal Medicine of the Maugeri Care and Research Institute of Tradate (VA), Italy. All the patients were transferred from emergency or medical departments of other hospitals for respiratory symptoms and radiological findings of COVID-19 pneumonia [8]. The diagnosis of viral infection was confirmed in all patients by nasopharyngeal swab [8]. The hospital staff of our Department included internists, cardiologists, and pneumologists.
At admission, the initial evaluation included a detailed clinical examination, 12-lead ECG, laboratory tests and arterial blood gas test. The presence of comorbidities was defined according to documented medical history, as collected by physicians at study site-level. This assessment was performed by any physician during the clinical interview with the patient and by searching through medical records.
For protocol, ECG was also recorded at discharge from hospital and when worsening clinical conditions or significant changes in laboratory tests occurred.
Laboratory parameters were assessed using standard techniques. PaO2/FIO2 ratio was used to estimate the severity of respiratory dysfunction [9]. Arterial blood gas test was also performed at the time of significant ECG changes or worsening clinical conditions.
ECG was recorded with 25 mm/s and 1 mV/cm calibration, and 0.05–150 Hz filter setting. ECG tracings were coded and were analyzed off-line.
We measured the following ECG parameters: heart rate (HR), corrected QT interval (msec), Cornell voltage (mm), and presence of ST-T abnormalities (Yes vs No). The QT interval was measured as the time between the start of the Q wave and the end of the T wave and corrected by HR according to the Bazett's formula. Measures of PR (interval between the beginning of the P wave and the end of the R wave) and QRS (interval from the beginning of the Q wave to the end of the S wave), were also included. Presence and type of arrhythmias was also taken into account.
We computed the Cornell voltage as the sum of the amplitudes of S wave in V3 and R wave in aVL [10]. ST-T changes were analyzed according to the Minnesota Coding [11]. Criteria for ST-T changes were any of the following: (1) coexistence, in any leads I, II, aVL or V3-V6 of ST-segment horizontal or downward sloping depression ≥ 0.05 mV (code 4–1 or 4–2) plus T-wave asymmetric inversion (code 5–1 or 5–2); (2) ST-J depression 〈0.05 mV with ST-segment downward sloping and segment or T-wave nadir 〉 0.05 mV below P-R baseline, in any of leads I, II, aVL or V2–V6 (code 4–3); (3) ST-J depression of ≥ 0.10 mV and ST-segment upward sloping or U-shaped, in any of leads I, II, aVL or V2-V6 (code 4–4); (4) T-wave amplitude zero (flat), negative or diphasic (negative–positive type only) with < 0.10 mV negative phase in lead I, II, V3–V6, or in lead aVL when R-wave amplitude is ≥ 0.5 mV (code 5–3): (5) T-wave amplitude positive and T- to R-wave amplitude ratio < 1:20 in any of leads I, II, aVL or V3-V6 when R-wave amplitude in the corresponding leads is ≥ 1.0 mV (code 5–4) [11].
To detect left ventricular (LV) hypertrophy at ECG we used the body mass index (BMI)-corrected Perugia score [12]. For computation of the BMI-corrected Perugia score, the Cornell voltage was amplified proportionally to BMI, thereby providing a simple correction for voltage attenuation at the skin surface [12]. Thus, ECG LV hypertrophy was defined by a Cornell-BMI product ([R wave amplitude in lead aVL + S wave depth in lead V3] x BMI) > 604 mm∙kg/m2 or typical strain pattern (as defined by a ≥ 0.5 mm depression of the J point, T-wave inversion with asymmetric branches and rapid return to baseline) [13]. Main ECG changes related to cardiovascular (CV) complications were classified according to current Guidelines [14], [15], [16].
We used STATA 15 (StataCorp, USA) and R software version 3 (R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org) for data analysis.
We present data as mean ± standard deviation (SD) for continuous variables and proportions for categorical variables. We analyzed differences in proportions between groups using the χ2 test. Mean values of variables were compared by paired or independent sample t-test. Logistic regression model tested the relationship between the demographic, clinical and laboratory findings with the occurrence of ECG abnormalities. In 2-tailed tests, p values <0.05 were considered statistically significant
3. Results
Overall, we studied 50 patients with complete clinical data, laboratory tests and 12-lead ECGs.
Table 1 shows the main characteristics of patients. Mean age was 64 years. The most prevalent comorbidity was hypertension (50%). Current smokers were 10%. Baseline BP was 126/80 mmHg. Overall, 49 patients showed sinus rhythm at baseline and mean HR was 75 ± 17 b.p.m.
Table 1.
Baseline main characteristics of study population according to ECG abnormalities documented during hospitalization for COVID-19 pneumonia.
| Variable | Overall | New ECG changes | p | |
|---|---|---|---|---|
| (N = 50) | No (N = 37) | Yes (N = 13) | ||
| Age (years) | 64±15 | 64±12 | 65±20 | 0.776 |
| Sex (male,%) | 72 | 68 | 85 | 0.239 |
| BMI (Kg/m2) | 26.8 ± 4.4 | 27.4 ± 4.2 | 25.4 ± 4.7 | 0.157 |
| Systolic BP (mmHg) | 126±19 | 126±19 | 126±18 | 0.957 |
| Diastolic BP (mmHg) | 80±12 | 82±11 | 74±11 | 0.051 |
| Pulse pressure (mmHg) | 46±14 | 44±14 | 49±15 | 0.300 |
| Hypertension (%) | 50 | 46 | 62 | 0.333 |
| Current smoker (%) | 10 | 8 | 15 | 0.705 |
| Diabetes (%) | 12 | 14 | 8 | 0.578 |
| Coronary artery disease (%) | 10 | 8 | 15 | 0.452 |
| Heart failure (%) | 6 | 3 | 15 | 0.098 |
| COPD (%) | 2 | 3 | 0 | 0.549 |
| Antiretroviral (%) | 54 | 68 | 15 | 0.001 |
| Hydroxychloroquine (%) | 82 | 89 | 62 | 0.026 |
| Macrolides (%) | 56 | 57 | 54 | 0.856 |
| Enoxaparin (%) | 76 | 78 | 69 | 0.506 |
| RAS blockers (%) | 18 | 19 | 15 | 0.081 |
| PaO2/FIO2 ratio (mmHg) | 346±111 | 349±121 | 336±77 | 0.708 |
| pH | 7.44±0.03 | 7.45±0.02 | 7.44±0.04 | 0.437 |
| Hemoglobin (g/dl) | 12.6 ± 1.3 | 12.7 ± 1.2 | 12.3 ± 1.6 | 0.414 |
| White blood cell count (x103) | 7.0 ± 2.9 | 7.2 ± 2.8 | 6.3 ± 3.0 | 0.328 |
| Creatinine (mg/dl) | 0.83±0.22 | 0.80±0.15 | 0.90±0.3 | 0.063 |
| Potassium (mEq/l) | 4.3 ± 0.4 | 4.3 ± 0.5 | 4.3 ± 0.4 | 0.806 |
| CRP (mg/dl) | 3.1 ± 3.8 | 3.0 ± 3.8 | 3.5 ± 3.8 | 0.639 |
| HS-troponin I (pg/ml) | 8.04±9.45 | 7.18±8.32 | 10.72±12.38 | 0.264 |
| Blood urea nitrogen (mg/dl) | 35.2 ± 18.5 | 33.9 ± 16.9 | 38.2 ± 22.6 | 0.513 |
| Heart rate (/min) | 75±17 | 76±16 | 73±21 | 0.547 |
| PR interval (msec) | 164±26 | 161±18 | 173±41 | 0.178 |
| QRS duration (msec) | 99±13 | 98±12 | 101±16 | 0.532 |
| QTc (msec) | 428±26 | 427±23 | 432±36 | 0.533 |
| ST-T abnormalities (%) | 30 | 27 | 38 | 0.439 |
| LV hypertrophy (%) | 33 | 31 | 40 | 0.318 |
Legend: ECG=electrocardiographic; BMI=body mass index; BP=blood pressure; COPD=chronic obstructive pulmonary disease; RAS=renin-angiotensin system; CRP=C-reactive protein; HS=high sensitivity; LV=left ventricular. Normal value of HS-troponin I<15.6 pg/ml.
Table 1 also summarizes measured ECG parameters at baseline. ST-T abnormalities were relatively common (30%) and prevalence of LV hypertrophy was 33%.
During hospitalization, 13 patients (26%) developed new ECG abnormalities which included atrial fibrillation (6%), brady-tachy syndrome (2%), persistent ST-T changes not associated with raise in troponin I levels nor pericardial effusion (2%, Fig. 1 ) and persistent ST-T changes associated with acute pericarditis (12%, Fig. 2 ). Two patients (4%) were transferred to an intensive care unit (ICU) for the development of right bundle branch block due to massive pulmonary embolism and ST-T ischemic changes for non-ST elevation myocardial infarction.
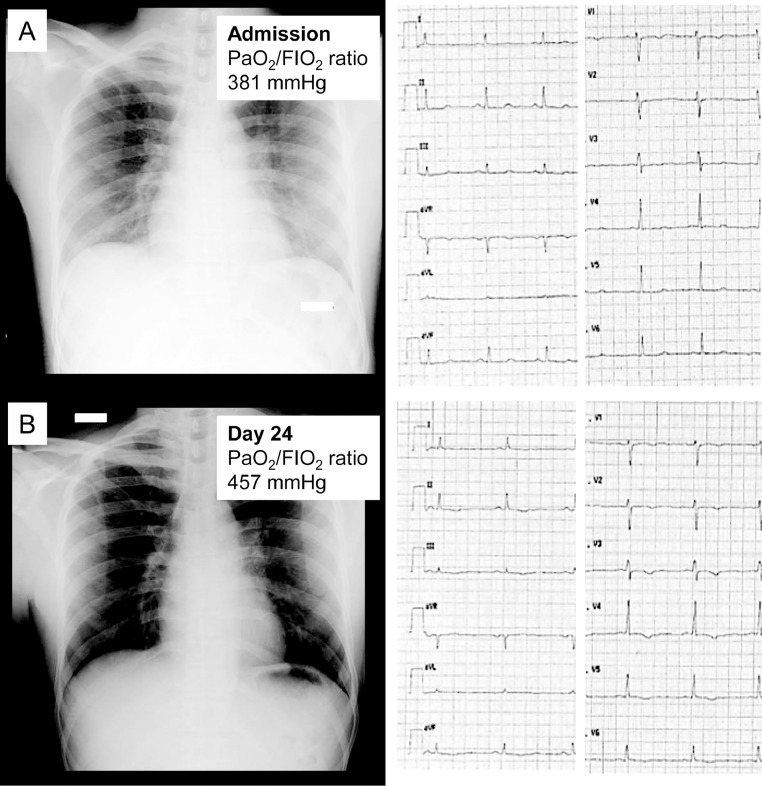
Fig. 1.
An healthy 23-year old white man without previous history of cardiovascular disease. At admission he reported fever, cough, and severe fatigue. Anteroposterior chest radiograph showed vague hazy densities and lung opacities (A). After recovery (day 22 from admission), he developed T inversion at 12-leads ECG (B). There was no pericardial effusion, nor left ventricular systolic dysfunction. High-sensitivity troponin I levels were persistently normal.
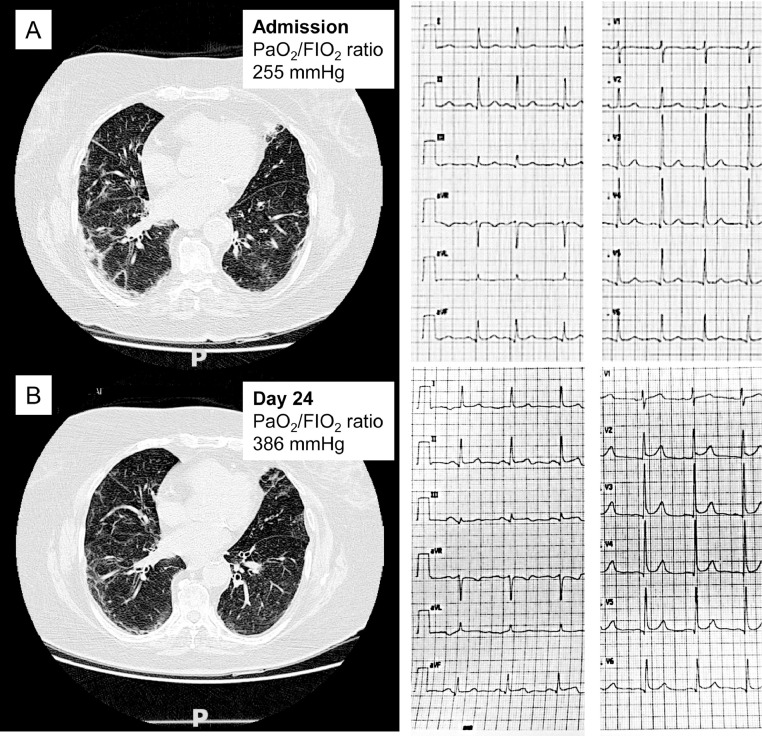
Fig. 2.
Pulmonary and cardiac involvement in a 79-year-old white woman. Computed tomographic (CT) images at middle level recorded at admission (A) and after 24 days (B). Despite a significant improvement in respiratory function detected by PaO2/FiO2 ratio and CT images, the patient developed chest pain and ECG signs of acute pericarditis (new widespread concave ST elevation and reciprocal ST depression in aVR). At day 26 the patient showed significant pericardial effusion.
In the overall cohort, 41 patients (82%) were treated with hydroxychloroquine (including 1 of the 2 patients transferred to an ICU), 27 (54%) with antiretrovirals, and 28 (56%) with macrolides.
Patients without new ECG changes during hospitalization were more likely to receive treatment with antiretrovirals (68% vs 15%, p = 0.001) and hydroxychloroquine (89% vs 62%, p = 0.026) than those who developed ECG abnormalities and, more importantly, treatments with these agents were associated with significant reduced odds of presenting ECG abnormalities during the hospital phase (odds ratios 0.20 and 0.10, respectively, all p<0.01) regardless of age, sex, and presence of comorbidities or cardiovascular risk factors (all p>0.05).
Mean duration of treatment with hydroxychloroquine was not significantly different between patients with or without new ECG abnormalities (13 vs 12 days, p = 0.349). Similar results were also obtained for treatment with antiretrovirals (14 vs 12 days, p = 0.169).
The distribution of other concomitant treatment (including enoxaparin, renin-angiotensin system blockers, and macrolides) was not dissimilar between the two groups. Gender distribution, BP values, PaO2/FIO2 ratio, serum markers of inflammation, troponin I levels, and prevalence of comorbidities were also similar (all p>0.05, Table 1). The measured ECG parameters (including QTc, HR, QRS duration and PR interval) did not differ between the two groups (all p>0.05, Table 1).
Excluding the two patients transferred to ICU, main ECG features recorded at discharge and related to ventricular depolarization and repolarization did not show significant changes from baseline (all p>0.05, Table 2 ). The time from the depolarization of the sinus node to the onset of ventricular depolarization (PR interval) exhibited a prolongation when compared to admission. There was also a slight decrease in the Cornell voltage (13±6 vs 11±5 mm, p = 0.0001). At discharge, only 1 patient exhibited a prolonged QTc interval (≥ 500 msec with a QRS ≤120 milliseconds) and 4 patients had a PR interval > 200 msec (ranging from 205 to 215 msec). Of note, none of the two patients transferred to an ICU showed prolonged QTc at the time of the cardiovascular event (for the patient with pulmonary embolism, we corrected QTc for new right bundle branch block [17]).
Table 2.
Changes in main ECG features between admission and pre-discharge.
| Feature | Admission | Pre-discharge | p |
|---|---|---|---|
| HR (/min) | 75±17 | 72±13 | 0.151 |
| PR interval (msec) | 161±19 | 167±20 | 0.009 |
| QRS duration (msec) | 98±12 | 96±11 | 0.163 |
| QTc (msec) | 426±25 | 420±39 | 0.291 |
| ST-T abnormalities (%) | 29 | 13 | 0.235 |
| Cornell voltage (mm) | 13±6 | 11±5 | 0.0001 |
Legend: HR=heart rate.
The development of ECG abnormalities during hospitalization was unrelated to the severity of respiratory function. In particular, the average PaO2/FIO2 ratio at the time of development of ECG changes was significantly increased from baseline (mean difference +51, p = 0.013). To further elucidate the relationship between the respiratory function and the new development of ECG abnormalities we grouped patients using categories of PaO2/FIO2 ratio [9]. During the development of ECG abnormalities, 5 patients (38%) were reclassified to a higher category, 7 patients (54%) to the same category, and only 1 patient (8%) to a lower category of PaO2/FIO2 ratio when compared to admission evaluation.
Furthermore, among patients with new ECG changes, abnormal serum levels of HS-troponin I were recorded in the 38% of cases.
The development of ECG abnormalities significantly affected the length of in-hospital stay. ECG abnormalities occurred after averages of 30 and 20 days from onset of COVID-19 symptoms (range 12–51 days) and admission to hospital (range 2–29 days), respectively. Notably, a large proportion (54%) of patients with ECG abnormalities had two 2 prior negative nasopharyngeal swabs.
4. Discussion
The classical clinical picture of COVID-19 is characterized by a flu-like syndrome of mild severity in most cases, but in about 15% of cases it is complicated by interstitial pneumonia with a variable degree of respiratory failure [18], [19]. Nonetheless, some case reports and systematic reviews drawn attention to the CV adverse effects associated with COVID-19 [4], [5], [6], [7], [20], [21], [22], [23]. Specifically, COVID-19 has been associated with complete hart block, acute coronary syndromes, myocarditis, decompensated heart failure, and pulmonary embolisms [5], [6], [20], [22], [23]. Despite the important role of ECG in diagnosing CV complications during the acute phase, to our knowledge there are no studies focused on ECG features and their changes during hospitalization for COVID-19 pneumonia. In this regard, we analyzed data from 50 patients consecutively admitted to hospital for proven COVID-19 pneumonia. Results of our analysis offer some key issues which deserve to be mentioned.
First, ECG abnormalities developed during hospitalization for COVID-19 pneumonia reflect a wide spectrum of cardiovascular complications including acute coronary syndromes, rhythm disorders, ST-T ischemic changes (Fig. 1), acute pericarditis (Fig. 2), and pulmonary embolism. These findings support the notion that ECG abnormalities developed during hospitalization may have a relevant clinical impact on the course of the disease and that COVID-19 infection may also be linked with an increased long-term cardiovascular risk.
The most common manifestation was ECG signs of acute pericarditis as diagnosed by new widespread concave ST elevation and PR depression throughout most of the limb (I, II, III, aVL, aVF) and precordial (V2-V6) leads, reciprocal ST depression and PR elevation in aVR, and a ST segment/T wave ratio> 0.25 [14]. COVID-19 induced pericarditis might reflect the expression of ACE2 receptors in epicardial adipocites, mediating the cell entry of SARS-CoV-2 [24], [25], [26], and possibly triggering local inflammation.
In this context, it is worth mentioning that epicardial fat has been linked to atrial electrical remodeling and the progression of atrial fibrillation [27], [28]. Although it is likely that atrial fibrillation may be related to COVID-19 infection (systemic hyperinflammation, fever, hypoxia, adrenergic tone), the involvement of epicardial adipocites (as demonstrated by ECG signs of pericarditis and development of pericardial effusion) during SARS-CoV-2 infection could predispose also to the development of atrial fibrillation. Taken together, these findings reinforce the recommendation to carefully re-asses the therapeutic choices of anticoagulation, balancing thromboembolic and bleeding risk.
In our cohort, 5 patients also exhibited a prolonged QT interval or a delayed conduction of the sinoatrial nodal impulse to the ventricle. The prolongation of PR interval observed in 4 patients did not exert a clinically significant effect because of a PR interval at discharge not exceeding 215 msec. The observation at discharge of a prolonged QTc (>500 msec) in only 1 patient suggests that current therapeutic approaches for COVID-19 [19] may exert limited effects on ventricular depolarization and repolarization [29]. More specifically, the use of hydroxychloroquine, which are structurally similar to quinidine and have QT-prolonging effects by blocking activation of the potassium channel IKr [30], does not appear to adversely affect QTc in COVID-19, although this was not among the primary aims of the present study.
More complex to understand is the observation of the reduction from baseline to discharge in Cornell voltages (13±6 vs 11±5 mm, p = 0.0001). This phenomenon might reflect the presence of pleural or pericardial effusions, [31], [32] epicardial edema, [33], or electric or conduction alternans associated with tachyarrhythmias.
Second, patient characteristics, previous vascular events, comorbidities and baseline ECG features had an insufficient discriminatory power to identify subjects at increased risk for the development of new ECG changes (including ST-T abnormalities, cardiac rhythm disturbances, clinically significant atrioventricular and interventricular delays, and ventricular depolarization and repolarization prolongations). Demographics and clinical characteristics, respiratory function, serum biomarkers of inflammation and myocardial injury, presence of comorbidities or previous vascular events, and baseline ECG features showed a similar distribution between patients with or without ECG changes recorded during hospitalization (all p>0.05, Table 1).
However, patients without new ECG changes during hospitalization were more likely to receive treatment with antiretrovirals and hydroxychloroquine and, more importantly, treatment with these agents were associated with a significant reduction in the risk of developing new ECG abnormalities during the hospital phase.
Finally, ECG abnormalities showed a late onset from hospitalization and initiation of COVID-19 symptoms. In our cohort, the average time for development of ECG abnormalities was 20 and 30 days from admission and onset of symptoms, respectively. Of note, a large proportion of patients (54%) experienced ECG abnormalities immediately before the scheduled discharge from hospital and after 2 consecutive negative nasopharyngeal swabs.
The present study should be interpreted in the context of its limitations. One, because 96% of our patients were Caucasians, we cannot extend the conclusions to different ethnic groups. Two, the period of observation in our cohort was limited to the hospital phase.
In conclusion, despite the evidence of multi-organ involvement in COVID-19, our observations suggest that the evolution of ECG abnormalities is independent from the severity of pulmonary tract infection. The ECG abnormalities exhibit a late onset, reflect a wide spectrum of cardiovascular complications and frequently occur after negative nasopharyngeal swabs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None of the authors of this study has financial or other reasons that could lead to a conflict of interest.
References
- 1.Cheng Z.J., Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48:155–163. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brussow H. The Novel Coronavirus - A Snapshot of Current Knowledge. Microb Biotechnol. 2020;13:607–612. doi: 10.1111/1751-7915.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akhmerov A., Marban E. COVID-19 and the Heart. Circ Res. 2020 doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 7.Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E. The Variety of Cardiovascular Presentations of COVID-19. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T. Diagnosis and clinical management of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: an operational recommendation of Peking Union Medical College Hospital (V2.0) Emerg Microbes Infect. 2020;9:582–585. doi: 10.1080/22221751.2020.1735265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonca A., Bruining H. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 10.Casale P.N., Devereux R.B., Kligfield P., Eisenberg R.R., Miller D.H., Chaudhary B.S. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 11.Prineas R., Crow R., Blackburn H. John Wright-PSG.; Littleton: 1982. The minnesota code manual of electrocardiographic findings: standards and procedures for measurement and classification. [Google Scholar]
- 12.Angeli F., Verdecchia P., Iacobellis G., Reboldi G. Usefulness of QRS voltage correction by body mass index to improve electrocardiographic detection of left ventricular hypertrophy in patients with systemic hypertension. Am J Cardiol. 2014;114:427–432. doi: 10.1016/j.amjcard.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Roman M.J., Kligfield P., Devereux R.B., Niles N.W., Hochreiter C., Halle A. Geometric and functional correlates of electrocardiographic repolarization and voltage abnormalities in aortic regurgitation. J Am Coll Cardiol. 1987;9:500–508. doi: 10.1016/s0735-1097(87)80041-4. [DOI] [PubMed] [Google Scholar]
- 14.Adler Y., Charron P., Imazio M., Badano L., Baron-Esquivias G., Bogaert J. ESC Guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J, 2015. 2015;36:2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calkins H. The 2019 ESC Guidelines for the Management of Patients with Supraventricular Tachycardia. Eur Heart J. 2019;40:3812–3813. doi: 10.1093/eurheartj/ehz837. [DOI] [PubMed] [Google Scholar]
- 16.Roffi M., Patrono C., Collet J.P., Mueller C., Valgimigli M., Andreotti F. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J, 2016. 2015;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 17.Talbot S. QT interval in right and left bundle-branch block. Br Heart J. 1973;35:288–291. doi: 10.1136/hrt.35.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linton N.M., Kobayashi T., Yang Y., Hayashi K., Akhmetzhanov A.R., Jung S.M. Incubation Period and Other Epidemiological Characteristics of 2019 Novel Coronavirus Infections with Right Truncation: a Statistical Analysis of Publicly Available Case Data. J Clin Med. 2020;9 doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poston J.T., Patel B.K., Davis A.M. Management of Critically Ill Adults With COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.4914. [DOI] [PubMed] [Google Scholar]
- 20.Azarkish M., Laleh Far V., Eslami M., Mollazadeh R. Transient complete heart block in a patient with critical COVID-19. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui Y., Tian M., Huang D., Wang X., Huang Y., Fan L. A 55-Day-Old Female Infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong N., Cai J., Zhou Y., Liu J., Li F. End-stage Heart Failure with COVID-19: strong Evidence of Myocardial Injury by 2019-nCoV. JACC Heart Fail. 2020 doi: 10.1016/j.jchf.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ullah W., Saeed R., Sarwar U., Patel R., Fischman D.L. COVID-19 complicated by Acute Pulmonary Embolism and Right-Sided Heart Failure. JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel V.B., Basu R., Oudit G.Y. ACE2/Ang 1-7 axis: a critical regulator of epicardial adipose tissue inflammation and cardiac dysfunction in obesity. Adipocyte. 2016;5:306–311. doi: 10.1080/21623945.2015.1131881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020 doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verdecchia P., Reboldi G., Cavallini C., Mazzotta G., Angeli F. [ACE-inhibitors, angiotensin receptor blockers and severe acute respiratory syndrome caused by coronavirus] G Ital Cardiol (Rome) 2020;21:321–327. doi: 10.1714/3343.33127. [DOI] [PubMed] [Google Scholar]
- 27.Friedman D.J., Wang N., Meigs J.B., Hoffmann U., Massaro J.M., Fox C.S. Pericardial fat is associated with atrial conduction: the Framingham Heart Study. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thanassoulis G., Massaro J.M., O'Donnell C.J., Hoffmann U., Levy D., Ellinor P.T. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3:345–350. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roden D.M., Harrington R.A., Poppas A., Russo A.M. Considerations for Drug Interactions on QTc in Exploratory COVID-19 (Coronavirus Disease 2019) Treatment. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047521. [DOI] [PubMed] [Google Scholar]
- 30.Traebert M., Dumotier B., Meister L., Hoffmann P., Dominguez-Estevez M., Suter W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol. 2004;484:41–48. doi: 10.1016/j.ejphar.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Berthoud B., Ennezat P.V. Electrical alternans due to large bilateral pleural effusion without pericardial effusion. Int J Cardiol. 2014;176:e125–e126. doi: 10.1016/j.ijcard.2014.07.230. [DOI] [PubMed] [Google Scholar]
- 32.Kanaporis G., Blatter L.A. Alternans in atria: mechanisms and clinical relevance. Medicina (Kaunas) 2017;53:139–149. doi: 10.1016/j.medici.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X., Wang R., Qu G., Wang Y., Liu P., Zhu Y. Histological findings of COVID‐19 based on autopsy: a case report. J Forensic Med. 2020;36:1–3. http://www.fyxzz.cn/CN/article/downloadArticleFile.do?attachType=PDF&id=23213 2020.36: 1-3; Available at: Accessed April 22, 2020. [Google Scholar]